Abstract
[Purpose]
Several epidemiological studies have demonstrated that there are positive correlations between vascular disorders and bone loss in postmenopausal women. The aim of the present study was to examine the effect of different types of exercise (e.g., climbing and swimming) for preventing endothelial dysfunction of arteries and bone loss in ovariectomized rats.
[Methods]
Twenty Sprague-Dawley female rats were randomly divided into three groups: ovariectomy (OVX) plus treatment with vitamin D3 and nicotine (VDN) (control rats [Con], n = 7), which is an animal model for endothelial dysfunction and bone loss; voluntary climbing resistance exercise with OVX plus VDN (climbing rats [Clim], n = 6), and swimming exercise with OVX plus VDN (swimming rats [Swim], n = 7). The period of exercise training was 8 weeks.
[Results]
The endothelin-1 (ET-1) protein levels were significantly lower in the Clim and Swim groups than in the Con. The endothelial nitric oxide synthase protein levels were significantly higher in the Swim group than in the Con, but they did not differ between the Clim and Con groups. The cortical bone mineral density in the tibia and breaking energy of the femur were significantly higher in the Clim group than in the Con, but this positive effect was not seen in the Swim group.
[Conclusion]
Voluntary climbing exercise decreased arterial ET-1 protein levels and prevented bone loss in a postmenopause-model rat combining OVX and VDN. Conversely, swimming suppressed endothelial dysfunction of the arteries but did not prevent bone loss. Thus, the type of exercise should be cautiously chosen for enhancing vascular function and bone status, especially in females after menopause.
Keywords: weight-bearing and non-weight-bearing exercise, endothelial dysfunction, bone loss, ovariectomized rat
INTRODUCTION
Aging increase the risk factors for vascular disease and osteoporosis [1,2]. Especially, the incidence of vascular disorders rapidly increases within a few years after menopause in women [3,4]. Additionally, women lose about 15% of their total bone within the first 5 years after menopause [5,6]. Recently, several epidemiological studies have demonstrated that there are positive correlations between vascular disorders and bone loss [7,8]. According to present findings, strategies for preventing increases in vascular disorders and bone loss may be beneficial for postmenopausal women.
Resistance training, which is important for maintaining bone and muscle health in the elderly, has been reported to have unfavorable effects on central arterial stiffness or compliance [9-11], being considered that resistance training in the elderly may not be beneficial on improvement of vascular function. Conversely, swimming, a popular and safe exercise for the elderly, offers an alternative to weight-bearing exercise. Swimming may improve impaired vascular function in the elderly [12,13], yet conflicting data exist on the effects of water exercise on bone loss [14]. Therefore, the intensity, duration, and type of exercise performed are of crucial importance for preventing these two disorders.
Endothelin-1 (ET-1), a potent vasoconstrictor peptide derived from endothelium, contributes to the increase in arterial stiffness during the progression of hypertension and atherosclerosis when overexpressed [15,16]. In contrast, nitric oxide (NO), which is produced by the endothelial isoform of NO synthase (eNOS) in the vascular endothelial cells, is a potent vasodilator that has anti-atherosclerotic properties [17,18]. Thus, ET-1 and NO play important roles in the regulation of vascular homeostasis. We previously developed a combined model of ovariectomy (OVX) and vitamin D3 and nicotine (VDN) in female rats, which showed vascular disorder and bone loss [19]. In the results, we suggested that an imbalance of the ET-1- and NO-producing system in the arterial tissue during periods of rapid bone loss caused by estrogen deficiency may be one of several possible mechanisms underlying the emergence of a relationship between vascular disorder and bone loss after menopause in females.
Therefore, strategies for preventing impairment of the ET-1- and NO-producing system and the progression of bone loss may be beneficial in postmenopausal women with progressive arterial disease. However, it is unclear whether different types of exercise training during elevations in endothelial dysfunction after menopause modulates eNOS expression and ET-1 levels in the aorta tissue. To our knowledge, limited studies have investigated the effects of different exercises for preventing endothelial dysfunction of arteries and bone loss simultaneously in animals with an estrogen deficiency. Accordingly, the aim of the present study was to examine the effects of different types of exercise (e.g., climbing and swimming) for preventing endothelial dysfunction of arteries and bone loss in OVX rats treated with VDN.
