Abstract
This study aimed to investigate effects of restricted calcium intake on cortical and trabecular bone density in white rats. Low Ca diet was fed for six weeks, and bone density and bone metabolism parameters were assessed in blood. This study was carried out on 12 male white rats aged 12 weeks (Sprague-Dawley; SD). These rats were bred for 1 week and randomly assigned to the standard calcium diet group (SCa group, n = 6) and the low calcium diet group (LCa group; n = 6). The SCa group was given a modified AIN-93M mineral mix (with 0.5% Ca), which was made by adding calcium to a standard AIN93 diet, and the LCa Group was fed a modified AIN-93 Mineral mix (with 0.1% Ca). Femoral BMD and BMC were measured by DEXA in each rat. After trabecular bone was separated from cortical bone, volumetric bone mineral density (vBMD) was measured using pQCT. Serum Ca and P levels were measured as parameters of bone metabolism, and S-ALP, S-TrACP and-Dpd levels were also measured. The results revealed no significant differences in weight, growth rate, feed consumption and feed efficiency between the two groups before and after calcium-restricted diet (p > .05). No significant differences were also observed in bone length and bone mass between the two groups (p > .05). Although bilateral femoral BMDs were not significantly different between the two groups, bilateral femoral BMCs significantly decreased in the LCa group, compared with the SCa group (p = .023, p = .047). Bilateral cortical MDs were not significantly different between the two groups, either. However, trabecular BMD significantly decreased in the LCa group, compared with the SCa group (p = .041). U-Dpd and S-TrACP levels significantly declined in the LCa group, compared to the SCa group (p = .039, p = .010). There were no significant differences in serum Ca and P levels between the two groups (p > .05). However, a significant decrease in urinary Ca level (p = .001) and a significant increase in urinary P (p = .001) were observed in the LCa group, compared to the Sca group. These findings described that six-week low calcium diet led to decreased trabecular bone density, reduced urinary excretion of Ca and increased urinary excretion of P. As a result, Ca hemeostasis can be maintained.
Keywords: restriction of dietary calcium, trabecular bone, cortical bone, bone mineral density (BMD), urinary Ca, urinary P, rats
INTRODUCTION
The incidence of osteoporosis steadily increases in the world population, and social costs associated with the treatment of oseoporosis is also rising. According to the U.S. National Data Center, nearly 10 million US population suffer osteoporosis, and about 34 million American people have osteopenia, which is on the rise[1. In the U.S., 2 million people have bone fracture every year and nearly U$19 billion is spent on treatment of bone fractures. It is estimated that nearly 3 million people have bone fracture in 2025 and that U$25.3 billion is required to treat them [2]. Oteoporosis refers to bone atrophy that results from systemic metabolic disorders. The age-related bone loss makes bone weak and prone to fractures [3]. According to WHO definition, osteopenia is defined as a T score of 1.0 or lower, and osteoporosis is defined as a T score of -2.5 or lower [4]. In Korea, there have been not many studies done on the population with osteoporosis. Rowe et al. [5] reported 3.3 people per 10,000 population suffered bone fracture in 50+ age group in Gwangju city, Jeonnam in Korea in 1991. But osteoporosis patients rose four times to 13.3 per 10,000 in 2001, showing a steep rise over the decade. According to the report of Health Insurance Review and Assessment Service from 2007 to 2011, the number of people who sought treatment due to osteoporosis increased from 535,000 in 2007 to 773,000 in 2011, showing about 44.3% increase. Total medical costs also jumped to KW72.2 billion from KW53.5 billion, at average annual increase of 7.9%, during the same period [6].
The structure of bone can be divided into four regions: cortical bone, trabecular bone, periosteum and endosteum[7]. The trabecular bone is composed of small pillar-shaped bony trabecular that includes plates. Unlike cortical bone, plates are arranged in a simple structure. Most skeletal diseases such as osteoporosis and bone fracture are affected by the structure of trabecular bone rather than cortical bone [8]. Although DEXA is commonly used as a diagnostic tool for osteoporosis, it measures bond density only, making it insufficient to evaluate bone strength [4]. DEXA has a limitation of measuring the integral bone mass of both trabecular and cortical bones as a whole [9]. Endotrabecular bone formation is higher than endocortical bone formation, making trabecular bone more suitable to evaluate early biochemical changes of bone metabolism, including osteoporosis [10]. However, effects of exercise and nutrition on both trabecular and cortical bone densities have been rarely documented. Recently developed peripheral quantitative computed tomography (pQCT) is useful in measuring volumetric bone mineral density vBMD; g/cm3 separately for cortical and trabecular bones in small animals.
