Abstract
Bone diseases and injuries are highly incapacitating and result in a high demand for tissue substitutes with specific biomechanical and structural features. Tissue engineering has already proven to be effective in regenerating bone tissue, but has not yet been able to become an economically viable solution due to the complexity of the tissue, which is very difficult to be replicated, eventually requiring the utilization of highly labor-intensive processes. Process automation is seen as the solution for mass production of cellularized bone tissue substitutes at an affordable cost by being able to reduce human intervention as well as reducing product variability. The combination of tools such as medical imaging, computer-aided fabrication, and bioreactor technologies, which are currently used in tissue engineering, shows the potential to generate automated production ecosystems, which will, in turn, enable the generation of commercially available products with widespread clinical application.
Introduction
Bone is a dense and specialized form of connective tissue responsible for supporting and protecting the body and its organs. Its complex architecture is built from type I collagen and calcium phosphate in the form of hydroxyapatite resulting in unique biomechanical properties, which are difficult to mimic artificially. Bone diseases and injuries are therefore highly incapacitating and are increasingly becoming a major socioeconomic issue.1 About 2 million bone grafting procedures take place annually worldwide to ensure adequate bone healing in many skeletal problems generating a turnover of about 1 billion US dollars a year.2 Autotransplantation employing bone harvested from patients' donor sites is the most common procedure due to its inherent histocompatibility and nonimmunogenicity.3 However, the sourcing of grafts in the patient's body enhances tissue morbidity, blood loss, risk of infection and fracture, operative time and cost, and results in long immobilization periods and postoperative pain. To eliminate these drawbacks, synthetic products such as Ostim® (Aap Implantable AG), Kasios® and Jectos® (Kasios), and Pro Osteon® (Biomet, Inc.) composed of hydroxyapatite and/or calcium phosphates and natural products of xenogeneic origin such as Bio-Oss® (Geistlich Pharma) have been introduced in the market. However, these products have high production cost and are mainly provided in the form of granules or pastes showing reduced ability to repair complex and/or high load demanding defects.
Over the last two decades, tissue engineering has shown great promise in regenerating human tissues by employing exogenously generated substitutes. Since then, various types of tissue substitutes, such as bone,4 cartilage,5 or skin,6 have been successfully generated in vitro. Despite initial projections (80 billion USD market by 2012) and extensive corporate investment, the translation of these ground-breaking technologies from the laboratory to a widespread clinical application revealed to be modest.7 The most commercially successful tissue-engineered product so far was Apligraf®, a skin substitute produced by Organogenesis, Inc. which, despite its proven effectiveness and relative simplicity, reached a very small part of its potential market given its labor-intensive and costly production process.7 Since bone tissue is more complex and difficult to regenerate than skin, mainly due to its structural and biomechanical properties, the task of creating commercially viable tissue-engineered bone replacement products is even more arduous. Automation is probably one of the key issues for enabling the generation of bone substitutes with enhanced complexity in a time- and cost-effective manner, allowing an effective shift from labor-intensive production to mass production.
In this article, we review several production steps of the process for the generation of bone substitutes focusing on the available options that rely on automated tools and strategies, which are currently applied in tissue engineering, namely, three-dimensional (3D) medical imaging, computer-aided design (CAD), additive manufacturing, and bioreactor technologies (Fig. 1). Unlike the currently existing literature that address these technologies separately,8,9 this review article considers that they can all be combined together into a highly integrated and automated ecosystem.
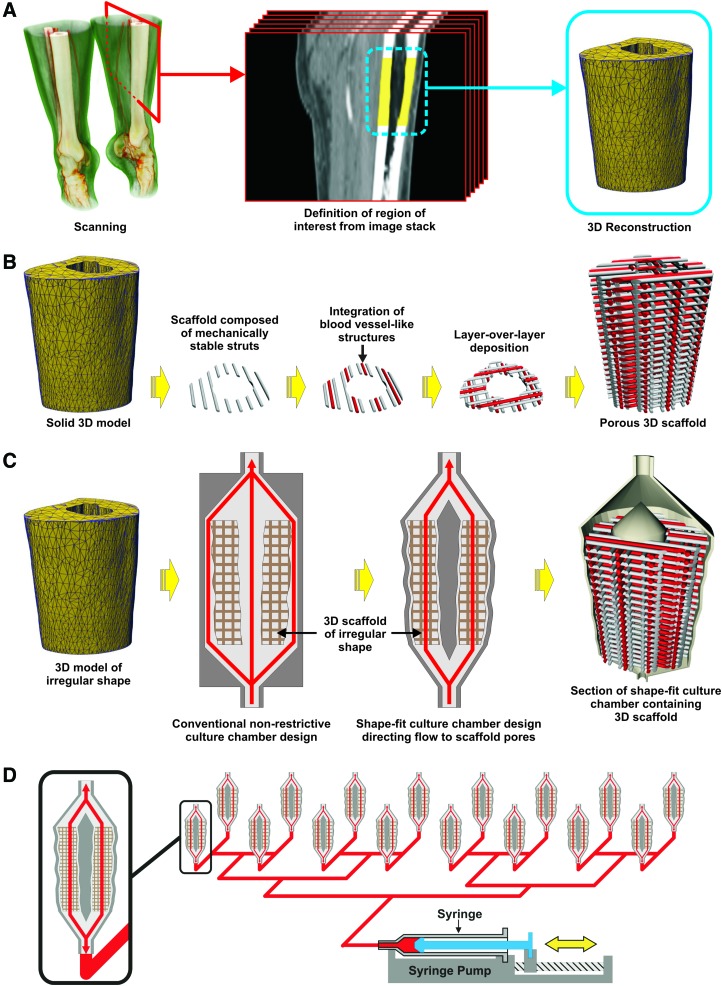
FIG. 1.
