Abstract
[Purpose]
The purpose of this study was to investigate the effect that muscle contraction induced NAD metabolism via NAMPT has on mitochondrial biogenesis.
[Methods]
Primary skeletal muscle cells were isolated from the gastrocnemius in C57BL/6 mice. The muscle cells were stimulated by electrical current at 1Hz for 3 minutes in conditions of normal or NAD metabolism related inhibitor treatment. NAD/NADH level, Sirt1 and mitochondria biogenesis related signal factor’s changes were examined in normal or NAD metabolism related inhibitor treated cells.
[Results]
Electrical stimulation (ES) induced muscle contractions significantly increased NAD/NADH levels, NAMPT inhibitor FK-866 inhibited ES-induced NAD formation, which caused SIRT1 expression and PGC-1α deacetylation to decrease. Moreover, NAMPT inhibition decreased mitochondrial biogenesis related mRNA, COX-1 and Tfam levels. Along with AMPK inhibitor, compound C decreases SIRT1 expression, PGC-1α deacetylation and muscle contraction induced mitochondrial biogenesis related mRNA increment. These results indicated that the AMPK-NAMPT signal is a key player for muscle contraction induced SIRT1 expression and PGC-1α deacetylation, which influences mitochondrial biogenesis. Inhibition of the AMPK upregulator, Camkkβ, STO-609 decreased AMPK phosphorylation and SIRT1 expression but did not decrease PGC-1α deacetylation. However, CAMKII inhibition via AIP decreased PGC-1α deacetylation.
[Conclusion]
In conclusion, the results indicate that NAMPT plays an important role in NAD metabolism and mitochondrial biogenesis. However, mitochondrial biogenesis is also controlled by different calcium binding protein signals including Camkkβ and CAMKII. [Keyword] Muscle contraction, NAD metabolism, SIRT1, PGC-1 α, mitochondria biogenesis.
Keywords: Muscle contraction, NAD metabolism, SIRT1, PGC-1 α, mitochondria biogenesis
INTRODUCTION
NAD metabolism is a critical factor that regulates the metabolism, energy production and DNA repair of cells [1]. It is known that muscle contraction elevates NAD/NADH levels and regulates various signal proteins including SIRT 1, which is one of the histone deactylation proteins that requires NAD as a substrate [2]. In skeletal muscle, SIRT1 deacetylates a number of transcription factors such as nuclear factor kappa β, p53, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and MyoD, which are all involved in the inflammatory process, mitochondrial biogenesis (energy metabolism) and skeletal muscle differentiation [3-5]. However, SIRT1 activation is also associated with the improvement of insulin sensitivity and suppression of oxidative and inflammatory stress [6].
During skeletal muscle contraction, AMP/ATP and NAD/NADH ratios increase. This has been shown to increase the activity of AMP activated protein kinase (AMPK) and the activities of many other NAD metabolism related protein kinases including nicotinamide phosphoribosyltransferase (NAMPT) [7]. NAMPT, also known as visfatin, is an enzyme that converts nitotinamide into nicotinamide mononucleotides, and is the rate limiting enzyme in this reaction [8]. It is well known that synthesized NAD is an essential factor for many intercellular processes. Increased NAD levels influence energy metabolism for muscle contraction and mitochondrial biogenesis, and elevated NAD levels can also increase SIRT 1 expression [9]. As a result, upregulated SIRT1 increases the deacetylation of signal proteins such as PGC-1α, which is associated with the upregulation of genes involved in mitochondrial fatty acid oxidation [10,11]. In addition, NAD acts as an electron acceptor in energy producing processes such as the TCA cycle and electron transport system [12].
In skeletal muscle, NAMPT is increased by exercise or calorie restriction and is dependent on AMPK activation [13]. Treatment with the AMPK activator AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide) and exercise both increased NAMPT expression and activity in skeletal muscle [14]. AMPK γ knock down mice showed a decrease in exercise induced NAMPT expression indicating that AMPK is likely a tightly regulated NAMPT transcription factor [15].