Bone is very active tissue that is sensitive to external mechanical stimuli and changes in bone metabolism. Changes in bone density and metabolism are varied by mechanical stress. Those changes are also affected by human race [12], nutrition [13] and environmental factors [14] in addition to genes [11]. Especially nutrition plays a crucial role in bone stock. It is well known that sufficient calcium intake increases bone density. The relationship between calcium intake and bone fracture is still unproven [15]. Some claim Ca intake alone is insufficient for the treatment of osteoporosis [16].Thus amounts of Ca intake appears to be closely associated to changes in bone stock. Decreased Ca intake or less efficient intestinal absorption of calcium with age is known to induce a secondary hyperparathyroidism, which in turn results in osteopenia [17]. According to the 2009 Korean National Health and Nutrition Examination Survey results[18], calcium intake was 447mg in women between 19 and 29 years and 441mg in women between 30 and 39, which accounted for 63.8% and 63.0%, respectively, of recommended calcium intake for Koreans set by the Institute of Medicine Dietary Reference Intakes (DRIs) in 2010 [19]. Some studies stated that vegetables provided 58.1% of calcium intake in the Korean population although vegetable calcium have a low absorption rate [18]. Also, phosphoric acid, phytic acid and oxalic acid contained in grains and vegetables undermine calcium absorption [20]. Actual absorption of calcium can be much lower than the amount of calcium intake [21]. The compounded effects of low calcium intake and low absorption rate of calcium appear to be significant on bone quality. However, effects of low calcium dietary intake on bone density and bone mineral content are still poorly documented. No studies have ever separated trabecular bone from cortical bone to investigated effects of low calcium diet on each of these bones.
This study aimed to evaluate changes in overall bone density in white rats following six-week restricted calcium intake using DEXA. At the same time, we determined effects of restricted calcium intake on bone stock in different areas after separating trabecular bone from cortical bone using
METHODS
Animal care
Experiment was carried out in 12 male white rats aged 12 weeks (Sprague-Dawley; SD) with the mean weight of 254.8 ± 10.9g. These mice were bred for 1 week and randomly assigned to the standard calcium diet group (SCa group, n = 6) and the low calcium diet group (LCa group; n = 6). For the control group, standard feed,AMI-93 Mineral mix (05% Ca content), was made by adding calcium to a standard AIN-93M. Calcium-restricted feed AIN-93 Mineral mix (0.1% Ca content) was made for the low-calcium diet group (Table 1). Rates were given free access to drinking distilled water during the experiment period. Rats were individually housed in cages (26×48× 20cm3) in a temperature-, humidity and light -controlled breeding room (23 ± 1℃, 50 ± 5% and dark phase 9:00-21:00, 12 h light/dark cycle). Rat's weight was measured once a week, and feed intake was measured every morning at 09:00∼10:00 using an electronic scale. Individual feed intake indicates the average amount of feed consumed per day. Feed efficiency was calculated based on the following equation:
Table 1.
Composition of the rat diet
| Ingredient (%) | low Ca (0.1%) | normal Ca |
|---|---|---|
| Cornstarch | 45.4988 | 45.749 |
| Alpha Cornstarch | 15.5 | 15.5 |
| Albumin | 15 | 15 |
| Sucrose | 10 | 10 |
| Corn oil | 4 | 4 |
| Cellulose | 5 | 5 |
| AIN-93 Mineral mix (normal Calcium) | 0 | 3.5 |
| AIN-93 Mineral mix (Low Calcium) | 3.5 | 0 |
| Vitamin mix | 1 | 1 |
| Choline Bitartrate | 0.25 | 0.25 |
| Biotin | 0.0004 | 0.0004 |
| CaCO3 | 0.25 | 0 |
| t-Butylhydroquinone | 0.0008 | 0.0008 |
| 100% | 100% |
Feed efficiency (%) = Weight gain during the experiment (g) ÷ total feed consumption during the experiment (g)×100 Before the experiment was over, white rats were then transferred to individual metabolism cages, and urine was collected over 24 hours and stored at temperature of -30℃ for analysis.
Bone parameters measurement
After the calcium-restricted diet was finished, the mixture of Ketamine (0.15 ml per 100g weight) and saline solution in equal amounts was injected by intra-abdominal injection for anesthesia. After blood collection, rats were killed. Serum was separated from collected blood by a centrifuge and stored at -30℃ for analysis. To measure bone density, low limbs were dissected from dead rats, and then bilateral femurs were harvested. After removing all soft tissues including muscles and ligaments, the bones were stored in 70% alcohol at 4℃ for bone density measurements. Bone weight (0.1 mm) was measured using an electronic scale (EB-430DW, Shimazu Co Ltd., Japan) and bone length is measured with a Vernier calliper. Bone mineral density, t-BMD; mg/cm2 and bone mineral content, t-BMC; mg were measured with a double-energy X-ray absorptionmetry (Lunar DPXL, USA, Software version 1.0C). After separation of trabecular bone from cortical bone, volumetric bone mineral density (vBMD; mg/cm3) was measured using peripheral quantitative computed tomography (pQCT, XCT960A, Norland Srtatec, Germany; ver 5.21). The point of measurement for trabecular vBMD was set in the distal metaphyseal area, 3.0mm off the end of the femur. Cortical vBMD was measured in femur midshaft shape, 12.0mm off the end of the femur. Structural strength of the femur was also evaluated by measuring fracture energy and stiffness with a three-point bending test and a measuring device (Autograph AGS-100D, Shimadzu, Japan). Support span was set at 12mm for femoral fracture, and compression was placed at the center point in femoral length. And load weight and loading speed was 50kg and 5mm/min, respectively.
