Abstract
Neonatal diabetes mellitus (NDM) results from impaired insulin secretion, occurring within the first 6 months of life. NDM is classified as transient NDM (TNDM) or permanent NDM. To date there are no universal guidelines regarding its management. Intravenous insulin infusion represents the first and most adequate therapeutic approach for sustained hyperglycemia, but this can provide only a short-term solution. Several factors should be taken into account in the choice of the long-term treatment. We describe our experience with two infants affected by TNDM. The first child was treated with continuous subcutaneous insulin infusion, whereas the second infant was treated with subcutaneous insulin glargine injections. Our experience shows that the two different therapeutic approaches, if properly managed, are equally effective.
Introduction
Neonatal diabetes mellitus (NDM) is a rare genetic disease characterized by persisting hyperglycemia, requiring insulin treatment, and occurring within the first 6 months of life.1 In Italy, NDM has an estimated incidence of 1:90,000 live births.2 Study of human leukocyte antigens does not show a haplotype predisposing to type 1 diabetes mellitus, but occasionally there is a protective one (DQA1, DQB1); islet cell antibodies and other type 1 diabetes mellitus–specific antibodies are absent.3 There are two forms of NDM: transient (TNDM) and permanent (PNDM), which are characterized by different pathogenesis and clinical evolution.1,4
The genetic causes of NDM have been identified in 90% of TNDM and 70% of PNDM cases.5 The most frequent cause of TNDM is overexpression of the PLAG/ZAC and HYMAI imprinted genes, which are located within region 6q24. Three different genetic mechanisms result in the overexpression of these genes: paternal uniparental disomy of chromosome 6, paternal duplication of the critical region 6q24, or loss of methylation on the maternally derived allele. In addition, mutations in the KCNJ11 and ABCC8 genes coding for the Kir 6.2 and SUR1 subunits, respectively, of K+ channels have been associated with TNDM.6 Genetic bases of PNDM are more heterogeneous; the most frequent causes of PNDM are KCNJ11-activating mutations, accounting for 30% of cases, followed by ABCC8 mutations, but other genetic causes have been identified involving FOXP3, CD25, EIF2AK3, IPF1, GLIS3, PTF1A, HNF1β, and GCK.7
TNDM is characterized by dehydration and low birth weight. Macroglossia and hiatal hernia are often present.6 The disease begins in the first days of life, in the absence of ketoacidosis, and requires the administration of exogenous insulin in order to correct hyperglycemia and allow catch-up growth.1,8 Insulin treatment is required for a variable period, and usually TNDM resolves within 3 months; however, recurrence later in life, more often during adolescence, occurs in a significant number of cases.9,10
Treatment of TNDM is complex because of the paucity of subcutaneous fat (especially in low-birth-weight infants), the minute doses of insulin required, the different sensibility to exogenous insulin, and, in particular, the high risk of hypoglycemia with the potential for neuronal damage.11
We describe our experience with two infants affected by TNDM. The first child was treated with continuous subcutaneous insulin infusion (CSII), and the second was treated with subcutaneous insulin glargine injections. Our experience shows that the two different therapeutic approaches, if properly managed, are equally effective.
Case Reports
Infant 1
S.K., the second child born of healthy consanguineous Indian parents, was born by spontaneous delivery, at 37 weeks 6 days of gestational age. The pregnancy was complicated by threatened abortion and intrauterine growth retardation. The birth weight was 2,000 g. The family history was negative for both type 1 and type 2 diabetes.
She was referred to our department at the age of 15 days for hyperglycemia without ketosis, macroglossia, and cutaneous xerosis. Laboratory evaluation showed normal thyroid, renal, and liver function; insulin (2.0 IU/mL; normal range, 2.6–24.9 IU/mL) and C-peptide (0.8 ng/mL; normal range, 0.8–4.2 ng/mL) levels were at the lower limits of the reference range. Islet cell autoantibodies and glutamic acid decarboxylase autoantibodies were negative. The patient underwent ophthalmology and cardiology evaluation, abdominal ultrasound, and cerebral ultrasound, which revealed no abnormalities. Dermatological examination confirmed the presence of diffuse cutaneous xerosis detected at birth (the father and sister also suffered the same condition).