Schematic representation of process for mass production of personalized bone substitutes. The process starts with a three-dimensional (3D) reconstruction obtained by medical imaging (A), which allows to produce scaffolds replicating the shape and structure of the target tissues (B) as well as shape-specific culture chambers (C) into which scaffolds are optimally seeded and cultured with cells in large scale (D). Color images available online at www.liebertpub.com/teb
Imaging Tools for Design
Technological advancement has enabled the visualization of human tissues and organs to levels of detail as never seen before and, by doing so, it has become possible to understand the structure–function relationship at the level of cells, tissues, and organs. With the advent of tissue engineering and regenerative medicine, it becomes now possible to not only observe but to mimic those same structures in such a way that the replacement and regeneration of damaged tissues and organs are possible.
Currently existing technologies for noninvasive imaging allow for body parts or whole bodies to be analyzed without any damage to the target tissues. Technologies, such as magnetic resonance imaging (MRI) and, in particular, computerized tomography (CT), enable the collection of data from bone tissue sites that need to be repaired (Table 1). Given the high degree of resolution provided by such technologies, it becomes possible to obtain not only information related to the outer shape of the defect and organ but also information related to their more complex inner porosity, density, and microstructure. This data can even be used for indirectly assessing other tissue parameters, such as mechanical properties, by comparison with calibration phantoms.10
Table 1.
Features of Various Medical Imaging, Additive Manufacturing, and Bioreactor Technologies Utilized in Bone Tissue Engineering Applications
| Technology | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Medical imaging technologies | ||||
| Computerized tomography | Utilizes an X-ray source that is variably attenuated when crossing tissues with different densities | Short scanning time. High contrast in cortical bone | Exposure to radiation. Limited contrast in newly formed bone | 13,61 |
| Magnetic resonance imaging | Utilizes magnetic fields that induce variable rates of oscillation of hydrogen atoms generating contrast between different tissues | No exposure to radiation. Enables visualization of newly formed bone | Long scanning time. Limited contrast in cortical bone | 61,62 |
| Additive manufacturing technologies | ||||
| Fused deposition modeling | Extrusion of molten material from a nozzle forming a thin filament that is laid down layer-by-layer over a deposition surface moving over three axes relative to the extrusion nozzle | Self-supportive structures do not require support material. Multimaterial prints possible | Material usually needs to be preprocessed into filament format | 30 |
| Selective laser sintering | Fusion of particles contained in sequentially stacked layers of powder material by means of directed laser radiation | Degree of detail mainly dependent on powder grain size. Easily prints, blends composite materials by mixing material powders | Utilizes support material that may be difficult to remove. Limited to single material prints | 28,29 |
| Bioprinting | Extrusion of materials such as gels and/or cellular aggregates from a nozzle forming a thin filament that is laid down layer-by-layer over a deposition surface moving over three axes relative to the extrusion nozzle | Ability to utilize gels and cells as deposition material. Multimaterial prints possible | Utilizes support material that may be difficult to remove. Produced structures are very delicate | 31 |
| Bioreactor technologies | ||||
| Perfusion bioreactor | Fluid forced through scaffold generates shear stress, which stimulates cells by deforming their structure | Can perform cellular seeding. Very simple and inexpensive systems | Requires a fluid circulation circuit | 38–40,42,43 |
| Direct mechanical compression bioreactor | Mechanical compression/relaxation of scaffolds deforms cells attached to scaffold surfaces | Closely mimics compressive forces felt by native tissues during movement | Requires the utilization of complex mechanical actuators. Exerts forces over cells indirectly | 44 |
| Hydrostatic compression bioreactor | Compressed fluid generates mechanical forces, which directly deform the membranes of cells contained in scaffolds | Self-contained. Exerts forces directly over cellular membrane | Requires the utilization of high-pressure equipment | 45 |
Softwares such as Mimics (Materialise NV), the CTAn+CTVol package (SkyScan NV),11 or Invesalius (Renato Archer Technology of Information Center, Brazil)12 are specialized in converting raw CT/MRI data (in the form of sequential two-dimensional (2D) images made of density-based grayscale-colored pixels) into 3D models by combining these 2D images into 3D stacks. According to a desired density threshold, density-based shapes and volumes can therefore be defined in 3D and selected out of the 3D stack. Finally, the obtained shapes are used to generate a volume, a 3D model, consisting of the volume contained in its interior (Fig. 1A). The analysis of parameters such as densities, volumes, and porosities may differ according to the software-specific algorithms, the scanning equipment, and contrasting agents used. Bone tissue also exhibits very irregular outer shapes, which are difficult to mimic by other means than CT or MRI. In a work by Grayson et al.13 medical CT was used to scan a bovine temporomandibular joint condylar bone and then fabricate an anatomically shaped scaffold replica by computerized numerically controlled milling of a bone explant block. This solution was very successful in generating a tailor-made functional bone substitute despite the above-mentioned drawbacks associated with bone explants.
Design by Replication of Real Tissue Models
Apart from replication, current technological development allows to improve on the natural design of tissues and organs and maximize the functionality of de novo generated substitutes as well as facilitating their manufacture.