Blood and urine bone metabolic markers analysis
Blood samples were taken after more than 12 hours of fasting following the calcium-restricted diet. Harvested blood was stored at 4℃ for 30 min and centrifuged at 3000 rpm for 20 minutes and stored at –30℃. Serum and 24 hr urine Ca levels were determined with an ultraviolet visible spectrophotometer (UV-1200, Shimazu, Japan) using OCPC method (orthocresol-phthalein complexion; Ca-test kit, Wako, Japan). Measured data was analyzed using molybdenum blue method (iP-test kit, Wako, Japan). The activation of serum alkalinephosphatase (S-ALP) was evaluated using p-Nitro-phenylphosphate method (ALP-test kit, Wako, Japan), and the activation of serum tartrate-resistance acid phosphatase (S-TrACP) was evaluated using p-Nitrophenylphosphate method (ACP-test kit, Wako, Japan). Measured deoxypyridinoline crosslinks (U-Dpd) in 24 hour urine was analyzed using a commercial ELISA kit (Osteolinks, Sumitomo, Japan).
Statistical analysis
Statistical analysis was performed by calculating mean values and SD for each group using SPSS for Windows (ver. Windows (ver. 18.0). The difference in the means from variables measured for the two groups was determined by Independent-Samples T-test. P value of less than 5% (p < .05) was considered statistically significant.
RESULTS
Comparison of body weight, growth rate, food intake and food efficiency.
Weight, growth rate, feed consumption and feed efficiency between the two groups before and after calcium-restricted diet were presented in Table 2. Weight was not significantly changed after 6-week calcium-restricted diet in the two groups, compared with baseline weight. No significant differences were observed in growth rate, feed intake and feed efficiency between the two groups in which daily average weight gain was 1g and corresponding feed efficiency rate was 5.8%.
Table 2.
The comparison of body weight, growth rate, food intake and food efficiency between two groups after 6weeks restriction of dietary calcium.
| SCa | LCa | p | |
|---|---|---|---|
| Initial body weight (g) | 266.2 ± 9.4 | 263.023.2 | .765 |
| Final body weight (g) | 323.1 ± 15.5 | 310.5 ± 19.0 | .283 |
| Growth rate (g/day) | 1.14 ± 0.16 | 0.95 ± 0.62 | .486 |
| Food intake (g/day) | 17.3 ± 1.2 | 16.2 ± 0.6 | .208 |
| Food efficiency (%)* | 6.2 ± 0.4 | 5.54.3 | .682 |
values are means ± SD. SCa, standard normal Ca diet group; LCa, low Ca diet group; Food efficiency,
:Calculated by [body weight gain/amount of food intake×100]. p, p-value by Independent T-test.
Comparison of femur BMD by DEXA and pQCT and other bone parameters.
Table 3 indicates bilateral femoral BMDs, BMCs, bone lengths and bone mass measured with DEXA and pQCT after six-week calcium-restricted diet. No significant differences were exhibited in bilateral femoral bone length and bone mass. Although DEXA revealed no significantly different bilateral femoral BMDs between the two groups, femoral BMCs significantly decreased by 15% in the LCa group and 11% in the SCa group (p = .023, p = .047). PQCT also revealed no significant differences in bilateral femoral total BMDs and total area, trabecular bone area (Trab area), cortical bone BMD (Crt BMD), cortical bone area (Crt area), cortical bone thickness (Crt thk) between the two groups. However, trabecular bone BMD (Trab BMD) was significantly decreased by 18% in the LCa group when compared with the SCa group (p = .041). No significant difference was also noticed in bone toughness measured in fracture energy and stiffness between the two groups.
Table 3.