Molecular typing alleles revealed the presence of the following human leukocyte antigen haplotype: DR3/DQ2 (DRB1 *03,*03; DQA1 *0501,*0501; DQB1 *0201,*0201), which predisposes to type 1 diabetes mellitus. Genetic analysis of region 6q24 was performed in the patient and the parents. A methylation-specific polymerase chain reaction using two primers, each specific for the maternal or the paternal allele, after DNA treatment with thiosulfite, was performed. The methylation-specific polymerase chain reaction showed only one peak of amplification for nonmethylated microsatellite analysis, ruling out a paternal uniparental disomy and suggesting a defective methylation in the maternal allele determining the overexpression of PLAG1/ZAC genes. This defect resulted from an isolated imprinting mutation of the differentially methylated region because no mutations of ZFP57 were found. Genetic testing was performed in the Molecular Genetic Laboratory, Bianchi Melacrino Morelli Hospital, Reggio Calabria, Italy.
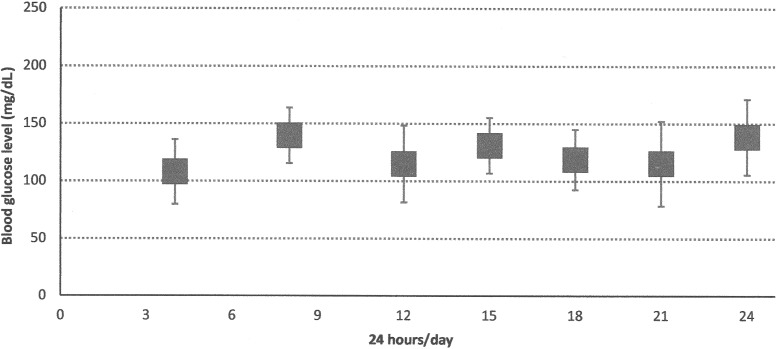
Insulin treatment was immediately begun intravenously at the dose of 0.01 unit/kg/h. After a good glycemic control was obtained, the subcutaneous insulin pump was started at a basal rate of 0.05 IU/h. The feeding pattern of the infant was based on seven formula bottles per day, at 3-h intervals, from 6:00 to 24:00. Blood glucose determinations were performed seven times a day. No premeal boluses were required because, as shown in Figure 1, there were moderate changes in blood glucose levels across daytime.
FIG. 1.
Case 1: weekly blood glucose profiles during the last week of hospitalization with continuous subcutaneous insulin infusion. Data are mean±SD values.
The baby was discharged at 36 days of life after the parents had been educated to manage the insulin pump. Insulin requirement rapidly decreased until discontinuation at 56 days of life, after 40 days of therapy. All further periodically performed blood glucose tests and hemoglobin A1c levels were normal.
At 5 years of age, auxological and neuropsychological development was appropriate, and she had no medical problems, except persistent xerosis treated with emollients.
Infant 2
This female infant was born at 37 weeks of gestation by cesarean section, weighing 1,600 g. Intrauterine growth retardation had been diagnosed antenatally. The family history was negative for any form of diabetes. Persistent hyperglycemia was noted from 12 h of age. Glycosuria was present, but ketonuria and ketosis were absent. Clinically, she showed mild dehydration, and subcutaneous tissue was scarce.
Results of laboratory and ultrasound exams were normal, except insulin levels, which were at the lower limits of the reference range. Islet cell autoantibodies and glutamic acid decarboxylase autoantibodies were negative.
Intravenous insulin infusion was started at the dose of 0.01 U/kg/h, achieving a good glycemic control in a few days. The minimal subcutaneous tissue prompted us not to use pump therapy, and, on Day 5, an attempt was made to introduce subcutaneous neutral protamine Hagedorn (NPH), administered twice daily at the dose of 0.6 unit/kg/day (0.55 unit/dose) into the buttock. Because of the minute dose to be administered, a 1:10 dilution of insulin NPH in 0.9% NaCl was prepared. The patient did not tolerate this regimen, which resulted in severe hypoglycemia within a few hours postdosing (<50 mg/dL), as well as hyperglycemia afterward (>250 mg/dL).