METHODS AND MATERIALS
Animals and protocol
Female Sprague-Dawley rats (6 weeks old) were obtained (CLEA Experimental Animals Supply Co. Ltd., Japan) and cared for according to the Guiding Principles for the Care and Use of Animals, based on the Helsinki Declaration of 1964. The animal care and experimental procedure were approved by the Committee on Animal Research at the University of Tsukuba. Twenty female rats were randomly divided into three groups: ovariectomy (OVX) plus treatment with VDN (control rats [Con], n = 7), which is an animal model for endothelial dysfunction and bone loss; voluntary climbing resistance exercise with OVX plus VDN (climbing rats [Clim], n = 6; one rat was excluded due to OVX failure); and swimming with OVX plus VDN (swimming rats [Swim], n = 7). After 1 week of postoperative recovery, the three groups received vitamin D3 (300,000 IU/kg, intramuscularly; cholecalciferol; Sigma Chemical Co., St. Louis, MO, USA) and nicotine (25 mg/kg, 5 mL/kg, per os; nicotine hydrogen tartrate,; Sigma Chemical Co.) at 0900 h [19]. One week after treatment, the Clim group was housed in metal cages with a wire mesh tower that had a water bottle at the top [20]. At the beginning of the exercise training, the water bottle was set at 20 cm high. The set point of the water bottle was gradually elevated to 2 m over 1 week. The Swim group was forced to swim in a bath for 1 h, six times a week, without any additional mass. The duration of the exercise was gradually increased to 1 h over 2 weeks. The temperature of the bath water was 34℃. The exercised rats performed each regimen for 8 weeks. The Con group was kept in individual cages. All rats were allowed access to food and distilled water ad libitum. Food consumption and body weight gain were measured every second day. The room temperature was maintained at 24 ± 1℃ with humidity at 50 ± 5%. Fluorescent lights were on from 0800 to 2000 h. Eight weeks after the start of exercise training, all the rats were deprived of food overnight. After measuring their body weight, blood samples from the abdominal aorta under ether anesthesia under ether anesthesia. The aortas were isolated and rinsed with cold saline and were stored at -80℃ to measure the protein levels of ET-1 and eNOS by using sandwich-enzyme immunoassay and electrophoresis and immunoblot analysis. The blood samples was drawn and mixed with disodium edetic acid and aprotinin (500 kallikrein inactivator units/mL). The plasma was separated by centrifugation at 1,000 × g for 10 min at 4℃ and stored at -80℃ to measure the calcium concentration. The left and right tibiae of all rats were isolated and freed from any muscle and connective tissue and were immersed in 70% ethanol solution to measure bone mineral density (BMD). The femur samples were also collected to measure bone strength.
Calcium concentration in the plasma
Plasma calcium concentration was measured by using the inductively coupled plasma atomic emission spectroscopy (ICAP-AES-575vNippon Jarrell-Ash Co., Ltd., Kyoto, Japan).
Sandwich-enzyme immunoassay of the aortas
The ET-1 levels in the aorta tissue extracts were determined by using a sandwich-enzyme immunoassay kit (Immuno-Biological Laboratories Co. Ltd., Gunma, Japan). The reported cross-reactivity of the antibody was ≤ 0.1% for all big ETs, ≤ 0.1% for ET-3, and 3.3% for ET-2.