The comparison of femoral BMD by DEXA and pQCT, and other bone parameters between two groups after 6weeks restriction of dietary calcium.
| side | SCa | LCa | p | |
|---|---|---|---|---|
| Bone length (mm) | R | 36.5 ± 0.4 | 36.9 ± 1.2 | .438 |
| L | 36.4 ± 0.6 | 36.9 ± 1.4 | .387 | |
| Bone mass (mg) | R | 936.6 ± 46.7 | 922.0 ± 67.1 | .726 |
| L | 946.7 ± 69.3 | 919.3 ± 26.7 | .479 | |
| by DEXA | ||||
| BMD (mg/cm2) | R | 220.5 ± 14.4 | 205.3 ± 7.6 | .091 |
| L | 221.8 ± 13.8 | 208.8 ± 5.7 | .116 | |
| BMC (mg) | R | 365.7 ± 29.2 | 312.0 ± 30.5* | .023 |
| L | 360.2 ± 23.3 | 320.5 ± 30.4* | .047 | |
| by pQCT | ||||
| total BMD (mg/cm3) | R | 641.5 ± 51.3 | 623.648.3 | .595 |
| total area (mm2) | R | 19.4 ± 2.2 | 16.9 ± 1.0 | .068 |
| Trab BMD (mg/cm3) | R | 296.931.0 | 244.4 ± 37.2* | .041 |
| Trab area (mm2) | R | 3.941.87 | 5.32±1.23 | .235 |
| Crt BMD (mg/cm3) | R | 948.1 ± 13.2 | 964.3 ± 28.6 | .254 |
| Crt area (mm2) | R | 7.79 ± 1.41 | 6.81 ± 0.53 | .225 |
| Crt thk (mm) | R. | 0.565 ± 0.099 | 0.530 ± 0.060 | .547 |
| Bone intensity by 3PBT | ||||
| Energy (mJ) | R | 71.7 ± 11.9 | 72.9 ± 7.0 | .860 |
| L | 74.8 ± 18.8 | 68.5 ± 10.3 | .563 | |
| Stiffness (N/mm) | R. | 302.7 ± 27.6 | 300.3 ± 82.6 | .949 |
| L | 321.0 ± 70.5 | 351.671.4 | .522 |
values are means ± SD. SCa, standard normal Ca diet group; LCa, low Ca diet group; R, right side; L, left side; DEXA, dual energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; 3PBT, three-point bending test; BMD, bone mineral density; BMC, bone mineral content; Trab, trabecular bone; crt, cortical bone; thk, thickness;
:p < .05 vs. SCa group. p, p-value by Independent T-test.
Comparison of serum and urine bone metabolism markers.
Weight, growth rate, feed consumption and feed efficiency between the two groups before and after calcium-restricted diet were presented in Table 4. After 6-week calcium- restricted diet, 24 hours urinary excretion of deoxypyri-dinoline (U-Dpd) was reduced by 29% from baseline data (p = .039), which was much significant when compared to the changes in the control group. More significant decrease (31%) was also observed in serum tartrate-resistance acid phosphatase (S-TrACP) in the LCa group, compared with the control group (p = .010). The difference in serum alkaline phosphatase (S-ALP) between the two groups was not significant.
Table 4.
The comparison of bone metabolism markers between two groups after 6weeks restriction of dietary calcium.
| SCa | LCa | P | |
|---|---|---|---|
| U-Dpd (Nmol/24h) | 12.6 ± 2.5 | 9.0 ± 1.6* | .039 |
| S-ALP (IU/l) | 38.5 ± 15.5 | 35.9 ± 8.6 | .767 |
| S-TrACP (IU/l) | 11.2 ± 1.5 | 7.71.8. | .010 |
values are means ± SD. SCa, standard normal Ca diet group; LCa, low Ca diet group; U-Dpd, 24 hours urinary excretion of deoxypyridinoline; U-Cre, 24 hours urinary excretion of creatinine; S-ALP, serum alkaline phosphatase; S-TrACP, serum tartrate-resistance acid phosphatase.
:p < .05 vs. SCa group. p, p-value by Independent T-test.
Comparison of Ca and P metabolism in serum and urine
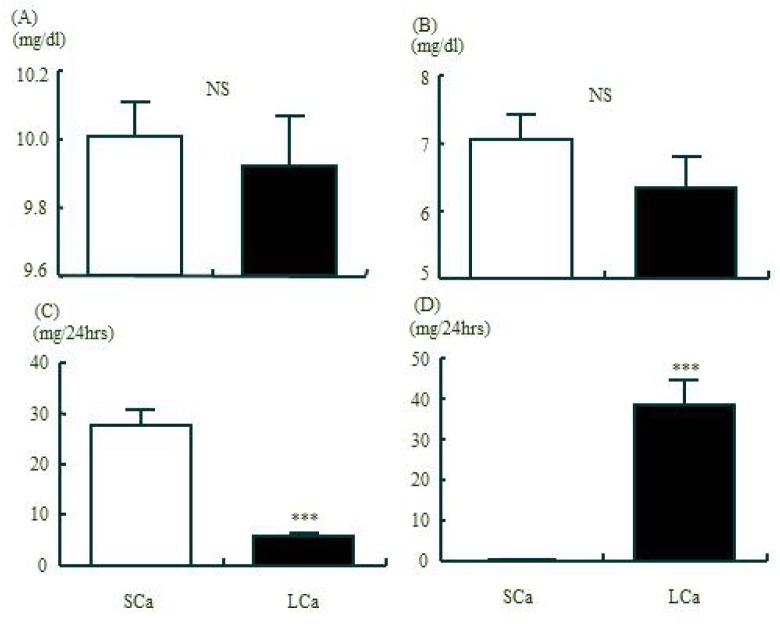
Fig 1 indicates the comparison of serum and urine Ca and P values after 6-week calcium-restricted diet. No significant differences were exhibited in serum Ca and P concentration. However, urine Ca excretion was significantly decreased while urine P level was significantly increased in the LCa group, compared with the control group (p = .0001 each).