The replication of tissue parts by reconstruction of CT or MRI scans usually involves a certain extent of design modifications, which are applied to CT- or MRI-generated 3D models (such as reorientation, edge smoothing, and mesh simplification operations), to facilitate and accelerate the 3D model manipulation, fabrication, and integration within the target site. The reconstructed 3D model can also be manually redesigned or combined with other 3D models (CT/MRI originated or CAD designed) to improve their functionality. Otherwise, in cases where there is a complete or substantial lack of tissue to be scanned and reconstructed, it becomes necessary to resort to other reference tissues possessing similar shapes and properties in order for their 3D models to be adapted to the target site by reverse engineering.14
The most simple way of using a CT/MRI-generated 3D model for generating tissue substitutes is by simply converting the reconstructed 3D model (which is initially solid) into a model containing repeating porosity patterns (which will later originate a porous implant). Porosity and pore interconnectivity are crucial parameters in the efficiency of tissue-engineered implantable devices since they allow preseeded cells to proliferate and populate the inner parts of the device while receiving sufficient nutrition as well as neovascularization and tissue ingrowth coming from the native tissues surrounding the device upon implantation.15–17 Porosity, together with pore interconnectivity and architecture, can also be greatly responsible for other features such as the mechanical properties of the device. The mechanical properties of scaffolds can also be adjusted according to numerically generated models to meet the desired requirements.18
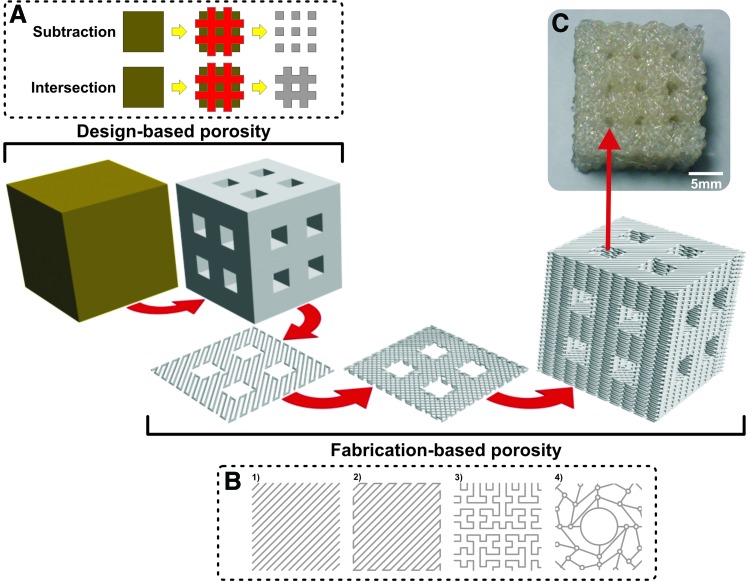
Porosity patterns can be generated by using design-based methods19 and/or fabrication-based methods20 either by simple repetition of predetermined subunits21 or generated by means of mathematical models developed by analysis of the target tissue structure22,23 (Fig. 2). Design-based methods imply further manipulation and modification of the 3D model and rely on the generation of a regular 3D matrix, which overlaps the 3D model. This 3D matrix is populated by subunits possessing predetermined geometries, which are used for locally performing either intersection or subtraction Boolean operations over the 3D model (Fig. 2A). A Boolean object resulting from an intersection operation will contain only the volume that was common to both original objects (3D model and subunits), while a Boolean object resulting from a subtraction operation will consist of the volume of the original object (3D model) with the intersection volume (subunits) subtracted from it. The main restriction in the design of these subunits is that they must be able to adjacently intersect with each other at some point. In the case of intersection Boolean operations, this feature becomes crucial since, upon fabrication, this allows for the device to maintain its structural and mechanical integrity. When performing subtraction Boolean operations, this feature is mostly important in keeping the interconnectivity of pores. Fabrication-based methods on the other hand imply the manipulation of operating parameters utilized in the process of fabrication itself. When ready for fabrication, the 3D model is horizontally sliced and each slice filled with patterned lines is then used by the fabrication machine-controlling software as guiding pathways for activating and moving the machine's tools. Pattern types can vary from just simple sets of parallel lines to more complex mathematically calculated patterns (Fig. 2B). Slice thickness and line spacing are other common examples of simple parameters that can be easily manipulated to substantially change the amount, size, and interconnectivity of pores. As in design-based methods, the only vital requirement in the generation of these patterns is that, upon fabrication, all the patterned layers are attached to each other at some point and forming a single object capable of maintaining its structural and mechanical integrity. Both design-based and fabrication-based methods provide an enormous array of possibilities when developing tissue engineering devices. Furthermore, the combination of design-based and fabrication-based methods is also an option and can generate an even more immense array of design possibilities. The combination of both can be used for generating distinct, but integrated types of porosity into one single device (Fig. 2C).
FIG. 2.
Schematic representation of generation of porosity into a 3D model by means of design- and fabrication-based methods. (A) Boolean operations employed in design-based porosity generation. (B) Examples of patterns of varied complexity employed in fabrication-based porosity generation. (C) Scaffold possessing design- and fabrication-based porosity. B4 modified with permission from Chen et al.22 Color images available online at www.liebertpub.com/teb
Fabrication with Accuracy and Reproducibility
To be able to fabricate structures as complex as tissues and organs, high-precision tools need to be employed. These tools must allow to accurately position cell and material building blocks, but also enable the 3D positioning necessary to generate fully functional 3D constructs.
Additive manufacturing is a highly automated layer-by-layer process that, unlike subtractive rapid prototyping, involves the sequential building of layers of material by deposition of new layers on top of previously laid layers of material. The first main application of additive manufacturing in the medical field was in helping to plan surgeries. By building real-size models accurately mimicking tissue features contained in the interior of the body, it was possible for surgeons to better plan surgical procedures.24 Furthermore, surgical guides for tool orientation were also built by additive manufacturing enabling as well a better execution of the surgical procedure itself.25,26
In general, an additive rapid prototyping material must be convertible to a more versatile form, such as a liquid, a colloidal, or a powder form, (typically by applying high temperatures or solvents), to be selectively and accurately added to layers. After deposition, the chosen material must be able as well to directly or indirectly attach back together in order for adjacent layers to be efficiently joined together during the layer-by-layer process.