Electrophoresis and immunoblot analysis of the aortas
The aorta tissue was homogenized with 10 volumes of 10 mM Tris-HCL (pH 7.8), 1 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1% NP 40, and 1 mM phenylmethylsulfonyl fluoride on ice using a potter tissue homogenizer (model PT10SK/35; Kinematica, Lucerne, Switzerland) and were then rotated for 90 min at 4℃. The homogenate was centrifuged at 10,000 × g for 30 min at 4℃. The resulting supernatant (cytosolic and membrane fraction) was stored at -80℃ until eNOS protein assays. The protein concentrations were determined by the bicinchoninic acid protein assay reagents (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard. The samples (15 μg protein) were followed by heat denaturation at 96℃ for 5 min with β-mercaptoethanol and sodium dodecylsulfate (SDS) sample buffer (62.5 mM Tris-HCl buffer with pH 6.8, containing 25% glycerol and 2% SDS). Each cytosolic and membrane fraction preparation of the aorta was separated on an SDS-polyacrylamide gel (7.5%) and then transferred to polyvinylidene difluoride (Millipore, Tokyo, Japan) membranes at 2.5 mA/cm2 for 60 min. After the membrane was treated with blocking buffer of 1% skim milk (eNOS) in a phosphatebuffered saline containing 0.05% Tween 20 (PBS-T) for 1 h at room temperature, the membrane was probed with monoclonal anti-eNOS antibody (Transduction Laboratories, Lexington, KY USA; 1:800 dilution with blocking buffer) for 12 h at 4℃, washed with PBS-T three times, and then incubated with a horseradish peroxidase-conjugated secondary antibody, which is an anti-mouse immunoglobulin antibody (Amersham Life Science, Buckinghamshire, UK; 1:2000 dilution with blocking buffer) for 1 h at room temperature. After this reaction, the membrane was washed with PBS-T five times. Finally, the eNOS were detected by the ECL Plus system (Amersham Life Science) and exposed to Hyper film (Amersham Life Science). The photograph was scanned using a Canoscan 600 (Canon, Tokyo, Japan), and quantification was performed by a computer with MacBAS software (Fuji Film, Tokyo, Japan).
Bone mineral density of the tibial proximal metaphysis
The BMD values of the tibia were measured by dual-energy x-ray absorptiometry (DXA) (DCS-600R; Aloka Co., Tokyo, Japan). We examined the proximal one-fifth of the tibia, including the epimetaphyseal region, which represents the cancellous region, and the middle two-fifth of tibia, which represents the cortical diaphyseal region [21].
Bone strength testing
The strength of the femoral mid-shaft was assessed by a three-point bending test (DYN-1255; IIO DENKI, Tokyo, Japan) using previously described methods (distance between the fulcrum 1 cm, plunger speed 100 mm/min, full scale 50 kg, chart speed 120 cm/min) [22]. The breaking energy refers to the workload that resulted in the breaking of the bone (workload was moved when the power of 1 dyn was reached).
Statistical analysis
All data were expressed as the mean ± standard error of mean and were analyzed using SPSS, version 21.0J (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to test for statistically significant differences among the groups, and the post hoc Tukey test was used for detecting a significant difference among the groups. A significance level of p < 0.05 was used for all comparisons.
RESULTS
The final body weight, body weight gain, food intake, and food efficiency did not differ among the groups (Table 1). There were no differences in plasma calcium concentration among the groups (8.95 ± 0.20 mg/dl, 8.75 ± 0.17 mg/dl, and 8.71 ± 0.20 mg/dl in the Con, Clim, and Swim groups, respectively).
Table 1.
Body weight, food intake, and food efficiency
| Final body weight (g) | Body weight gain (g/day) | Food intake (g/day) | Food efficiency | |
|---|---|---|---|---|
| Con | 366.3±8.2 | 3.16±0.22 | 16.98±0.86 | 0.19±0.00 |
| Clim | 357.3±10.4 | 2.85±0.15 | 15.98±0.42 | 0.17±0.01 |
| Swim | 344.6±9.6 | 2.64±0.19 | 15.96±0.59 | 0.17±0.01 |
Data show means ± SE. Ovariectomy (OVX) plus Vitamin D3 and Nicotine (VDN) group with non-exercise control group (Con). OVX plus VDN group with climbing resistance exercise group (Clim) and with forced swimming exercise group (Swim, 1h/day, 6 times /wk). The VDN was administered a week after OVX operation. Exercise started a week after VDN administration. The period of exercise was 8 weeks.