AMPK activity is regulated by allosteric AMP binding [16], while AMPK phosphorylation is regulated by calmodulin kinase kinase (CaMKK) [17]. In addition, Camkkβ also phosphorylates Ser-27 and Ser-47 on SIRT1 in brain tissue, which increases SIRT1’s anti-inflammatory capacity [18]. Moreover, mitochondrial biogenesis and energy production can be regulated by CAMKII where phosphorylation can be regulated by various factors [19]. These results indicate that AMPK phosphorylation and calcium signal related protein activation regulate mitochondrial biogenesis via deacetylation regulating proteins including SIRT1 [20,21]. However, a few studies have reported that NAMPT activation or expression is induced by not only exercise or muscle contraction but also by a calcium related SIRT1 regulating mechanism.
In this study, we examined the effect of NAMPT inhibition using FK-866 (NAMPT specific inhibitor) on ES-induced muscle contraction mediated SIRT1-PGC-1α signals and on mitochondrial biogenesis. Because NAD has a robust effect on SIRT1 related PGC-1α deacetylation, we hypothesized that the NAMPT signal pathway would be an important upregulator in genes involved in mitochondrial biogenesis gene regulation and muscle contraction. Moreover, we examined the calmodulin activated protein kinase inhibitor on these signals. Overall, the purpose of this study was to investigate the effect that NAD metabolism via NAMPT regulation and SIRT1 activation have on mitochondrial biogenesis during skeletal muscle cell contraction.
METHODS
Experimental animal
8-week-old C57BL/6 mice were used for this study. All mice were housed in plastic cages and were given standard food and water. The temperature and humidity in the room were maintained at 23~25℃ and 60~70% respectively. In addition, the light was controlled in 12 hour cycles. All mice weighed approximately 25 ± 1.32g. Management and experimental procedures for the animals used in this study were in compliance with ethics regulations of the Animal Testing Ethics Committee of Chonbuk University (2014-1-0039).
Separation of skeletal muscle primary muscle cell
In order to obtain a single muscle fiber for experimental purposes, each C57BL/6 mice were sacrificed by cervical dislocation and the gastrocnemius harvested. The skeletal muscle was then put into a high glucose Eagle’s minimal essential medium (DMEM) buffer containing 0.2% type Ⅰ collagenase and incubated at 80~90 RPM for two hours in a 37℃ shaking water bath. When enzymatic separation was finished, a separated muscle fiber was collected by using a pasteur pipette and moved to a petri dish. The muscle fiber was then moved to a new culture dish containing a DMEM-FBS buffer that comprised of 10% horse serum and 10% fatal bovine serum (FBS) and stored in a CO2 incubator until the experiment.
Assessment of mitochondrial mRNA regulation using real-time PCR methodology.
Mitochondrial mRNA was quantitatively analyzed in skeletal muscle cells using ABI 7300 real-time PCR (Applied Biosystems, CA, USA). Primers were designed within the mitochondrial genome (Table 1).
Table 1.
Real-time PCR primer sequences.
| Gene | Forward primer (5’ → 3’) | Reverse primer (5’ → 3’) |
|---|---|---|
| PGC-1α | ttccaccaagagcaagtat | cgctgtcccatgaggtatt |
| Tfam | gaagggaatgggaaaggtaga | aacaggacatggaaagcagat |
| SIRT1 | acttgtacgac gaagacgac | cagaaggttatctcggtacc |
| NAMPT | acagatactgtggcgggattg | tgatatccacgccatctccttg |
| COX-I | ctagccgcaggcattactat | tgcccaaagaatcagaacag |
Electrical stimulation on single muscle fiber
Electrical stimulation on single muscle fibers was conducted based on an experiment by Rosenblatt et al. [22]. The skeletal muscle single fibers stored in DMEM medium buffer were moved to a Krebs Ringer buffer (115 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 1.2 mM Na2SO4, 2.5 mM CaCl2, 25 mM NaHCO3, 5 mM glucose, pH 7.4) and 70~80 muscle fibers were transferred to a 1.5 ml tube. After stabilization in a 37℃ water tank for five minutes, the fibers were electrically stimulated by an electronic stimulator (DS2A mk2. digitimer. England) using a carbon electrode. Electrical stimulation was performed at 1Hz frequency for three minutes [23]. The samples that completed the electrical stimulation were immediately frozen with liquid nitrogen and stored in a -80℃ deep freezer until further experimentation.