Despite the existence of a wide array of materials able to be used in the additive manufacturing of bone scaffolds, polycaprolactone (PCL) and poly(lactic acid) (PLA) are the most commonly used materials (either alone or combined with other materials) due to their biodegradability and adequate mechanical properties as well as their approval for medical implantation. Another material of great interest for fabricating bone scaffolds is hydroxyapatite, which is a natural constituent of bone. Hydroxyapatite can be utilized in additive manufacturing by applying significantly higher processing temperatures than PCL or PLA when directly deposited. Alternatively, hydroxyapatite scaffolds can as well be indirectly fabricated by casting into sacrificial molds fabricated by additive manufacturing.27
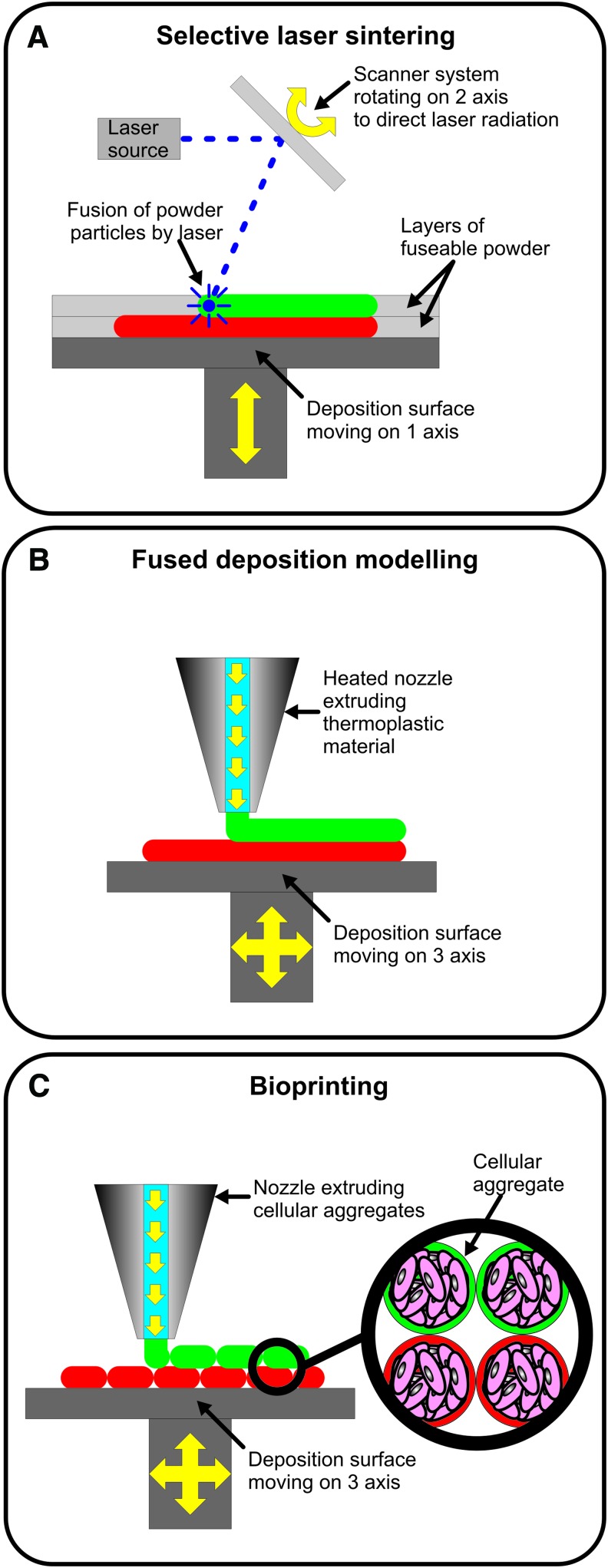
The array of specialized materials that can be utilized in additive rapid prototyping, although not as wide as in subtractive rapid prototyping, is still quite large and is constantly evolving given that there is currently a wide range of additive rapid prototyping technologies and that each one utilizes materials with specific properties for adequate processing (Table 1). Fused deposition modeling (FDM) and selective laser sintering (SLS) are the most commonly used additive manufacturing technologies in bone tissue engineering applications, mostly due to the possibility of manufacturing objects, which possess mechanical properties similar to the ones found in native bone, while at the same time maintaining a high degree of control over the outer and inner architecture of the manufactured object. SLS results from the fusion of particles contained in a powder layer by means of directed laser radiation.28,29 A thin layer of powder is first spread over a flat surface and then irradiated by a laser beam, which is oriented to selected locations of the powder layer. As a result, the irradiated powder particles are fused together forming 2D patterns. The laser beam is then stopped and a new layer of powder is spread over the precedent layer by means of a mechanical roller. The patterned fusion process is then repeated resulting in the fusion of the new patterned layer with the layer beneath. At the end of the process, the excess powder is removed uncovering the manufactured object (Fig. 3A). On the other hand, FDM consists of the extrusion of molten material from a heated extruder forming a thin filament that is laid down over a deposition surface moving over three axes relative to the extrusion nozzle.30 By coordinating the movement of the deposition table and the extrusion in the nozzle, highly detailed patterns of thermoplastic material can be created over the deposition surface. When the deposition of one layer of material is finished, the extrusion is stopped while the deposition table is slightly moved away from the extruder tip. The extrusion is then restarted and a new patterned layer is deposited over the precedent layer to which it adheres (Fig. 3B). FDM and SLS technologies may, however, need to be complemented with other additive manufacturing technologies, such as bioprinting, to replicate specific features contained into the bone tissue structure. Bioprinting results from the extrusion of material from a nozzle, but unlike FDM it does not involve heating. Therefore, the extrusion process can involve sensitive materials such as gels and cellular aggregates.31 Typically, the process is started with the patterned deposition of a gel filament, which forms a grooved layer. This gel layer then allows for further deposition of a second material, which is composed of small cellular aggregates. The deposition of those aggregates into the grooves formed by the gel layer ensures that the cellular aggregates are kept in their exact positions. This process is repeated layer over layer generating 3D shapes composed of cellular aggregates that, during further maturation, fuse together and generate continuous masses of cells possessing a predetermined shape (Fig. 3C). A particular application of bioprinting is in the formation of blood vessel networks contained in bone. Blood vessel networks are flexible and delicate structures possessing mechanical properties very different from the surrounding tissue. These structures have so far been mimicked by applying gel-based or even scaffold-free printing technologies, which are able to lay down intricate patterns of cells that later generate vessel-like structures. An