The arterial ET-1 protein levels were significantly lower in the Clim and Swim groups than in the Con, whereas there was no difference between the exercised groups (Fig. 1). Fig. 2 shows the eNOS protein levels in the artery. The arterial eNOS protein expression did not differ between the Con and Clim groups, but it was significantly higher in the Swim group than in the Con.
Fig. 1.
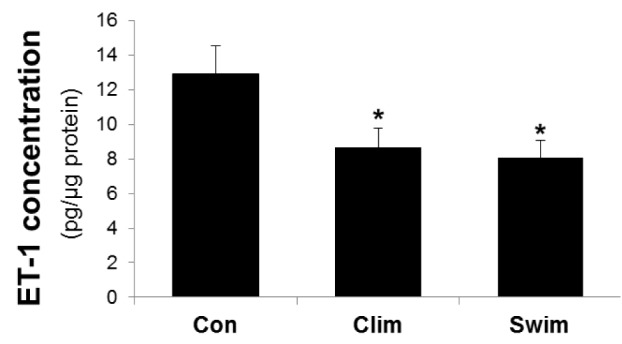
The endothelin-1 concentration in the thoracic aorta. The rat groups were the control (Con) with ovariectomy (OVX) plus vitamin D3 and nicotine (VDN) and no exercise; climbing (Clim) resistance exercise group with OVX plus VDN; and forced swimming (Swim) exercise group (1 h/day, 6 times/week). The VDN was administered a week after OVX. The exercises started a week after VDN administration. The exercise period was 8 weeks. * p< 0.05 vs. the Con. Values are expressed as the mean ± SE.
Fig. 2.
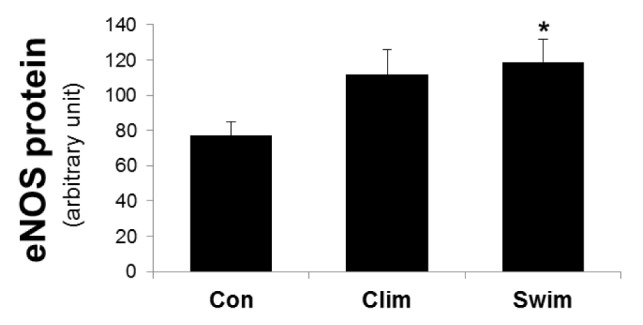
The expression of the endothelial nitric oxide (eNOS) protein in the thoracic aorta. The rat groups were the control (Con) with ovariectomy (OVX) plus vitamin D3 and nicotine (VDN) and no exercise; climbing (Clim) resistance group with OVX plus VDN; and forced swimming (Swim) exercise group (1 h/day, 6 times/week). The VDN was administered a week after OVX. The exercises started a week after VDN administration. The exercise period was 8 weeks. * p < 0.05 vs. the Con. Values are expressed as the mean ± SE.
Fig. 3 shows the BMD data of the tibiae. The BMD of the tibial proximal metaphysis tended to be higher in the Clim group than in the Con (p = 0.078), but it did not differ among the groups. The BMD of the tibial diaphysis was significantly higher in the Clim group than in the Con, but this result was not seen in the Swim group compared to the Con. The breaking energy of the femur was significantly higher in the Clim group than in the Con (Fig. 4), but this was not seen in the Swim group.
Fig. 3.
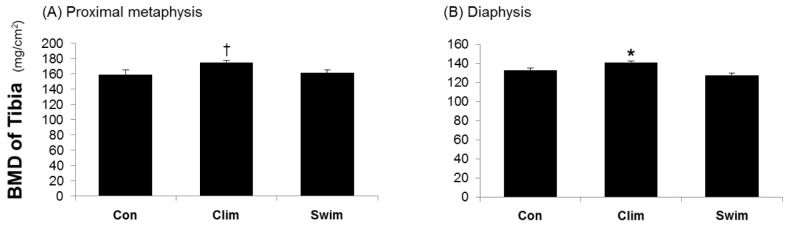
Bone mineral density of the proximal tibial metaphysis (A) and tibial diaphysis (B). The proximal one-fifth of the tibia, including the epimetaphyseal region, represents the cancellous region, and the middle two-fifth of tibia, represents the cortical diaphyseal region. The rat groups were the control (Con) with ovariectomy (OVX) plus vitamin D3 and nicotine (VDN) and no exercise; climbing (Clim) resistance exercise group with OVX plus VDN; and forced swimming (Swim) exercise group (1 h/day, 6 times/week). The VDN was administered a week after OVX. The exercises started a week after VDN administration. The exercise period was 8 weeks. † p = 0.078 vs. the Con; * p < 0.05 vs. the Con. Values are expressed as the mean ± SE.