Fig. 1.
The comparison of Ca and P metabolism in serum and urine between two groups after 6weeks restriction of dietary calcium. A, serum Ca; B, serum P; C, 24 hrs urinary Ca excretion; D, 24 hrs urinary P excretion. values are means ± SE. SCa, standard normal Ca diet group; LCa, low Ca diet group; ***:p < .001 vs. SCa group by Independent T-test . Serum Ca and P concentration of the LCa did not changed comparing to the SCa, but 24 hrs urinary Ca excretion of the LCa was significantly decreased (p < 0.001) and urinary P was significantly increased (p < 0.001) comparing to the SCa.
DISCUSSION
Bone is very active tissue that is sensitive to metabolic changes such as exercise and nutrition [14]. Increased calcium helps improve bone density [15]. Calcium intake has a significantly positive correlation with lumbar spine bone density [22]. Also, calcium supplements increase bone density and retard the loss of bone stock [23]. Thus the relationship between calcium intake and changes in bone stock are proven. But data in effects of calcium-deficiency diet on bone density and bone metabolism are scant. The 2009 Korean National Health and Nutrition Examination Survey results [18}, calcium intake in adult women falls much behind recommended calcium intake for Koreans set by the Institute of Medicine Dietary Reference Intakes (DRIs) in 2010. Song and Paik[24] also claimed the daily average calcium intake in female college students was very low with 487 ± 144mg. It was also reported that adults with osteoporosis consume less dairy products than those without osteoporosis. However data in effects of low dietary calcium intake on bone density is very scant. It is therefore necessary to review the mechanism involved in the association of restricted-calcium intake with a reduction in bone density and serum parameters related to bone metabolism.
The overall structure of bone can be divided into cortical bone and trabecular bone[7]. As trabecular bone is highly sensitive to changes, it serves as a better predictor to evaluate early biochemical changes of bone metabolism [10]. In this study, white rats were given calcium-restricted diet for 6 weeks. Harvested femur was divided into trabecular bone and cortical bone using pQCT. And then bone density was measured and serum and urinary parameters of bone metabolism were evaluated. In this study, we employed a novel approach to understanding effects of calcium-restricted diet on different bone components by separating trabecular bone from cortical bone.
The results of this study unveiled no significant differences in t-BMID between the two groups. However, t-BMC was significantly decreased in the LCa group, compared with that of the control group (p < .05). Although dual energy x-ray absortiometry (DEXA) is commonly used to measure bone density but rather inefficient to determine the difference in bone toughness between cortical and trabecular bones and to predict the risk of bone fracture[26, 27]. That is, DEXA-based bone mineral density indicates total femur bone mineral density expressed as total BMD or t-BMD, meaning that total mineral content of the femur as a whole [28]. It is therefore difficult to detect delicate changes that happen in different compartments of cortical or trabecular bones. In this study, calcium-restricted intake led to a significant decrease in t-BMC. But no significant changes were exhibited in t-BMD even though it was decreased as a whole. This means that low calcium-induced changes in bone density may vary with bone components but they are insignificant when combined.
On the other hand, bone remodeling is triggered by osteoblast and osteoclast. The surface-to-volume of trabecular bone volume is nearly 10 times that of cortical bone, so its contribution to activation of bone metabolism is greater. It is therefore assumed that the decrease in bone density caused by calcium deficiency may occur in trabecular bone sooner than cortical bone. To overcome the limitations of DEXA, new scanning techniques have been introduced. Among them, peripheral quantitative computer tomography (pQCT) is designed to reconstruct the target bone in 3D and measure volumetric bone mineral density (v-BMD) from each of cortical and trabecular bones, providing a better prediction of fracture risk [29,30]. Bilateral cortical v-BMDs were not significantly different between the two groups, either. However, trabecular v-BMD was significantly decreased in the LCa group, compared with the SCa group (p = .041). This finding supported the assumption that trabecular bone density is affected sooner by low calcium intake. Approximately 95% of the lumbar spine consists of trabecular bone, making the bone less sensitive to nutrition and hormone [31]. The lifetime loss of bone is estimated at 50% in trabecular bone and 35% in cortical bone [32]. In this study, the loss of trabecular bone density was also greater than that of cortical bone. Zebaze et al. [33] suggested faster resorption of trabecular bone than cortical bone in presence of osteoporosis in their study investigating distal radius BMD in 122 women aged between 27 and 98 after separating trabecular bone from cortical bone using pQCT. Di Leo et al. [34] also asserted a greater loss of tracecular bone density in postmenopausal women with the average age of 63 based on pQCT-based bone density. Therefore, a similar pattern is observed in reduced bone density whether is caused by either calcium restricted diet or menopause.
