Abstract
Background: Europeans and Americans are gradually accepting the hemoglobin A1c (HbA1c) threshold of 6.5% for diagnosing diabetes proposed by the American Diabetes Association, but the cutoff of HbA1c for the Chinese population is unclear. We evaluated the diagnostic efficiency of HbA1c for diagnosing newly diagnosed diabetes and prediabetes in community-based Chinese adults 40 years of age or older.
Subjects and Methods: In this study 8,239 subjects (5,496 women) 40–90 years of age underwent HbA1c and oral glucose tolerance test measurement after an overnight fast. Diabetes and prediabetes were defined by the World Health Organization criteria. The area under the receiver operating characteristic curve (AUC) was used to evaluate the diagnostic efficiency of HbA1c, and the optimal cutoff was defined as the point on the receiver operating characteristic curve with the largest Youden index. Spearman correlation was used for correlation analysis.
Results: The prevalence of newly diagnosed diabetes and prediabetes was 10.7% (880/8,239) and 19.0% (1,564/8,239), respectively. Fasting plasma glucose and postprandial plasma glucose were positively correlated with HbA1c level (r=0.725 and r=0.673, both P<0.001, respectively). For diagnosing diabetes, the AUC was 0.857 (95% confidence interval, 0.841–0.873), and the optimal cutoff for HbA1c was 6.3%, with the largest Youden index being 0.581. For diagnosing prediabetes, the AUC was 0.681 (95% confidence interval, 0.666–0.697), and the optimal cutoff for HbA1c was 5.9%, with the largest Youden index being 0.280.
Conclusions: An HbA1c threshold of 6.3% was highly valuable for diagnosing newly diagnosed diabetes, and a value of 5.9% was weakly valuable for diagnosing prediabetes in community-based Chinese adults 40 years of age or older.
Introduction
The prevalence of type 2 diabetes in China is increasing as a result of the improved lifestyle and population aging. Among adults 20 years of age or older in China, an estimated 92.4 million have diabetes, and 148.2 million have prediabetes.1 It is alarming that approximately 60% of diabetes has not been diagnosed.1,2 The onset of diabetes occurs at least 4–7 years before the clinical diagnosis, and more than 20% of people have diabetic retinopathy at the time of diagnosis.3 However, an improved lifestyle could prevent the onset of type 2 diabetes or delay its progression.4,5 Therefore, early diagnosis and intervention are of paramount importance.
Hemoglobin A1c (HbA1c) reflects the 2–3-month mean plasma glucose level with low short-term variability.6 HbA1c level has been the “gold standard” of glucose control for decades and is more intimately related to the risk of complications than a single or episodic measurement of glucose levels.7 However, clinical practice recommendations from the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation have incorporated HbA1c for diagnosing diabetes mellitus.8,9 Although the initiative was supported by the results of many large-scale cross-sectional epidemiological surveys, whether HbA1c can move from a diabetes monitoring indicator to a diagnostic indicator is the focus of debate.
Europeans and Americans are gradually accepting the HbA1c threshold of 6.5% for diagnosing diabetes proposeded by the American Diabetes Association,8 but the cutoff of HbA1c for the Chinese population is unclear. We aimed to evaluate the diagnostic efficiency of HbA1c for newly diagnosed diabetes and prediabetes (both impaired fasting glucose and impaired glucose tolerance) in community-based Chinese adults 40 years of age or older.
Subjects and Methods
Study design and subjects
The present work was one part of the baseline survey from the REACTION Study investigating the association of diabetes and cancer, which was conducted among 259,657 adults, 40 years of age or older, in 25 communities across mainland China, from 2011 to 2012.10–12 During February and March 2012, we investigated 10,028 subjects (6,458 women) 40–90 years of age from four urban communities (one from Jinan city and three from Jining city) in Shandong Province, China. The average response rate was 91.5%. In the present study, we excluded 1,789 subjects with previously diagnosed diabetes (n=1,624), cancer (n=106), gastrointestinal surgery (n=12), splenectomy (n=5), chronic liver disease (n=21), end-stage renal disease (n=3), or glucocorticoid treatment (n=18). Finally, 8,239 subjects (5,496 women) were eligible for the analysis. The institutional review board at the Department of Endocrinology and Metabolic Disease, Ruijin Hospital, Shanghai Jiaotong University School of Medicine approved the study protocol. All the subjects gave their informed consent.