Fig. 4.
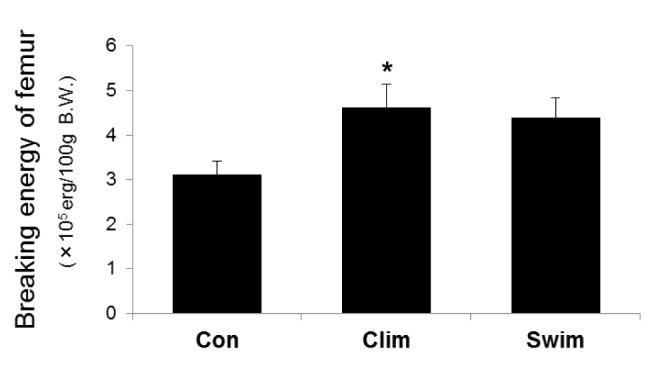
The breaking energy of the femurs. The breaking energy of the femoral diaphysis was determined by a three-point bending test. The rat groups were the control (Con) with ovariectomy (OVX) plus vitamin D3 and nicotine (VDN) and no exercise; climbing (Clim) resistance exercise group with OVX plus VDN; and the forced swimming (Swim) exercise group (1 h/day, 6 times/week). The VDN was administered a week after OVX. The exercises started a week after VDN administration. The exercise period was 8 weeks. * p < 0.05 vs. the Con. Values are expressed as the mean ± SE.
DISCUSSION
The major finding of the present study was that the climbing exercise decreased arterial ET-1 protein levels and increased BMD and bone strength in the OVX rats treated with VDN. Conversely, swimming clearly suppressed the endothelial dysfunction of arteries but not bone loss.
In our previous study, a combined rat model with OVX and VDN treatment showed marked increases in bone loss and the impairment of arterial endothelial function, such as a decrease in eNOS and an elevation of the ET-1 protein levels [19]. It also showed that the arterial eNOS protein levels did not decrease 4 weeks after VDN treatment after OVX, but the levels decreased at 8 weeks. The increase in arterial ET-1 protein levels and bone loss occurred at 4 and 8 weeks, respectively. Thus, the present rat model during the experimental period of 8 weeks was considered to be suited for examining the inhibitory effect of various types of exercise on the progression of arterial dysfunction and bone loss.
Regular aerobic exercise has a beneficial effect on the endothelial function of arteries. Aerobic exercise training increases the circulating plasma nitrite/nitrate levels (the stable end product of NO) and reduces plasma ET-1 levels in postmenopausal women [23,24]. In contrast to the beneficial effects of regular aerobic exercise [23-26], resistance training has been reported as having unfavorable effects on central arterial stiffness or compliance modulated by endothelial function [9-11]. The present study revealed for the first time that in the aorta of rats, the production of ET-1 was increased by climbing resistance exercise, although it did not reach a significant level in eNOS. The reason for the different results of ET-1 between our study and other studies may be the fact that the climbing resistance used in the present study was a voluntary, light-intensity resistance exercise [20]. Masatsugu et al. [27] reported that hemodynamic shear stress downregulated the messenger ribonucleic acid expression of ET-1 in an intensity- and time-dependent manner in endothelial cells. Thus, performing intermittent weight-bearing physical activity with a light intensity may have a favorable effect on the prevention of endothelial dysfunction of the arteries.