In this study, no significant differences were displayed in bone strength measured as fracture energy and stiffness using a three-point bending test (p > .05). Bone density does not provide accurate prediction of fracture risk [35]. According to NIH Consensus definition, bone density is responsible for approximately 60-80% of bone strength. In addition, the structure of trabecular and cortical bones, bone turnover rate, delicate bone fracture and mineralization affect bone strength [36]. The contribution proportions of trabecular and cortical bones to bone strength are not established. But it is known that cortical bone has a greater effect on bone strength. In this study, fracture strength was measured at the center point of the femur. Since the femoral diaphysis consists of cortical bone, it is said that structural parameters of cortical bone reflect bone strength. Michlotti and Clark[37] also claimed the association of cortical bone thickness of femur neck to fracture risk. In this study, effects of low calcium diet on cortical bone density were not significant, compared to those in trabecular bone density. There was also no significant difference in bone strength between the two groups, supporting a positive correlation between cortical bone and bone strength.
We also found that low-calcium diet significantly decreased bone resorption markers U-Dpd and S-TrACP (p = .039, p = .010). Dpd is one of bone metabolism parameters representing osteolysis. Dpd is generated when bone matrix is broken down by osteoclasts and discovered in bone tissue. Dpd does not undergo metabolism and excreted in urine, so urinary excretion of Dpd is measured [39]. Dpd increases in postmenopausal women [40]. TrACP is one of osteoclast markers, and serum TrACP levels reflect the efficacy of bone resorption. An earlier study reported a higher serum TrACP level in the above 50 age group, compared with the other younger age groups (below 20 and between 20 and50 age groups), confirming the effectiveness of serum TrACP as a bone resorption marker [41]. In this study, low urinary Dpd and serum TrACP levels were observed in the LCa group showing reduced bone stock. That is, calcium deficiency inhibited bone turnover and subsequently bone formation. However, resulting bone stock reduction in this study differs from age-related osteopenia caused by osteoclast- induced bone resorption disorders in postmenopausal women. Further study is needed to clarify the difference between low calcium-induced loss and age-related loss of bone stock.
Bone sensitively responds to changes in calcium intake [42] and maintains the balance between bone resorption [43] and bone formation [44].Thus bone plays an important role in calcium homeostasis. Calcium metabolism is affected by various factors, including physical activity, nutrition and hormones. When calcium level falls due to low calcium intake [45] and increased excretion in urine [46], PTH release rises [47], provoking bone resorption stronger than bone building-up. And then the body will pull calcium from bones to maintain adequate serum calcium levels [45]. If more calcium is repeatedly taken out of bones than is replaced, osteoporosis will eventually occur. Blood P level is regulated by intestinal absorption of P and urinary P excretion and affected by 1,25(OH)2D3 and PTH [49]. Metabolic acidifica-tion can cause a steep decrease in bone stock when it is coupled with abnormalities in urinary P excretion [50]. In this study, changes in serum Ca and P were not significant (p > .05). However, calcium-restricted diet significantly decreased urinary Ca excretion (p = .001) but increased urinary P excretion (p = .001). We therefore conclude that low calcium diet signals the body to pull Ca from the bones to keep blood Ca level up and inhibit urinary Ca excretion by accelerating P excretion in order to maintain Ca and P homeostasis. Bone stock is therefore decreased as a result.
REFERENCES
- 1.National Resource Center National Institutes of Health Osteoporosis and related bone diseases. 2009 Available at: http://www.niams.nih.gov/Health_Info/Bone/Osteoporosis. Accessed October 20, 2013.
- 2.National Osteoporosis Foundation What is osteoporosis? 2013 Retrieved June 7, 2013, from http://www.nof.org/articles/7s. Accessed October 20, 2013.
- 3.Kynaston RU. On osteoporosis of the spine with spontaneous fracture. Guys Hosp Gaz. 1950;64(1615):241–242. [PubMed] [Google Scholar]
- 4.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 5.Rowe SM, Song EK, Kim JS, Lee JY, Park YB, Bae BH, Hur CI. Rising incidence of hip fracture in Gwangju City and Chonnam Province, Korea. J Korean Med Sci. 2005;20(4):655–8. doi: 10.3346/jkms.2005.20.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health Insurance Review & Assessment Service January 24 Report material. 2013 Retrieved June 7, 2013, from http://www.hira.or.kr/dummy.do?pgmid=HIRAA02 0041000000&cmsurl/cms/notice/02/1316013_13390.html.