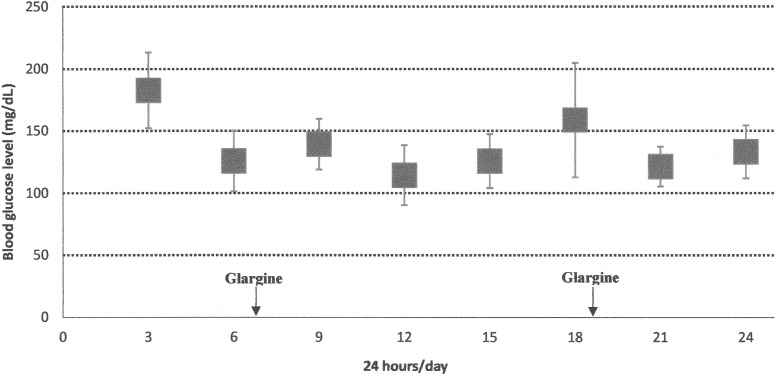
Therefore, we started insulin glargine, once every 24 h at the initial dose of 0.45 unit/kg/day, also diluted 1:10, and administered into the buttock. The daily insulin dose was slightly lowered compared with the NPH dose in order to reduce the risk of hypoglycemic episodes. After 2 weeks, as blood glucose measurements still showed occasional hypoglycemia, we started to further reduce the daily dose of insulin glargine, which was progressively lowered to 0.2 IU/kg/day. We also split the dose twice daily (every 12 h) to facilitate dose adjustments, if necessary. Because of the very small volume to be administered, we preferred to use a 1:100 dilution, which makes the drug more manageable and reduces risk of mistakes by the parents. This regimen allowed a satisfactory blood glucose control, as shown in Figure 2.
FIG. 2.
Case 2: weekly blood glucose profiles during the last week of hospitalization with insulin glargine. Data are mean±SD values.
The feeding pattern of the infant was based on eight formula bottles a day, at 3-h intervals, during the first 2 weeks of life and seven formula bottles a day, at 3-h intervals, from 6:00 to 24:00 h afterward. Blood glucose determinations were performed eight times a day.
The baby was discharged at 1 month of age. After 4 months, persistent euglycemia prompted discontinuation of insulin glargine.
To date, the patient is 3 years old, and she is in optimal general health and shows normal glucose and hemoglobin A1c levels. Genetic tests excluded abnormalities of chromosome 6q24 and mutations of Kir 6.2. Further investigations are in progress to establish the cause of TNDM in this patient.
Discussion
NDM (TNDM or PNDM) has increasingly been identified and described over the last decade because of evolution and expansion of genetic investigations.1 However, to date, there has been no universal guidelines regarding its management. Intravenous insulin infusion remains the first and most adequate therapeutic approach for sustained hyperglycemia, in order to obtain satisfactory blood glucose control in a titratable manner.12 In addition, infants with NDM are often dehydrated at presentation, and this would limit absorption of subcutaneous insulin. For this reason, both our infants were initially managed with intravenous short-acting insulin infusions. However, this can provide only a short-term solution because long-term intravenous infusions may be hazardous in small infants.13 Few data are available on the best mode of insulin delivery in the treatment of NDM.
The following aspects should be considered: the small amount of insulin to be delivered, insulin absorption, frequency and type of meals, and high risk of hypoglycemia and brain damage. Several factors should be taken into account in the choice of the long-term treatment. Absorption of drugs from subcutaneous injection sites is affected by several variables, such as local blood flow, muscle mass, and amount of adipose tissue. Absorption may also be affected by physiochemical characteristics of drugs, such as pH, ease of diffusion through capillary membranes, and surface area over which the injection volume spreads.14 Subcutaneous drug absorption is usually reduced in low-birth-weight infants because of a lower regional perfusion and reservoir mass.15 Doses of insulin required for these patients are minimal; therefore, adjustments of the dose require minimal changes that are not easily feasible. Frequent meals, typical of small babies, require frequent control of blood glucose levels and harbor the risk of frequent pre- and postmeal glucose fluctuations, although this did not occur in our two patients.