Data collection
A standard questionnaire was used to obtain data on demographic characteristics and lifestyle by face-to-face interviews. All investigators received extensive training relative to the study questionnaire and outcome measures before conducting the investigation. Data were collected on family history of diabetes and history of coronary heart disease or stroke. The anthropometric data collected included height, weight, and blood pressure. Body mass index was calculated by weight (kg) divided by height squared (m2). Blood pressure was measured on the right arm three times consecutively at 1-min intervals; the mean of the three measurements was used for analysis.
Blood samples were collected in the morning after at least 10 h of overnight fasting and 2 h after a 75-g oral glucose tolerance test and assayed by the glucose oxidase method with use of an automatic clinical chemistry analyzer. HbA1c was determined on ion-exchange high-performance liquid chromatography with use of an automatic glycated hemoglobin meter (Variant™; Bio-Rad, Hercules, CA). Levels of serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and creatinine were determined by the phosphoglycerol oxidase method, colorimetric enzyme assay, homogeneous assay, homogeneous assay, and picric acid method, respectively. All clinical features were measured following a standardized manual of operations.
Definitions and diagnostic criteria
According to 1999 World Health Organization diagnostic criteria,13 newly diagnosed diabetes was defined as a fasting plasma glucose level of ≥7.0 mmol/L and/or 2-h postprandial plasma glucose level of ≥11.1 mmol/L. Prediabetes features impaired fasting glucose and impaired glucose tolerance. Impaired fasting glucose was defined as a fasting plasma glucose level of ≥6.1 mmol/L and <7.0 mmol/L and a 2-h postprandial plasma glucose level of <7.8 mmol/L. Impaired glucose tolerance was defined as a fasting plasma glucose level of <7.0 mmol/L and a 2-h postprandial plasma glucose level of ≥7.8 mmol/L and <11.1 mmol/L.
Statistical analysis
Continuous variables with normal distribution were expressed as mean±SD values, and variables with skewed distribution were expressed as median values with interquartile range and logarithmically transformed to satisfy normal distribution for statistical analysis. Categorical variables are described with number (proportion). Differences in means among groups were tested by univariate analysis of variance, and those in frequencies were tested by χ2 test. Correlation was examined by Spearman's correlation analysis. The area under the receiver operating characteristic curve (AUC) was determined to evaluate the diagnostic efficiency of HbA1c for newly diagnosed diabetes and prediabetes. Diagnostic testing determined sensitivity, specificity, positive predictive value, and negative predictive value. The point with the largest Youden index, equal to (sensitivity+specificity – 1), was defined as the optimal cutoff. SPSS version 21.0 software (SPSS, Inc., Chicago, IL) was used for data analyses. Two-sided P<0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of study participants divided into three groups (normoglycemia, prediabetes, and newly diagnosed diabetes), according to diagnosis by oral glucose tolerance test. The prevalence of newly diagnosed diabetes and prediabetes was 10.7% (880/8,239) and 19.0% (1,564/8,239), respectively. The three groups differed statistically in all characteristics but sex ratio. Although there were more females than males in all three groups, this does not reflect the sex ratio in the population but rather the willingness to participate.
Table 1.