Measurement of NAMPT activity.
The NAMPT activity was measured using a NAMPT mouse activity ELISA kit (AG-45A-0007EK-KI01) from Enzo life science.
Measurement of intracellular NAD and NADH levels.
Levels of NAD and NADH were measured using NAD/NADH (ab65348) Assay Kits from Abcam (USA). Briefly, 80-90 cells were transferred to a 1.5-ml tube containing 100 μl of phenol red-free DMEM and treated with ES or with inhibitors. The stimulated cells were immediately frozen in liquid nitrogen and stored at -80℃ until measurement. Intracellular NAD, NADH, NADP, and NADPH levels were measured according to the manufacturer’s instructions.
Immunoprecipitation and western blotting
The frozen sample was lysed in a lysis buffer (Invitrogen, USA) and immunoprecipitation was performed according to the immunoprecipitation protocol provided by Abcam. 20ul of protein G (p7700, Sigma-Aldrich, USA) was added for three hours to preclear immunoglobin and then centrifuged to obtain supernatant at 15000 Rpm for 10 minutes. PGC-1α antibody (1:1000) (AB3242, millipore, USA) and 30ul of protein G were added to the precleared supernatant then incubated for 12 hours at 4℃. The protein G agarose was washed three times with cold PBS for 15 minutes. The immunoprecipitated protein was visualized and blotted using the western blotting method. PGC-1α deacetylation was measured using acetyl-lysine antibody (9441, ABcam, USA). Expression of proteins or acetylation of proteins was compared by general western blotting. SIRT1 (07-131, Millipore, USA), NAMPT (sc-166866, Santacruz, USA), AMPK (sc-74461, Santacruz, USA), and p-AMPK thr 172 (sc-33524, Santacruz, USA) antibodies were used for western blotting.
Statistical analysis
All data are expressed as mean ± SEM. Statistical analyses were performed using a One-way ANOVA when comparing each group in the in vitro and in vivo studies. All analyses were performed using SPSS 20.0 (SPSS Inc. USA). Each reported value is the mean of at least two separate experiments in each group. P < 0.05 was considered statistically significant.
RESULTS
Muscle contraction induced NAMPT activation influence on intracellular NAD/NADH level
To examine the effect of muscle contraction on intracellular NAD metabolism, we measured NAD/NAD levels and NAMPT expression. Muscle contraction by electrical stimulation (ES) significantly increased NAD (p < 0.05, p = 0.477, t = -8.581, Df = 4) and NADH (p < 0.05, p = 0.020, t = -3.742, Df = 4) levels (Fig. 1A). Moreover, NAD/NADH levels were also significantly increased by ES (0.45/0.06 to 0.999/0.018) (Fig. 1B). NAMPT expression (p < 0.05, p = 0.001, t = -9.514, Df = 8) and mRNA (p < 0.05, p = 0.001, t = -8.033, Df = 6) levels were also significantly increased by ES. These results indicate that muscle contraction increases the intracellular NAD/NADH levels as well as NAMPT expression.
Fig. 1.