- 7.Stephen C, Cowin . bone Mechanics Hand book. 2001. [Google Scholar]
- 8.Senthil K, Eswaran, Harun H, Bayraktar, Mark F, Adams, Atu lGupta, Paul F, Hoffmann, David C, Lee, Panayiotis Papadopoulos, Tony M, Keaveny The micro-mechanics of cortical shell removal in the human vertebral body. Computer methods in applied mechanics and engineering. 2007;196:3025–3032. [Google Scholar]
- 9.Lochmüller EM, Groll O, Kuhn V, Eckstein F. Mechanical strength of the proximal femur as predicted from geometric and densitometric bone properties at the lower limb versus the distal radius. Bone. 2002;30(1):207–216. doi: 10.1016/s8756-3282(01)00621-4. [DOI] [PubMed] [Google Scholar]
- 10.Lochmüller EM, Miller P, Burklein D, Wehr U, Rambeck W, et al. In situ femoral dual-energy X-ray absorptiometry related to ash weight, bone size and density, and its relationship with mechanical failure loads of the proximal femur. Osteoporos Int. 2000;11(4):361–367. doi: 10.1007/s001980070126. [DOI] [PubMed] [Google Scholar]
- 11.Zmuda JM, Sheu YT, Moffett SP. Genetic epidemiology of osteoporosis: past, present, and future. Curr Osteoporos Rep. 2005;3(3):111–115. doi: 10.1007/s11914-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 12.Ross PD, Fujiwara S, Huang C, Davis JW, Epstein RS, Wasnich RD, Kodama K, Melton LJ. Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the US. Int J Epidemiol. 1995;324(6):1171–7. doi: 10.1093/ije/24.6.1171. [DOI] [PubMed] [Google Scholar]
- 13.Mundy GR. Nutritional modulators of bone remodeling during aging. Am J Clin Nutr. 2006;83(2):427S–430S. doi: 10.1093/ajcn/83.2.427S. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari SL. Osteoporosis, a complex disorder of aging with multiple genetic and environmental determinants. World Rev Nutr Diet. 2005;95:35–51. doi: 10.1159/000088271. [DOI] [PubMed] [Google Scholar]
- 15.Wickham CA, Walsh K, Cooper C. Dietary calcium, physical activity and risk of hip fracture. A prospective study. BMJ. 1989;299:889–892. doi: 10.1136/bmj.299.6704.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal woman: a randomized controlled trial. AM J Med. 1995;98:331–335. doi: 10.1016/S0002-9343(99)80310-6. [DOI] [PubMed] [Google Scholar]
- 17.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health, Welfare and Family Affairs. Korea Center for Disease Control and Prevention Korea health statistics 2009: Korea national health and nutrition examination survey (KNHANES Ⅳ-3) 2010 Korea Center for Disease Control and Prevention, Seoul, Korea.
- 19.Korean Nutrition Society . Dietary reference intakes for Koreans. Seoul, Korea: 2010. [Google Scholar]
- 20.Choi HM. 21 century nutrition. 2. Paju, Korea: Kyumunsa; [Google Scholar]
- 21.Choi JH, Kim SK. Comparison of the dietary factors between normal and osteopenia groups by bone mineral density in Korean female college students. J Korean Soc Food Sci Nutr. 2008;37:869–878. [Google Scholar]
- 22.Oh JJ, Hong ES, Baik IK, Lee HS, Lim HS. Effects of dietary calcium protein and phosphorus intakes in bone mineral density in Korean premenopausal women. Korean J Nutrition. 1996;29:59–69. [Google Scholar]
- 23.Na HB, Kim HJ, Park J. Effects of calcium supplementation and exercise on bone mineral density in middle-aged women. Korean J Nutrition. 2002;35:962–969. [Google Scholar]
- 24.Song YJ, Paik HY. Effect of dietary factors on bone mineral density in Korean college women. Korean J Nutr. 2002;35:464–472. [Google Scholar]
- 25.Yu CH, Lee JS, Lee LH, Kim SH, Lee SS, Kang SA. Nutritional factors related to bone mineral density in the different age groups of Korean men. Korean J Nutr. 2004;37:132–142. [Google Scholar]
- 26.Holzer G, von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009;24:468–474. doi: 10.1359/jbmr.081108. [DOI] [PubMed] [Google Scholar]
- 27.Mautalen CA, Vega EM, Einhorn TA. Are the etiologies of cervical and trochanteric hip fractures different? Bone. 1996;18:133S–137S. doi: 10.1016/8756-3282(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 28.Mazess RB, Barden HS. Measurement of bone by dual-photon absorptiometry (DPA) and dual-energy X-ray absorptiometry (DEXA) Ann Chir Gynaecol. 1988;77(5-6):197–203. [PubMed] [Google Scholar]