CSII allows an easy control of insulin delivery and blood glucose levels.16,17 Short-acting insulin analogs (e.g., insulin aspart and lispro), which have the advantage of rapid onset, short duration of action, and predictability, are commonly used.18 CSII allows delivery of smaller infusion volumes and more precise dose titration as well as the ability to switch off insulin delivery quickly. Hence, CSII reduces the risk of hypoglycemia with ensuing neuronal damage.19 CSII also allows a better control of hyperglycemia by bolus administration. Tubiana-Rufi20 reported experience with the use of CSII in NDM during 18 years, describing 17 newborns affected by NDM, eight of them with TNDM, and highlighting how CSII therapy is safe, more physiological and accurate, and easier to manage than intermittent insulin injections. Bharucha et al.13 described two infants who attained euglycemia once transitioned to CSII after failing to maintain glycemic control by subcutaneous insulin injections. Wintergerst et al.21 described a premature baby boy with severe growth retardation who developed diabetes on Day 2 of life. In this case, glycemic control had been also affected by medical complications, such as necrotizing enterocolitis, poor weight gain, and cholestatic jaundice. This required a continued use of intravenous insulin infusion until Day 108 of life, when the infant was placed on CSII with success. Beardsall et al.22 reported the combination of continuous glucose monitoring and insulin pump therapy in a preterm infant with neonatal diabetes with satisfactory results, reducing the risk of hypoglycemia.
Despite the obvious benefits, some specific factors should be considered before starting treatment with an insulin pump. This treatment requires a dedicated specialized multidisciplinary team with experience in managing CSII. Parents need to be properly educated about the correct use of the pump before the patient is discharged from the neonatal unit, in order to be aware of problems associated with insulin delivery system, such as checks of line, syringe, and cannula for kinks and for air bubbles and disconnection, and to be able to adopt specific corrective measures.13
Insulin glargine, a long-acting recombinant human insulin analog, is given as a 24-h dosing regimen and has a flat pharmacokinetics with no peaks. Although this approach compared with CSII is less physiologic and provides less control on the amount of insulin administered, dosing insulin glargine every 12 h allows a short window of time to evaluate the pharmacokinetics and to better make dose adjustments. Mitamura et al.23 presented the case of an infant with TNDM who attained control of blood glucose concentration with ultralente insulin treatment without any episodes of hypoglycemia. Jeha et al.24 described three cases of successful management of TNDM with insulin glargine, suggesting this therapeutic approach because of its flat pharmacokinetic profile, which might prove useful in this condition. Two of the three cases initially failed transition from intravenously insulin to subcutaneous NPH insulin, similar to what we have reported with our second infant. Furthermore, release of insulin glargine depends on the rise in ambient pH upon administration, and it is less influenced than other subcutaneous drugs by the lower regional perfusion and reservoir mass resulting from minimal subcutaneous tissue. Barone et al.11 reported, for the first time, the successful management of TNDM in an extremely low-birth-weight neonate with the long-acting subcutaneous insulin glargine. These authors believed that the release pattern of glargine, as a truly “peak-less” insulin, might be ideal for TNDM management during the neonatal period and early infancy, when patients are frequently or continuously fed.
Conclusions
The absence of clinical guidelines for the management of NDM allowed us to practice two different therapeutic approaches: CSII and long-acting insulin injections. We think that CSII is the more physiological mode with which to deliver insulin, and therefore we prefer it if the patient and the family do not present contraindications. However, the experience with our patients indicates that the two different modes of insulin delivery in the treatment of TNDM are equally effective and free from complications, particularly hypoglycemia, if properly managed. Therefore, we believe that the choice of the best therapeutic approach should take several factors into account and should be personalized in order to obtain a fast control of blood glucose levels and avoid severe complications.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aguilar-Bryan L, Bryan J: Neonatal diabetes mellitus. Endocr Rev 2008;29:265–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iafusco D, Massa O, Pasquino B, Colombo C, Iughetti L, Bizzarri C, Mammì C, Lo Presti D, Suprani T, Schiaffini R, Nichols CG, Russo L, Grasso V, Meschi F, Bonfanti R, Brescianini S, Barbetti F; Early Diabetes Study Group of ISPED: Minimal incidence of neonatal/infancy onset diabetes in Italy is 1:90,000 live births. Acta Diabetol 2012;49:405–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, Cherubini V, Barbetti F, Martinetti M, Cerutti F, Prisco F; Early Onset Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology: Permanent diabetes mellitus in the first year of life. Diabetologia 2002;45:798–804 [DOI] [PubMed] [Google Scholar]