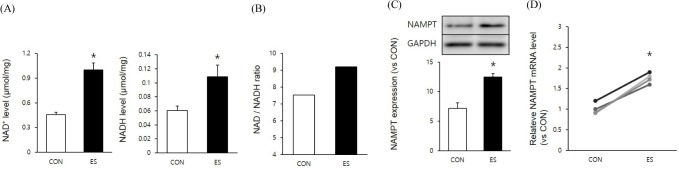
Muscle contraction increased NAMPT expression and intracellular NAD/NADH level. A : ES-induced muscle contraction increased intracellular NAD/NADH level. B : Calculation of NAD/NADH ratio. C : ES-induced muscle contraction increased NAMPT expression D : ES-induced muscle contraction increased NAMPT mRNA. * p < .05 between con and ES. P values were calculated by t-test
NAMPT inhibitor FK-866 effect on ES-induced intracellular NAD/NADH level
To identify whether NAMPT inhibition influences ESinduced intracellular NAD/NADH levels, we applied the NAMPT inhibitor FK-866 to skeletal muscle. Cells treated with the NAMPT inhibitor had significantly decreased NAD (p < 0.05, F = 215.457, Df = 14) and NADH (p < 0.05, F = 215.457, Df = 14) level (Fig. 2A). These results indicated that NAMPT inhibition only influenced the NAD formation mechanism during ES-induced muscle contraction. Indeed, FK-866 treatment significantly reduced ES-induced NAMPT activity (p < 0.05, F = 211.977, Df = 14) and expression compared to ES (p < 0.05, F = 26.006, Df = 14).
Fig. 2.
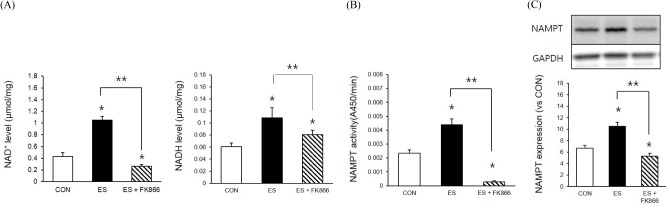
NAMPT inhibition influenced ES-induced and NAPT expression and NAD metabolism. A : NAMPT inhibitor decreased ES-induced intracellular NAD level. B : NAMPT inhibitor decreased ES-induced intracellular NADH level. C : NAMPT inhibitor decreased ES-induced NAMPT mRNA level. D : C : NAMPT inhibitor decreased ES-induced NAMPT expression. * p < .05 between CON and ES. **,* p < .05 between ES and FK-866 treatment P values were calculated by one-way ANOVA
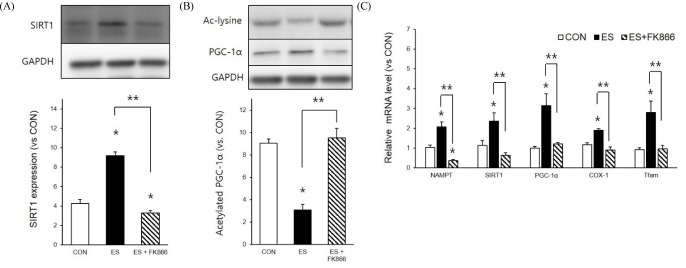
NAMPT inhibitor effect on ES-induced SIRT1 expression and PGC-1α deacetylation
Due to the effect of NAMPT inhibition on ES-induced intracellular NAD/NADH level demonstrated in our results, we checked ES-induced SIRT1 expression and PGC-1α deacetylation levels in the presence and absence of the NAMPT inhibitor. Indeed, ES-induced SIRT1 expression (p < 0.05, F = 297.679, Df = 14) and PGC-1α deacetylation (p < 0.05, F = 228.719, Df = 14) were significantly decreased by the NAMPT inhibitor. Next, since SIRT1 activation and PGC-1α both influenced mitochondrial biogenesis, we measured ES-induced mitochondrial mRNA related to energy metabolism. Not only NAMPT (F = 55.028), SIRT1 (F = 17.799), and PGC-1α mRNA (F = 22.178) but also cyclooxygenase-1 (COX-1) (F = 55.922) and mitochondrial transcription factor A (Tfam) mRNA levels (F = 18.961) were significantly decreased by the NAMPT inhibitor. These results indicate that ES-induced muscle contraction elevated NAD level via NAMPT as well as ES-induced NAD metabolism play key roles in mitochondrial biogenesis.