example of that is shown in a work by Norotte et al.,32 where bioprinting was utilized to concomitantly lay down aggregates of various cell types according to predefined patterns, which during further maturation fused together and generated stratified 3D vessel-like structures. Furthermore, previous works show as well that soft materials such as gels can be further integrated into the structure of more stiff thermoplastic scaffolds resulting in scaffolds possessing optimal mechanical properties.33,34 By using a similar strategy and applying design principles mimicking the natural organization of blood vessels,35–37 the concomitant integration of bioprinted blood vessels into the structure of more mechanically stable scaffolds produced by technologies such as FDM or SLS would potentially enable the generation of mechanically stable vascularized bone tissue substitutes (Fig. 1B).
FIG. 3.
Schematic representation of the mode of operation of selective laser sintering (A), fused deposition modeling (B), and bioprinting (C). Color images available online at www.liebertpub.com/teb
Culturing Cells in 3D Templates
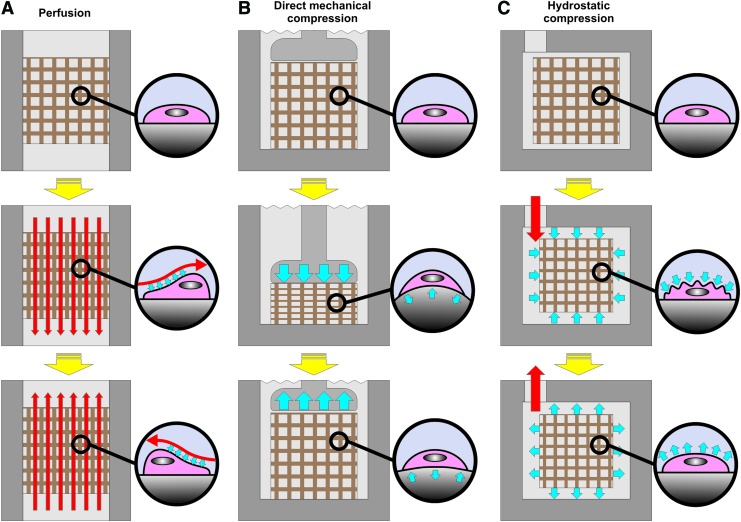
Despite being highly labor intensive and expensive, static culture is still the most widely used cell culture technique in tissue engineering strategies. This culturing technique is often characterized by nonhomogenous cell distribution, being the majority of seeded cells confined to the outer surfaces of the scaffold, which in turn results in nonhomogenous distribution of the in vitro-generated extracellular matrix. Furthermore, static culturing conditions are far from mimicking the dynamic environment found in vivo, which is responsible for many signals/stimuli that trigger cell development. Dynamic cell culture has been shown to avoid cell death in the construct's core by improving the mobility of nutrients into these most central regions as well as influencing cellular development.38–41 Hence, many dynamic culture devices possessing varying degrees of automation have so far been developed to overcome the limitations found in static culture. These systems are able to culture tissue-engineered constructs into highly controlled environments while providing a wide array of biomechanical stimuli (Fig. 4). Stimulatory signals applied in bone tissue engineering such as perfusion-based shear stress,42,43 direct mechanical compression,44 and hydrostatic compression45 have shown to be effective in improving the quality of generated constructs (Table 1). In fact, such results would be expected since such stimuli can be found influencing bone development in a similar way when applied in vivo.46,47 Bioreactors are able to modulate cellular development through a mechanism of mechanotransduction, which consists of triggering intracellular biochemical signals by means of mechanical deformation of the cellular structure.48 Perfusion bioreactors employ pumps to continuously perfuse the culture medium through the interconnected porous networks of cell-seeded scaffolds. Shear stress resulting from the movement of fluid over the surface of cells in the scaffolds results in the deformation of the structure of cells and triggers mechanotransductive downstream signaling (Fig. 4A). Given their simplicity, flow perfusion bioreactors enable seeding and culture of cells into scaffolds with a high degree of automation. In a different way, bioreactors performing direct mechanical compression are inspired by the mechanical compressive forces felt by tissues in their natural environment during movement. Cells contained in porous scaffolds are stimulated by the deformation that occurs in the structure of the scaffold during compression/relaxation (Fig. 4B). Shear stress can as well occur as a result from the movement of fluids from/to the interior of the scaffold's pores during deformation. Finally, in hydrostatic pressure bioreactors, mechanical forces act directly over the membranes of cells contained in porous scaffolds by means of a fluid. When the pressure of the fluid (culture medium) contained in the bioreactor (and in the scaffold's pores) is increased/decreased, it acts upon the cellular membrane by causing a compression/relaxation deformation (Fig. 4C). Apart from being able to simplify and automate the process of construct culture, bioreactors in general show as well the potential to generate constructs in a more standardized, traceable, cost-effective, safe, and regulatory-compliant way.9,49
FIG. 4.