- 4.Murphy R, Ellard S, Hattersley AT: Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008;4:200–213 [DOI] [PubMed] [Google Scholar]
- 5.Hamilton-Shield JP: Overview of neonatal diabetes. Endocr Dev 2007;12:12–23 [DOI] [PubMed] [Google Scholar]
- 6.Temple IK, Mackay DJG, Docherty LE: Diabetes Mellitus, 6q24-Related Transient Neonatal. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, eds. GeneReviews®. www.ncbi.nlm.nih.gov/books/NBK1534/ (accessed September17, 2012)
- 7.Greeley SA, Naylor RN, Philipson LH, Bell GI: Neonatal diabetes: an expanding list of genes allows for improved diagnosis and treatment. Curr Diab Rep 2011;11:519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beardsall K, Ogilvy-Stuart AL, Frystyk J, Chen JW, Thompson M, Ahluwalia J, Ong KK, Dunger DB: Early elective insulin therapy can reduce hyperglycemia and increase insulin-like growth factor-I levels in very low birth weight infants. J Pediatr 2007;151:611–617, 617.e1 [DOI] [PubMed] [Google Scholar]
- 9.Flechtner I, Vaxillaire M, Cavé H, Froguel P, Polak M: Neonatal diabetes: a disease linked to multiple mechanisms. Arch Pediatr 2007;14:1356–1365 [DOI] [PubMed] [Google Scholar]
- 10.Von Mühlendahl KE, Herkenhoff H: Long-term course of neonatal diabetes. N Engl J Med 1995;333:704–708 [DOI] [PubMed] [Google Scholar]
- 11.Barone JV, Tillman EM, Ferry RJ, Jr: Treatment of transient neonatal diabetes mellitus with subcutaneous insulin glargine in an extremely low birth weight neonate. J Pediatr Pharmacol Ther 2011;16:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodoyez-Goffaux F, Sodoyez JC: Transient diabetes mellitus in a neonate. Evaluation of insulin, glucagon, and growth hormone secretion and management with a continuous low-dose insulin infusion. J Pediatr 1977;91:395–399 [DOI] [PubMed] [Google Scholar]
- 13.Bharucha T, Brown J, McDonnell C, Gebert R, McDougall P, Cameron F, Werther G, Zacharin M: Neonatal diabetes mellitus: insulin pump as an alternative management strategy. J Paediatr Child Health 2005;41:522–526 [DOI] [PubMed] [Google Scholar]
- 14.Yaffe SJ, Aranda JA: Pediatric Pharmacology: Therapeutic Principles in Practice, 2nd ed. Philadelphia: W.B. Saunders Co., 1992 [Google Scholar]
- 15.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE: Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349:1157–1167 [DOI] [PubMed] [Google Scholar]
- 16.Weinzimer SA, Swan KL, Sikes KA, Ahern JH: Emerging evidence for the use of insulin pump therapy in infants, toddlers, and preschool-aged children with type 1 diabetes. Pediatr Diabetes 2006;7(Suppl 4):15–19 [DOI] [PubMed] [Google Scholar]
- 17.Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE: A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care 2005;28:15–19 [DOI] [PubMed] [Google Scholar]
- 18.Colquitt J, Royle P, Waugh N: Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta-analysis. Diabet Med 2003;20:863–866 [DOI] [PubMed] [Google Scholar]
- 19.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ: Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab 2009;297:E194–E201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tubiana-Rufi N: Insulin pump therapy in neonatal diabetes. Endocr Dev 2007;12:67–74 [DOI] [PubMed] [Google Scholar]
- 21.Wintergerst KA, Hargadon S, Hsiang HY: Continuous subcutaneous insulin infusion in neonatal diabetes mellitus. Pediatr Diabetes 2004;5:202–206 [DOI] [PubMed] [Google Scholar]
- 22.Beardsall K, Pesterfield CL, Acerini CL: Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed 2011;96:F223–F224 [DOI] [PubMed] [Google Scholar]
- 23.Mitamura R, Kimura H, Murakami Y, Nagaya K, Makita Y, Okuno A: Ultralente insulin treatment of transient neonatal diabetes mellitus. J Pediatr 1996;128:268–270 [DOI] [PubMed] [Google Scholar]
- 24.Jeha GS, Venkatesh MP, Edelen RC, Kienstra KA, Karaviti L, Fernandes CJ: Neonatal diabetes mellitus: patient reports and review of current knowledge and clinical practice. J Pediatr Endocrinol Metab 2005;18:1095–1102 [DOI] [PubMed] [Google Scholar]