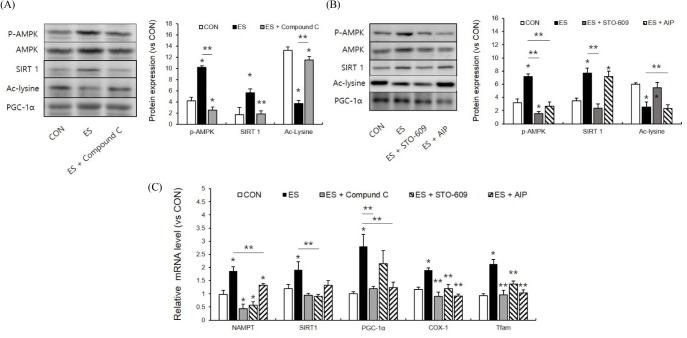
AMPK, calmodulin kinase inhibitor inhibited ES-induced PGC-1α deacetylation and mitochondrial biogenesis related mRNA up-regulation
Because NAMPT expression was influenced by muscle contraction induced AMPK phosphorylation, we next examined the effect of the AMPK inhibitor compound C on ES-induced SIRT and PGC-1α expression. P-AMPK levels were significantly reduced by the AMPK inhibitor (p < 0.05, F = 80.470, Df = 14). ES-induced SIRT1 expression (p < 0.05, F = 63.801, Df = 14) and PGC-1α deacetylation were also reduced (p < 0.05, F = 289.785, Df = 14) (Fig. 4A). These results indicate that AMPK phosphorylation plays a pivotal role in NAD metabolism. AMPK activity is controlled by allosteric interaction with AMP whereas AMPK phosphorylation is controlled by calmodulin kinase. We next used the calcium calmodulin kinase kinase β (Camkkβ) inhibitor, STO-609, and the calmodulin activated protein kinase II (CamkII) inhibitor, autocamtide-2-related inhibitory peptide (AIP), to identify the effects of calcium signals on ES-induced NAD metabolism and mitochondrial biogenesis. Camkkβ inhibitor significantly reduced ES-induced AMPK phosphorylation, and so did AIP (p < 0.05, F = 97.460, Df = 19). Camkk β inhibitor reduced ES-induced SIRT1 expression but AIP did not (p < 0.05, F = 57.189, Df = 19) (Fig. 4B). More interestingly, PGC-1α deacetylation was not influenced by Camkkβ inhibitor treatment (p < 0.05, F = 38.992, Df = 19). These results indicated that AMPK and Camkkβ signals were involved in SIRT1 expression whereas PGC-1α deacetylation was controlled by CamkII signaling. These inhibitor effects were also similarly displayed in ES-induced mitochondrial mRNA expression (Fig. 4C). However, Camkkβ and CamKII inhibitor reduced COX-1 and Tfam mRNA respectively. This also indicated that mitochondrial biogenesis could be controlled by both signals.
Fig. 4.