We previously reported that voluntary wheel running exercise and regular aerobic exercise training reversed the increase in ET-1 protein levels and the decrease in eNOS protein in OVX rats treated with VDN [28]. The present study revealed that swimming exercise training and physiologically aerobic exercise, also decreased the ET-1 protein levels and increased the eNOS protein levels. This result was similar with studies using arterial tissues of old rats, which reported that swimming exercise training suppressed the increase in ET-1 protein levels [25] and the decrease in eNOS protein levels [26]. Although the present study had less swimming time per day and a different animal model compared with that used by Tanabe et al., aerobic exercise may be beneficial for enhancing endothelial function of the arteries.
Weight-bearing exercise such as resistance exercise is well known to have a preventive and recovery effect on BMD and bone strength in postmenopausal women [29]. We confirmed that climbing resistance exercise clearly suppressed the progression of bone loss. This effect was shown in the cortical region of the tibial BMD and in the breaking energy of the femur at the cortical region. However, the present study revealed that swimming exercise did not affect the bone mass. Huang et al. [30] reported that swimming exercise increased the biomechanical properties of growing bone in male rats but not the BMD. However, swimming exercise in the present study did not affect the biomechanical properties or bone strength. Of course, swimming exercises are reported to have beneficial effects on increasing BMD [31], but it is obvious that swimming exercise may be less effective than resistance exercise in preventing bone loss.
In conclusion, we demonstrated that voluntary climbing exercise attenuated the increase in arterial ET-1 protein levels and bone loss in a postmenopause-model rat combining OVX and VDN. Conversely, swimming exercise clearly suppressed the endothelial dysfunction of the arteries but did not suppress bone loss. We suggest that the causality between vascular disorders and bone loss may not be the case, although positive correlations between vascular disorders and bone loss have been reported in several epidemiological studies. Thus, the type of exercise should be cautiously chosen for enhancing vascular function and bone status, especially in postmenopausal females.
Acknowledgments
The authors are grateful to all members of the Exercise and Nutrition Laboratory at the University of Tsukuba for their kind cooperation in the anatomy work. We also specially thank Motoyuki Iemitsu (Faculty of Sport and Health Science, Ritsumeikan University, Japan) and Seiji Maeda (Institute of Health and Sports Sciences, University of Tsukuba, Japan) for providing technical assistance. J.H.P. was supported by the SMART Research Professor Program of Konkuk University.
REFERENCES
- 1.Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the Health, Aging, and Body Composition Study. Calcif Tissue Int. 2006;79:102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer JR, Kammerer CM, Rainwater DL, O’Leary DH, Bruder JM, Bauer RL, Mitchell BD. Decreased bone mineral density is correlated with increased subclinical atherosclerosis in older, but not younger, Mexican American women and men: the San Antonio Family Osteoporosis Study. Calcif Tissue Int. 2007;81:430–441. doi: 10.1007/s00223-007-9079-0. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1997;96:2468–2482. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 4.Kuh D, Langenberg C, Hardy R, Kok H, Cooper R, Butterworth S, Wadsworth ME. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. BJOG. 2005;112:476–485. doi: 10.1111/j.1471-0528.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 5.Nordin BE, Polley KJ. Metabolic consequences of the menopause. A cross-sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcif Tissue Int. 1987;41:S1–59. [PubMed] [Google Scholar]
- 6.Fischer M, Raue F. Measurements of bone mineral density. Mineral density in metabolic bone disease. Q J Nucl Med. 1999;43:233–240. [PubMed] [Google Scholar]
- 7.Tanko` LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 8.Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A. Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab. 2003;88:2573–2578. doi: 10.1210/jc.2002-021511. [DOI] [PubMed] [Google Scholar]
- 9.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistancetrained men. Hypertension. 2003;41:130–135. doi: 10.1161/01.hyp.0000047649.62181.88. [DOI] [PubMed] [Google Scholar]