Scheme showing how perfusion, mechanical compression, and hydrostatic compression bioreactors are able to mechanically deform cells to stimulate their development. Red arrows indicate fluid movement and blue arrows indicate mechanical forces. (A) Perfusion bioreactor, (B) Direct mechanical compression bioreactor, and (C) Hydrostatic compression bioreactor. Color images available online at www.liebertpub.com/teb
Bioreactors must also be versatile, being able to adapt to various kinds of constructs possessing variable degrees of complexity and aimed at diverse applications. This is why bioreactors, as well as their fabrication process, must be easily adjustable to specific conditions and requirements on the fly. Recent work addressed this requirement by resorting to additive manufacturing not only to fabricate scaffolds but as well to simultaneously fabricate their enclosing culture chamber.50 In this way, complex-shaped constructs could be produced to replicate the shape of CT-scanned bone parts while contained in a culture chamber optimally designed for that specific construct (Fig. 1C).
Another aspect, which is important for the application of tissue engineering into the clinic, is process scalability. In a scenario of widespread adoption of tissue engineering-based therapies, significant amounts of sufficiently large constructs would need to be simultaneously produced to fulfill clinical demands (Fig. 1D). Many scalable and modular systems have been developed to address this requirement by resorting to various fabrication processes to enable the simultaneous culture of multiple and/or large constructs.40,51–53 A specific application, where this is already visible (although usually at a smaller construct scale), is in the high-throughput screening of biomaterials where cells contained in multiple simplified 3D constructs are submitted to varying culture environments to fully understand and optimize their development.54,55
As the human body, bioreactors must as well be able to continuously sense and accordingly react to all the events occurring in the construct and its surrounding environment during culture. Many kinds of sensors and analytical techniques have been so far integrated into bioreactor-based procedures. The most common ones are based on electrochemical and optical principles and are usually applied in an invasive, noninvasive, or shunt configuration.56 Some of them are commonly used in other types of fully scaled-up cultures such as the culture of yeasts or bacterial microorganisms for mass production of food and drug compounds as well as in clinical and physiological monitoring.57 A very interesting type of bioreactor, with particular utility for bone tissue engineering, is able to perform noninvasive high-resolution analysis of bone constructs under perfusion culture by means of micro-CT scanning.58,59 In this way, tissue constructs do not need to be removed from its culture chamber every time a scan is performed, hence reducing human labor as well as minimizing the possibility of contamination.
Personalized Bone Tissue Engineering Resulting from Integrated Manufacturing Ecosystems
The provision of personalized treatments for patient-specific requirements is seen as the next big step in healthcare toward cost-effective, efficient and improved patient outcomes. The ability to automate the design, fabrication, and culture steps in tissue engineering (Fig. 1) not only enables to reduce human intervention but as well to generate more complex and personalized constructs. The generation of personalized bone tissue substitutes has, in fact, been explored in the field of tissue engineering for over a decade in various ways. Back in the year 2000, Hollister et al. had already described a medical image-based computational method for generating 3D scaffolds closely mimicking the outer and inner architectures of bone parts.19 Despite the high potential of this method, the generated personalized bone-like structures were produced from epoxy resin by means of stereolithography and not from implantable materials, validating mostly the design method but not demonstrating its full in vitro or in vivo application. In a way, this work demonstrated that, by utilizing advanced technologies to make more complex scaffolds, it becomes also more challenging to seed and culture cells and tissues into them. In 2010, Grayson et al. addressed this issue by realizing that, together with customized implants, it was also necessary to produce customized cell and tissue culture environments (implant-specific bioreactor chambers) to successfully promote the formation of healthy and fully functional tissue substitutes into complex personalized scaffolds.13 However, the main disadvantage of such strategy was that, since it employed several different materials and technologies of varying degrees of automation to build both the implant and corresponding chamber, the manufacturing of such devices became overly scattered, complex, and laborious.
An efficient way of improving the productivity of a manufacturing system is by reducing the number of production steps to a minimum while also employing a reduced number of highly versatile tools. This simplifies the process of manufacturing highly customized products and facilitates the integration of all production tools into a smart and lean manufacturing ecosystem. A recent concept for generating personalized tissue-engineered constructs has followed such strategy by electing additive manufacturing as its main multipurpose tool.50 In a recent work employing this concept, additive manufacturing combined with medical imaging was utilized to simultaneously generate, in one single step, personalized scaffolds readily contained into personalized and readily automatable culture environments, into which cells and tissues could be cultured to generate bone-like constructs.60 This achievement was only possible since additive manufacturing, medical imaging, and bioreactor technologies are heavily based on software. The ability to manipulate digital information instead of physical objects enables an enormous degree of creative freedom that is simply not possible in the physical world. Furthermore, digital information is much easier to manage, combine, and modify, and therefore becomes the most suitable way of integrating the most diverse tools and technologies into common strategies.