AMPK and calcium/calmodulin kinase inhibition influenced mitochondrial biogenesis factor. A : The AMPK inhibitor, compound C, decreased ES-induced SIRT1 expression and PGC-1α deacetylation. B : Camkkβ inhibition and CAMKII inhibition regulate SIRT1 and PGC-1α deacetylation differently. C : Camkkβ inhibition and CAMKII inhibition regulate ES-induced mitochondria biogenesis related mRNA expression differently. * p < .05 between CON and ES. ** p < .05 between ES and inhibitor treatment. P values were calculated by one-way ANOVA
DISCUSSION
NAD metabolism plays a pivotal role in the energy production needed for skeletal muscle contraction as well as in the activation of diverse cellular signals. NAMPT produce NAD through the salvage pathway which regulates SIRT1 activity [24]. Due to NAD synthesis via NAMPT during muscle contraction, SIRT1 is activated by NAD which increases the biogenesis of myocyte mitochondria [20]. Although the exact process through which mitochondrial biogenesis occurs is still under investigation, the present study shows that muscle contraction either directly or indirectly activates NAMPT, thus elevating intracellular NAD/NADH levels. Therefore, it appears that this increase of SIRT1 expression and PGC-1α deacetylation also play regulatory roles in energy metabolism and mitochondrial biogenesis. Our data also shows that NAMPT upstream factors such as AMPK, Camkkβ and CAMKII have possible additional regulatory mechanisms for mitochondrial biogenesis induced by the mitochondrial biogenesis process.
For further clarification, NAMPT produces NAD through the salvage pathway which converts nicotinamide (NAM) to nicotinamide mononucleotide (NMN) in the nucleus and cytosol. This NAD biosynthetic machinery plays an important role in maintaining the mitochondrial NAD pool in response to various intracellular processes such as those mentioned previously [25]. The NAD biosynthetic machinery which shuttles NAD for glycolysis, TCA cycle and electron transport is evidently a highly regulated and important energy production pathway required for sustained muscle contraction [26]. Our present study showed that ES-induced muscle contraction causes an increase of NAMPT activity, expression and mRNA which influences intracellular NAD/NADH levels (Fig. 1B-D and Fig. 2A and B). SIRT1, an NAD-dependent protein deacetylase, is controlled by NAD/NADH levels which play an important role in the deacetylation of PGC-1α, a key transcription coactivator regulating mitochondrial biogenesis [27]. In our experiment, it appears that NAMPT inhibition either directly or indirectly inactivated SIRT1 and PGC-1α, which caused an overall decrease in mitochondrial biogenesis through alterations in mRNA levels including COX-1 and Tfam. Interestingly, a high fat diet and Aging are related to decreased production of NAD, which can readily result in the decrease of mitochondrial biogenesis factors [6,28]. In addition, exercise induced an increase in levels of NAMPT which has been shown to significantly increase mitochondrial concentration in congruence with the findings in our experiment [14]. However, mitochondrial biogenesis is controlled by various other factors. Other studies showed that SIRT1 alone is not sufficient for the elevation of mitochondrial biogenesis without the addition of other factors [29]. This lack of SIRT1’s ability to adequately induce mitochondrial biogenesis on its own led us to investigate other possible regulatory factors such as AMPK and calcium binding proteins and what role they may play in mitochondrial biogenesis.
AMPK is not only a key regulatory factor for energy metabolism but also for mitochondria biogenesis [4]. AMPK controls numerous factors required for gene transcription and protein kinase activity via phosphorylation of various proteins. AMPK controls NAMPT mRNA and AMPK knockout mice showed an overall decrease in NAMPT expression, which means that AMPK is important for intracellular NAD metabolism [7,14]. AMPK also regulated SIRT1 and PGC-1α, causing incremental elevations in mitochondrial biogenesis [13,30]. The present results showed that the AMPK inhibitor, compound C, decreased not only the levels of SIRT and the deacetylation of PGC-1α but also several mRNA levels of other proteins related to mitochondrial biogenesis. However, this implies that not only does AMPK inhibition disturb NAD synthesis but it could also alter AMPK related kinase inactivation or downstream gene regulation. AMPK could also directly control PGC-1α and other mitochondrial biogenesis factor such as calcium binding proteins [31,32].