- 10.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004;110:2858–2863. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 11.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Goto K, Miyauchi T. Effects of athletic strength and endurance exercise training in young humans on plasma endothelin-1 concentration and arterial distensibility. Exp Biol Med. 2006;231:789–793. [PubMed] [Google Scholar]
- 12.Kawasaki T, Sullivan CV, Ozoe N, Higaki H, Kawasaki J. A long-term, comprehensive exercise program that incorporates a variety of physical activities improved the blood pressure, lipid and glucose metabolism, arterial stiffness, and balance of middle-aged and elderly Japanese. Hypertens Res. 2011;34:1059–1066. doi: 10.1038/hr.2011.81. [DOI] [PubMed] [Google Scholar]
- 13.Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011;107:783–787. doi: 10.1016/j.amjcard.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Bruton A, Gónzalez-Agüero A, Gómez-Cabello A, Casajús JA, Vicente-Rodríguez G. Is bone tissue really affected by swimming? A systematic review. PLoS One. 2013;8: doi: 10.1371/journal.pone.0070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 16.Ramzy D, Rao V, Tumiati LC. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKCdependent pathway. Circulation. 2006;114:1319–1326. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- 17.Palmer RMJ, Ferrige AG, Moncada S. Nitric Oxide release accounts for the biological activity of endotheliumderived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 18.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 19.Park JH, Omi N, Iemitsu M, Maeda S, Kitajima A, Nosaka T, Ezawa I. Relationship between arterial calcification and bone loss in a new combined model rat by ovariectomy and vitamin D(3) plus nicotine. Calcif Tissue Int. 2008;83:192–201. doi: 10.1007/s00223-008-9162-1. [DOI] [PubMed] [Google Scholar]
- 20.Notomi T, Okimoto N, Okazaki Y, Nakamura T, Suzuki M. Tower climbing exercise started 3 months after ovariectomy recovers bone strength of the femur and lumbar vertebrae in aged osteopenic rats. J Bone Miner Res. 2003;18:140–149. doi: 10.1359/jbmr.2003.18.1.140. [DOI] [PubMed] [Google Scholar]
- 21.Omi N, Morikawa N, Ezawa I. The effect of voluntary exercise on bone mineral density and skeletal muscles in the rat model at ovariectomized and sham stages. Bone Miner. 1994;24:211–222. doi: 10.1016/s0169-6009(08)80138-9. [DOI] [PubMed] [Google Scholar]
- 22.Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995;17:163S–168S. doi: 10.1016/8756-3282(95)00329-c. [DOI] [PubMed] [Google Scholar]
- 23.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol. 2003;95:336–341. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 24.Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 25.Maeda S, Miyauchi T, Iemitsu M, Tanabe T, Yokota T, Goto K, Yamaguchi I, Matsuda M. Effects of exercise training on expression of endothelin-1 mRNA in the aorta of aged rats. Clin Sci (Lond) 2002;Suppl 48:118S–123S. doi: 10.1042/CS103S118S. [DOI] [PubMed] [Google Scholar]
- 26.Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, Irukayama-Tomobe Y, Yokota T, Ohmori H, Matsuda M. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 27.Masatsugu K1, Itoh H, Chun TH, Saito T, Yamashita J, Doi K, Inoue M, Sawada N, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. Shear stress attenuates endothelin and endothelin-converting enzyme expression through oxidative stress. Regul Pept. 2003;28:13–19. doi: 10.1016/s0167-0115(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Iemitsu M, Maeda S, Kitajima A, Nosaka T, Omi N. Voluntary running exercise attenuates the progression of endothelial dysfunction and arterial calcification in ovariectomized rats. Acta Physiol (Oxf) 2008;193:47–55. doi: 10.1111/j.1748-1716.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 29.Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, Wells G, Tugwell P, Cranney A. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002: doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 30.Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol. 2003;95:300–307. doi: 10.1152/japplphysiol.01076.2002. [DOI] [PubMed] [Google Scholar]
- 31.Ay A, Yurtkuran M. Influence of aquatic and weightbearing exercises on quantitative ultrasound variables in postmenopausal women. Am J Phys Med Rehabil. 2005;84:52–61. doi: 10.1097/01.phm.0000146500.85850.be. [DOI] [PubMed] [Google Scholar]