Concluding Remarks and Future Directions
The automation of tissue engineering is becoming a reality. Important advancements in this area allowed so far to mostly create and identify the basic enabling technologies necessary for the proper development of this field. Nonetheless, and given the high complexity of bone tissue, it becomes necessary to develop equally complex development strategies, but these must result in simpler methodologies and technologies that enable automation and thus, easier processing into industrial viable products that can reach large-scale clinical application. The rapid development in computer and automation technologies as well as the achievement of higher resolution powers in analysis and fabrication processes increasingly enables tissue engineers to more closely mimic the complex and highly dynamic environments found in native tissues. The convergence of these technologies allied to an increasingly better understanding of the mechanisms at the basis of tissue development will in the future allow for the generation of tissue replicas possessing similar or even improved features. Equally important is the training of highly skilled hybrid scientists capable of mastering and combining all the involved technologies into generating mass produced, tailor-made, patient-specific solutions.
Acknowledgments
The authors would like to acknowledge the partial support by the European Network of Excellence EXPERTISSUES (NMP3-CT-2004-500283). Pedro Costa would also like to acknowledge the Portuguese Foundation for Science and Technology for his PhD grant (SFRH/BD/62452/2009).
Disclosure Statement
No competing financial interests exist.
References
- 1.Brooks P.M.The burden of musculoskeletal disease—a global perspective. Clin Rheumatol 25,778, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bohner M.Resorbable biomaterials as bone graft substitutes. Mater Today 13,24, 2010 [Google Scholar]
- 3.Damien C.J., and Parsons J.R.Bone-graft and bone-graft substitutes—a review of current technology and applications. J Appl Biomater 2,187, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S.M., Mukhachev V., Lavroukov A., et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. New Engl J Med 344,385, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brun P., Dickinson S.C., Zavan B., Cortivo R., Hollander A.P., and Abatangelo G.Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther 10,R132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaglstein W.H., and Falanga V.Tissue engineering and the development of Apligraf(R), a human skin equivalent. Clin Ther 19,894, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Mason C.Automated tissue engineering: a major paradigm shift in health care. Med Device Technol 14,16, 2003 [PubMed] [Google Scholar]
- 8.Sun W., Darling A., Starly B., and Nam J.Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem 39,29, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Martin I., Smith T., and Wendt D.Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products. Trends Biotechnol 27,495, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Shefelbine S.J., Simon U., Claes L., Gold A., Gabet Y., Bab I., et al. Prediction of fracture callus mechanical properties using micro-CT images and voxel-based finite element analysis. Bone 36,480, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Tuan H.S., and Hutmacher D.W.Application of micro CT and computation modeling in bone tissue engineering. Comput Aided Design 37,1151, 2005 [Google Scholar]
- 12.Silva D.N., De Oliveira M.G., Meurer E., Meurer M.I., Da Silva JVL, and Santa-Barbara A.Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J Cranio Maxill Surg 36,443, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Grayson W.L., Frohlich M., Yeager K., Bhumiratana S., Chan M.E., Cannizzaro C., et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 107,3299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiffel A.J., Kafka C., Hernandez K.A., Popa S., Perez J.L., Zhou S., et al. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. Plos One 8,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang T.Y., Kang H.W., Hwang C.M., Lee S.J., Park J., Yoo J.J., et al. The realistic prediction of oxygen transport in a tissue-engineered scaffold by introducing time-varying effective diffusion coefficients. Acta Biomater 7,3345, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Otsuki B., Takemoto M., Fujibayashi S., Neo M., Kokubo T., and Nakamura T.Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 27,5892, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mavrogenis A.F., Dimitriou R., Parvizi J., and Babis G.C.Biology of implant osseointegration. J Musculoskel Neuron 9,61, 2009 [PubMed] [Google Scholar]
- 18.Eshraghi S., and Das S.Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone-hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater 8,3138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollister S.J., Levy R.A., Chu T.M., Halloran J.W., and Feinberg S.E.An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Max Surg 29,67, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Tellis B.C., Szivek J.A., Bliss C.L., Margolis D.S., Vaidyanathan R.K., and Calvert P.Trabecular scaffolds created using micro CT guided fused deposition modeling. Mat Sci Eng C Bio S 28,171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wettergreen M.A., Bucklen B.S., Starly B., Yuksel E., Sun W., and Liebschner M.A.K.Creation of a unit block library of architectures for use in assembled scaffold engineering. Comput Aided Design 37,1141, 2005 [Google Scholar]
- 22.Chen Z., Su Z., Ma S., Wu X., and Luo Z.Biomimetic modeling and three-dimension reconstruction of the artificial bone. Comput Meth Prog Bio 88,123, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Yasar O., Lan S.F., and Starly B.A Lindenmayer system-based approach for the design of nutrient delivery networks in tissue constructs. Biofabrication 1,045004, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kermer C., Rasse M., Lagogiannis G., Undt G., Wagner A., and Millesi W.Colour stereolithography for planning complex maxillofacial tumour surgery. J Cranio Maxill Surg 26,360, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Chow J., Hui E., Lee P.K.M., and Li W.Zygomatic implants - Protocol for immediate occlusal loading: a preliminary report. J Oral Maxil Surg 64,804, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ran W., Liu X.Z., Guo B., Shu D.L., and Tan Z.M.Removal of a foreign body from the skull base using a customized computer-designed guide bar. J Cranio Maxill Surg 38,279, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Chu T.M.G., Halloran J.W., Hollister S.J., and Feinberg S.E.Hydroxyapatite implants with designed internal architecture. J Mater Sci Mater M 12,471, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Williams J.M., Adewunmi A., Schek R.M., Flanagan C.L., Krebsbach P.H., Feinberg S.E., et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26,4817, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Eosoly S., Brabazon D., Lohfeld S., and Looney L.Selective laser sintering of hydroxyapatite/poly-ɛ-caprolactone scaffolds. Acta Biomater 6,2511, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Zein I., Hutmacher D.W., Tan K.C., and Teoh S.H.Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23,1169, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., and Forgacs G.Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norotte C., Marga F.S., Niklason L.E., and Forgacs G.Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30,5910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuurman W., Khristov V., Pot M.W., van Weeren P.R., Dhert W.J.A., and Malda J.Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3,2011 [DOI] [PubMed] [Google Scholar]