To examine the effect of calcium binding kinase inhibition on mitochondirial biogenesis, we used a Camkkβ specific inhibitor, STO-609, and a CAMKII specific inhibitor, AIP, for muscle contraction induced mitochondrial biogenesis. Intriguingly, our results showed that SIRT1 expression levels were only decreased by the Camkkβ specific inhibitor while PGC-1α deacyetlation was inhibited by only the CAMKII inhibitor (Fig. 4B). Camkkβ controls AMPK phosphorylation which regulates substrate metabolism [17]. Moreover, Camkk β is also involved in SIRT1 phosphorylation [18]. These studies indicate that Camkkβ could control AMPK and SIRT1 phosphorylation which influence mitochondrial biogenesis. Furthermore, CAMKII is an important regulator for mitochondrial biogenesis. However, it’s not reported in the literature as a regulator of SIRT1. Recently, CAMKII was found to control a PGC-1α activation mechanism via nuclear factor control. General control of amino-acid synthesis 5 (GCN5) [34] and GCN5 levels were also controlled by CAMKII levels [35]. However, in this study, we did not examine the effect of calcium binding protein inhibition to NAD metabolism and how this influences the NAD/NADH level. Additional studies using inhibition or gene knockout mice may be needed to investigate these factors further. There are many other transcriptional factors involved in muscle contraction which if investigated further would elucidate other possible pathways for the activation and upregulation of mitochondrial biogenesis.
CONCLUSION
This study aimed to examine how SIRT1, PGC-1α regulation and mitochondria biogenesis were influenced by NAMPT. We found that muscle contraction increased NAMPT activity and expression as well as mRNA which in turn caused elevations in the NAD/NADH levels during muscle contraction. In addition, NAMPT inhibition via FK-866 decreased SIRT1 expression, PGC-1α deacetylation and muscle contraction induced mitochondrial biogenesis related mRNA increment. The AMPK inhibitor, compound C, decreased SIRT1 expression, PGC-1α deacetylation and muscle contraction induced mitochondrial biogenesis related mRNA increase. The Camkkβ inhibitor, STO-609, decreased AMPK and SIRT1 expression but did not decrease PGC-1α deacetylation. Lastly, the CAMKII inhibitor, AIP, decreased AMPK and PGC-1α deacetylation but did not decrease SIRT1 expression. Overall, these results indicated that NAMPT plays an important role in NAD metabolism and mitochondrial biogenesis, however, mitochondrial biogenesis is controlled by different calcium binding protein signals including Camkk β and CAMKII. Further studies will be needed to identify this exact regulation mechanism.
Fig. 3.
NAMPT inhibition reduced ES-induced SIRT1 expression, PGC-1α deacetylation and mitochondria biogenesis related mRNA. A : NAMPT inhibitor decreased ES-induced SIRT1 expression B : NAMPT inhibitor decreased ES-induced PGC-1α deacetylation. C : NAMPT inhibitor decreased ES-induced mitochondria biogenesis related mRNA expression. * p < .05 between CON and ES. ** p < .05 between ES and FK-866 treatment. P values were calculated by one-way ANOVA
Acknowledgments
This paper was supported by research funds of Chonbuk National University in 2012.