- 29.Bousson V, Le Bras A, Roqueplan F, et al. Volumetric quantitative computed tomography of the proximal femur: relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos Int. 2006;17:855–864. doi: 10.1007/s00198-006-0074-5. [DOI] [PubMed] [Google Scholar]
- 30.Wachter NJ, Augat P, Krischak GD, Mentzel M, Kinzl L, Claes L. Prediction of cortical bone porosity in vitro by microcomputed tomography. Calcif Tissue Int. 2001;68:38–42. doi: 10.1007/s002230001182. [DOI] [PubMed] [Google Scholar]
- 31.Calvo MS. The effects of high phosphorus intake on calcium homeostasis. Adv Nurt Res. 1994;9:183–207. doi: 10.1007/978-1-4757-9092-4_11. [DOI] [PubMed] [Google Scholar]
- 32.Kim WY. Osteoorosis and dietary factors. Korean J Nutrition. 1994;27:636–645. [Google Scholar]
- 33.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 34.Di Leo C, Tarolo GL, Bagni B, Bestetti A, Tagliabue L, Pietrogrande L, Pepe L. Peripheral quantitative Computed Tomography (PQCT) in the evaluation of bone geometry, biomechanics and mineral density in postmenopausal women. Radiol Med. 2002;103(3):233–41. [PubMed] [Google Scholar]
- 35.Garber MA, Mc Dowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in rat model. Aviat Space Environ Med. 2000;71(6):586–592. [PubMed] [Google Scholar]
- 36.WHO Assessment of osteoporosis fracture risk and its role in screening for postmenopause osteoporotic. 1994 WHO technical report. Geneve.
- 37.Michelotti J, Clark J. Femoral neck length and hip fracture risk. J Bone Miner Res. 1999;14:1714–1720. doi: 10.1359/jbmr.1999.14.10.1714. [DOI] [PubMed] [Google Scholar]
- 38.Telci A, Cakatay U, Kurt BB, Kayali R, Sivas A, Akçay T, Gökyiğit A. Changes in bone turnover and deoxypyridinoline levels in epileptic patients. Clin Chem Lab Med. 2000;38(1):47–50. doi: 10.1515/CCLM.2000.008. [DOI] [PubMed] [Google Scholar]
- 39.Rubinacci A, Melzi R, Zampino M, Soldarini A, Villa I. Total and free deoxypyridinoline after acute osteoclast activity inhibition. Clin Chem. 1999;45:1510–1516. [PubMed] [Google Scholar]
- 40.Ceballos A, Mas R, Castano G, Fernandez L, Mendoza S, Menendez R, Gonzalez JJ, Illnait J, Gamez R, Fernandez J. The effect of D-003(10 mg/day) on biochemical parameters of bone remodelling in postmenopausal women: a randomized, double-blind study. Int J Clin Pharmacol Res. 2005;25:175–186. [PubMed] [Google Scholar]
- 41.Rico H, Iritia M, Arribas I, Revilla M. Biological profile of tartrate-resistant acid phosphatase as a marker of bone resorption. Rev Esp Fisiol. 1990;46(4):379–83. [PubMed] [Google Scholar]
- 42.Miyake Y. Heterotopic bone formation: participation of Ca metabolism. Nippon Hinyokika Gakkai Zasshi. 1965;56(11):1163–1171. doi: 10.5980/jpnjurol1928.56.11_1163. [DOI] [PubMed] [Google Scholar]
- 43.Chai BF, Tang XM, Zhou WR. Osteoclastic resorption of trabeculae in osteoporotic femoral head: a scanning electron microscopic study. Zhonghua Wai Ke Za Zhi. 1994;32(10):621–625. [PubMed] [Google Scholar]
- 44.Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405(6787):704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Orimo H. Physiological and pathological aging in bone-contribution of risk factors to bone loss with aging. Nippon Ronen Igakkai Zasshi. 1991;28(3):318–324. [PubMed] [Google Scholar]
- 46.Spencer H, Kramer L. Osteoporosis: calcium, fluoride, and aluminum interactions. J Am Coll Nutr. 1985;4(1):121–128. doi: 10.1080/07315724.1985.10720071. [DOI] [PubMed] [Google Scholar]
- 47.McKane WR, Khosla S, Egan KS, Robins SP, Burritt MF, Riggs BL. Role of calcium intake in modulating age-related increases in parathyroid function and bone resorption. J Clin Endocrinol Metab. 1996;81(5):1699–1703. doi: 10.1210/jcem.81.5.8626819. [DOI] [PubMed] [Google Scholar]
- 48.McSheehy PM, Chambers TJ. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986;119(4):1654–1659. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- 49.Dominguez JH, Gray RW, Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab. 1976;43(5):1056–68. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- 50.Ambuhl PM, Zajicek HK, Wang H, Puttaparthi K, Levi M. Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int. 1998;53(5):1288–98. doi: 10.1046/j.1523-1755.1998.00901.x. [DOI] [PubMed] [Google Scholar]