- 34.Jetze V., Benjamin P., Thijs J.B., Jelle B., Wouter J.A.D., Ferry P.W.M., et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5, 035007. 2013 [DOI] [PubMed] [Google Scholar]
- 35.Lemon G., Howard D., Tomlinson M.J., Buttery L.D., Rose F.R.A.J., Waters S.L., et al. Mathematical modelling of tissue-engineered angiogenesis. Math Biosci 221,101, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Manoussaki D.A mechanochemical model of angiogenesis and vasculogenesis. Esaim Math Model Num 37,581, 2003 [Google Scholar]
- 37.Merks R.M.H., and Koolwijk P.Modeling morphogenesis in silico and in vitro: towards quantitative, predictive, cell-based modeling. Math Model Nat Pheno 4,149, 2009 [Google Scholar]
- 38.Bancroft G.N., Sikavitsast V.I., van den Dolder J., Sheffield T.L., Ambrose C.G., Jansen J.A., et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteloblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 99,12600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartmell S.H., Porter B.D., Garcia A.J., and Guldberg R.E.Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng 9,1197, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Grayson W.L., Bhumiratana S., Cannizzaro C., Chao P.H.G., Lennon D.P., Caplan A.I., et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A 14,1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikavitsas V.I., Bancroft G.N., and Mikos A.G.Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res 62,136, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Sikavitsas V.I., Bancroft G.N., Holtorf H.L., Jansen J.A., and Mikos A.G.Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A 100,14683, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein A.S., Juarez T.M., Helmke C.D., Gustin M.C., and Mikos A.G.Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials 22,1279, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Endres S., Kratz M., Wunsch S., and Jones D.B.Zetos: a culture loading system for trabecular bone. Investigation of different loading signal intensities on bovine bone cylinders. J Musculoskel Neuron 9,173, 2009 [PubMed] [Google Scholar]
- 45.Henstock J.R., Rotherham M., Rose J.B., and El Haj A.J.Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone 53,468, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan S., Weimer D.A., Agans S.C., Bain S.D., and Gross T.S.Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res 17,1613, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allison S.J., Folland J.P., Rennie W.J., Summers G.D., and Brooke-Wavell K.High impact exercise increased femoral neck bone mineral density in older men: A randomised unilateral intervention. Bone 53,321, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Sikavitsas V.I., Temenoff J.S., and Mikos A.G.Biomaterials and bone mechanotransduction. Biomaterials 22,2581, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Gardel L.S., Serra L.A., Reis R.L., and Gomes M.E.Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng Part B Rev 20,126, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Costa P.F., Martins A., Vaquette C., Melchels F.P., Neves N.M., Gomes M.E., et al. Bioreactor composed of watertight chamber and internal matrix for the generation of cellularized medical implants. Patent application WO 2013103306, 2013 [Google Scholar]
- 51.Koch M.A., Vrij E.J., Engel E., Planell J.A., and Lacroix D.Perfusion cell seeding on large porous PLA/calcium phosphate composite scaffolds in a perfusion bioreactor system under varying perfusion parameters. J Biomed Mater Res A 95A,1011, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Janssen F.W., van Dijkhuizen-Radersma R., Van Oorschot A., Oostra J., de Bruijn J.D., and Van Blitterswijk C.A.Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen M 4,12, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Costa P.F., Martins A., Neves N.M., Gomes M.E., and Reis R.L.Multichamber bioreactor with bidirectional perfusion integrated in culture system for tissue engineering strategies. European Patent Application 090098632009 [Google Scholar]
- 54.Rotenberg M.Y., Ruvinov E., Armoza A., and Cohen S.A multi-shear perfusion bioreactor for investigating shear stress effects in endothelial cell constructs. Lab Chip 12,2696, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Figallo E., Cannizzaro C., Gerecht S., Burdick J.A., Langer R., Elvassore N., et al. Micro-bioreactor array for controlling cellular microenvironments. Lab Chip 7,710, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Rolfe P.Sensing in tissue bioreactors. Meas Sci Technol 17,578, 2006 [Google Scholar]
- 57.Santoro R., Krause C., Martin I., and Wendt D.On-line monitoring of oxygen as a non-destructive method to quantify cells in engineered 3D tissue constructs. J Tissue Eng Regen Med 6,696, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Porter B.D., Lin A.S.P., Peister A., Hutmacher D., and Guldberg R.E.Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials 28,2525, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Hagenmuller H., Hitz M., Merkle H.P., Meinel L., and Muller R.Design and validation of a novel bioreactor principle to combine online micro-computed tomography monitoring and mechanical loading in bone tissue engineering. Rev Sci Instrum 81,014303, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Costa P.F., Vaquette C., Baldwin J., Chhaya M., Gomes M.E., Reis R.L., Theodoropoulos C., et al.. Biofabrication of customized bone grafts by combination of additive manufacturing and bioreactor knowhow. Biofabrication, 2014. [Epub ahead of print]; DOI: 10.1088/1758-5082/6/3/035006 [DOI] [PubMed] [Google Scholar]
- 61.Washburn N.R., Weir M., Anderson P., and Potter K.Bone formation in polymeric scaffolds evaluated by proton magnetic resonance microscopy and X-ray microtomography. J Biomed Mater Res A 69A,738, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Xu H., Othman S.F., and Magin R.L.Monitoring tissue engineering using magnetic resonance imaging. J Biosci Bioeng 106,515, 2008 [DOI] [PubMed] [Google Scholar]