REFERENCES
- 1.Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–64. doi: 10.1046/j.1432-1327.2000.01187.x. [DOI] [PubMed] [Google Scholar]
- 2.White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. American Journal of Physiology-Endocrinology and Metabolism. 2012;303:E308–E21. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Molecular Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 4.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Developmental Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins - novel therapeutic targets to treat age-associated diseases. Nature Reviews Drug Discovery. 2008;7:841–53. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 6.Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Frontiers in Bioscience. 2009;14:2983–95. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandauer J, Vienberg SG, Andersen MA, Ringholm S, Risis S, Larsen PS, Kristensen JM, Frosig C, Leick L, Fentz J, Jorgensen S, Kiens B, Wojtaszewski JFP, Richter EA, Zierath JR, Goodyear LJ, Pilegaard H, Treebak JT. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. Journal of Physiology-London. 2013;591:5207–20. doi: 10.1113/jphysiol.2013.259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The Nicotinamide Phosphoribosyltransferase: A Molecular Link between Metabolism, Inflammation, and Cancer. Cancer Research. 2010;70:8–11. doi: 10.1158/0008-5472.CAN-09-2465. [DOI] [PubMed] [Google Scholar]
- 9.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420–8. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai S, Yoshino J. The importance of NAMPT/NAD/ SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15 Suppl 3:26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Pan R, Li R, Niemann B, Aurich AC, Chen Y, Rohrbach S. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-gamma coactivator-1alpha(PGC-1alpha) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes. 2011;60:157–67. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 13.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009;458:1056–U140. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. American Journal of Physiology-Endocrinology and Metabolism. 2010;298:E117–E26. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HL, Xu MJ, Lee J, He CY, Xie ZL. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. American Journal of Physiology-Endocrinology and Metabolism. 2012;303:E1234–E44. doi: 10.1152/ajpendo.00198.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–65. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott MJ, Edelman AM, Turcotte LP. CaMKK is an upstream signal of AMP-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2009;297:R1724–R32. doi: 10.1152/ajpregu.00179.2009. [DOI] [PubMed] [Google Scholar]
- 18.Wen L, Chen Z, Zhang F, Cui X, Sun W, Geary GG, Wang Y, Johnson DA, Zhu Y, Chien S, Shyy JY. Ca2+/calmodulin-dependent protein kinase kinase beta phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc Natl Acad Sci U S A. 2013;110:E2420–7. doi: 10.1073/pnas.1309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain SS, Paglialunga S, Vigna C, Ludzki A, Herbst EA, Lally JS, Schrauwen P, Hoeks J, Tupling AR, Bonen A, Holloway GP. High-Fat Diet-Induced Mitochondrial Biogenesis Is Regulated by Mitochondrial-Derived Reactive Oxygen Species Activation of CaMKII. Diabetes. 2014;63:1907–13. doi: 10.2337/db13-0816. [DOI] [PubMed] [Google Scholar]
- 20.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11: doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1alpha. Exp Gerontol. 2013;48:1343–50. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. in vitro Cell Dev Biol Anim. 1995;31:773–9. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg BR, Gilai A. Structural changes in single muscle fibers after stimulation at a low frequency. J Gen Physiol. 1979;74:1–16. doi: 10.1085/jgp.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang TL, Sauve AA, Kraus WL. Enzymes in the NAD(+) Salvage Pathway Regulate SIRT1 Activity at Target Gene Promoters. Journal of Biological Chemistry. 2009;284:20408–17. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Dash RK, Kim J, Saidel GM, Cabrera ME. Role of NADH/NAD+ transport activity and glycogen store on skeletal muscle energy metabolism during exercise: in silico studies. Am J Physiol Cell Physiol. 2009;296:C25–46. doi: 10.1152/ajpcell.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkle A. Poly(ADP-ribose). The most elaborate metabolite of NAD+ FEBS J. 2005;272:4576–89. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- 29.Gurd BJ, Little JP, Perry CG. Does SIRT1 determine exercise-induced skeletal muscle mitochondrial biogenesis: differences between in vitro and in vivo experiments? J Appl Physiol (1985) 2012;112:926–8. doi: 10.1152/japplphysiol.01262.2011. [DOI] [PubMed] [Google Scholar]
- 30.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan Z, Root-McCaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity(Silver Spring) 2014;22:730–8. doi: 10.1002/oby.20605. [DOI] [PubMed] [Google Scholar]
- 33.Wright DC. Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl Physiol Nutr Metab. 2007;32:840–5. doi: 10.1139/H07-062. [DOI] [PubMed] [Google Scholar]
- 34.Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptorgamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–70. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao X, Gluck N, Li D, Maine GN, Li H, Zaidi IW, Repaka A, Mayo MW, Burstein E. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-kappaB/RelA. Genes Dev. 2009;23:849–61. doi: 10.1101/gad.1748409. [DOI] [PMC free article] [PubMed] [Google Scholar]