Baseline Characteristics for Subjects with Normoglycemia, Prediabetes, and Newly Diagnosed Diabetes by Oral Glucose Tolerance Test
| Normoglycemia (n=5,795) | Prediabetes (n=1,564) | Newly diagnosed diabetes (n=880) | P value | |
|---|---|---|---|---|
| Age (years) | 57.11±9.79 | 60.04±9.87 | 60.88±9.78 | <0.01 |
| Women | 3,874 (66.9%) | 1,042 (66.6%) | 580 (65.9%) | 0.856 |
| Family history of diabetes | 681 (11.8%) | 213 (13.6%) | 142 (16.1%) | <0.01 |
| History of coronary heart disease | 435 (7.5%) | 190 (12.1%) | 184 (20.9%) | <0.01 |
| History of stroke | 49 (0.8%) | 28 (1.8%) | 25 (2.8%) | <0.01 |
| BMI (kg/m2) | 25.86±3.41 | 26.56±3.48 | 27.20±3.37 | <0.01 |
| Systolic blood pressure (mm Hg) | 137.26±20.42 | 140.82±20.61 | 145.79±20.22 | <0.01 |
| Diastolic blood pressure (mm Hg) | 79.53±11.51 | 80.96±11.52 | 82.64±12.26 | <0.01 |
| HbA1c (%) | 5.75±0.42 | 6.05±0.53 | 7.27±1.63 | <0.01 |
| Fasting plasma glucose (mmol/L) | 5.23±0.46 | 6.14±0.51 | 8.36±2.40 | <0.01 |
| Postprandial plasma glucose (mmol/L) | 5.36±1.06 | 7.57±1.68 | 12.20±4.26 | <0.01 |
| Triglycerides (mmol/L) | 1.24 (0.89, 1.75) | 1.41 (1.01, 1.97) | 1.58 (1.12, 2.22) | <0.01 |
| Total cholesterol (mmol/L) | 5.31±0.95 | 5.51±1.03 | 5.61±1.03 | <0.01 |
| HDL cholesterol (mmol/L) | 1.53±0.34 | 1.49±0.34 | 1.43±0.31 | <0.01 |
| LDL cholesterol (mmol/L) | 3.10±0.80 | 3.27±0.87 | 3.36±0.87 | <0.01 |
| Creatinine level (μmol/L) | 64.10±10.31 | 65.75±1 0.74 | 67.91±11.66 | <0.01 |
Data were expressed as mean±SD values (for variables with normal distribution), median value with interquartile range (for variables with skewed distribution), or number with percentage (for categorical variables) as indicated. Difference in means between groups was tested using univariate analysis of variance and in frequencies using the χ2 test.
BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
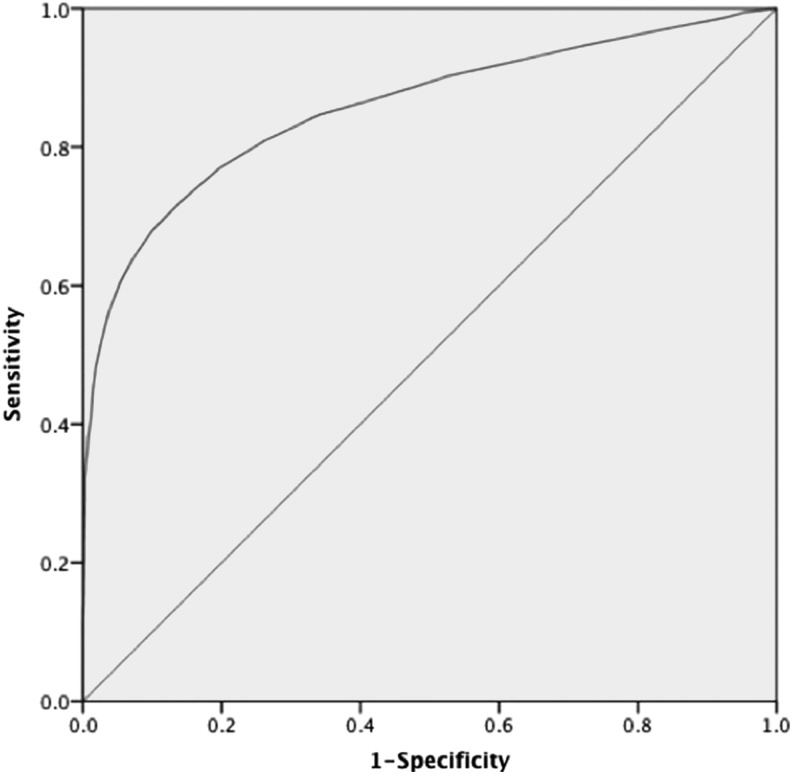
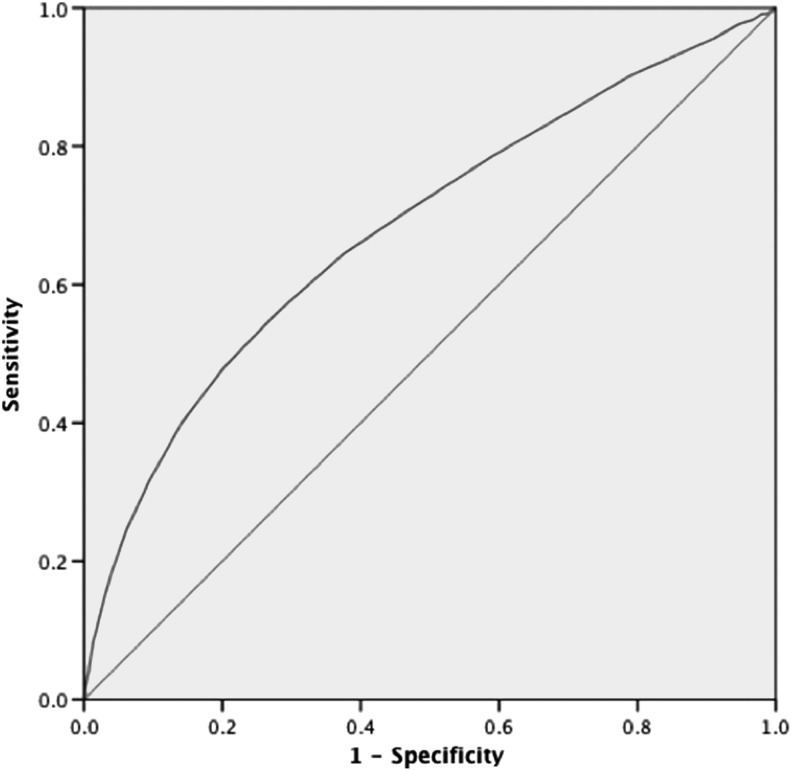
Fasting plasma glucose and postprandial plasma glucose were positively correlated with HbA1c (r=0.725 and r=0.673, both P<0.001). HbA1c was a highly predictive factor for identifying newly diagnosed diabetes and a relatively weak variable for diagnosing prediabetes (Figs. 1 and 2 and Table 2). For diagnosing diabetes, the AUC was 0.857 (95% confidence interval, 0.841–0.873), and with the largest Youden index of 0.581, the optimal cutoff for HbA1c was 6.3%, with sensitivity of 72.2%, specificity of 85.9%, positive predictive value of 0.378, and negative predictive value of 0.963. For diagnosing prediabetes, the AUC was 0.681 (95% confidence interval, 0.666–0.697), and with the largest Youden index of 0.280, the optimal cutoff for HbA1c was 5.9%, with sensitivity of 64.5%, specificity of 63.5%, positive predictive value of 0.317, and negative predictive value of 0.867.
FIG. 1.
Receiver operating characteristic curve for detecting newly diagnosed diabetes by hemoglobin A1c level. The area under the receiver operating characteristic curve was 0.857 (95% confidence interval, 0.841–0.873) for diagnosing diabetes.
FIG. 2.
Receiver operating characteristic curve for detecting prediabetes by hemoglobin A1c level. The area under the receiver operating characteristic curve was 0.681 (95% confidence interval, 0.666–0.697) for diagnosing prediabetes.
Table 2.
Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Youden Index for Detecting Newly Diagnosed Diabetes and Prediabetes at Different Hemoglobin A1c Thresholds by Receiver Operating Characteristic Curve
| Newly diagnosed diabetes | Prediabetes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c level (%) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Youden index | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Youden index |
| 5.6 | 94.8% | 27.1% | 0.135 | 0.977 | 0.219 | 84.7% | 30.3% | 0.247 | 0.880 | 0.150 |
| 5.7 | 92.6% | 36.7% | 0.149 | 0.977 | 0.293 | 78.6% | 40.9% | 0.264 | 0.876 | 0.195 |
| 5.8 | 90.2% | 47.3% | 0.170 | 0.976 | 0.375 | 71.2% | 52.4% | 0.287 | 0.871 | 0.236 |
| 5.9a | 87.3% | 56.8% | 0.195 | 0.974 | 0.441 | 64.5% | 63.5% | 0.317 | 0.867 | 0.280 |
| 6.0 | 84.7% | 65.9% | 0.229 | 0.973 | 0.506 | 56.0% | 71.8% | 0.350 | 0.859 | 0.278 |
| 6.1 | 80.9% | 73.8% | 0.270 | 0.970 | 0.547 | 48.1% | 79.5% | 0.390 | 0.851 | 0.276 |
| 6.2 | 76.9% | 80.4% | 0.319 | 0.967 | 0.573 | 40.0% | 85.9% | 0.433 | 0.841 | 0.259 |
| 6.3b | 72.2% | 85.9% | 0.379 | 0.963 | 0.581 | 31.6% | 90.6% | 0.475 | 0.831 | 0.222 |
| 6.4 | 68.0% | 90.0% | 0.449 | 0.959 | 0.580 | 24.4% | 93.9% | 0.520 | 0.822 | 0.183 |
| 6.5 | 63.9% | 92.8% | 0.516 | 0.956 | 0.567 | 18.7% | 95.9% | 0.553 | 0.814 | 0.146 |
Hemoglobin A1c (HbA1c) threshold found for prediabetes.
HbA1c threshold found for newly diagnosed diabetes.
Discussion
For decades, HbA1c has been recommended only as the gold standard of glucose control, which is an independent predictor of cardiovascular events in patients with diabetes and in individuals without diabetes.14 However, the January 2010 clinical practice recommendations from the American Diabetes Association advocated the use of HbA1c for the diagnosis of diabetes.8 According to the recommendation, an HbA1c threshold of 6.5% is considered diagnostic of diabetes, and subjects with an HbA1c level between 5.7% and 6.4% are considered at high risk of developing this condition. This HbA1c threshold has been accepted by Europeans and Americans. However, the cutoff of HbA1c for diagnosing diabetes in Chinese people is not clear.
We found an HbA1c threshold of 6.3% highly valuable for detecting undiagnosed diabetes. Our research result was supported by the same HbA1c threshold for detecting undiagnosed diabetes found for Shanghai adults, which demonstrated sensitivity and specificity of 63% and 96%, respectively.15 Other studies in East Asia countries showed an optimal HbA1c cutoff for diagnosing diabetes of 5.6% in Japan16 and 5.9% in Korea.17 In a Middle Eastern population, researchers found an HbA1c threshold of 6.4%.18 Therefore, HbA1c criteria for diagnosing diabetes in different populations are needed.
In screening newly diagnosed diabetes by the HbA1c threshold of 6.5% from the American Diabetes Association,8 we found a sensitivity of 63.9% and a specificity of 92.8%. Therefore, an HbA1c threshold of 6.3% may be used for diagnosing diabetes in community-based Chinese adults 40 years of age or older; this value provided the optimum balance of sensitivity (72.2%) and specificity (85.9%) and had the largest Youden index (0.581). However, people with an HbA1c level of <6.3% may still be at risk, depending on the presence of other risk factors of diabetes.
For diagnosing prediabetes in our study, the AUC was 0.681 (95% confidence interval, 0.666–0.697), and the optimal HbA1c threshold was 5.9%, with sensitivity of 64.5% and specificity of 63.5%. Therefore, HbA1c was a relatively weak diagnostic tool to detect prediabetes. This result was similar to another survey of community dwellers in Beijing, which found an optimal HbA1c cutoff of 5.7%, with sensitivity of 59.4% and specificity of 73.9% for diagnosing prediabetes.19 Although the subjects with a diagnosis of prediabetes by HbA1c showed more differences than detection by impaired fasting glucose, the predictive value for progression to diabetes was similar to that assessed by impaired fasting glucose alone.20 An HbA1c value of >6.0% may be a clinically useful marker to identify people at risk for the development of diabetes and also cardiovascular disease and death.14
Our survey was a cross-sectional epidemiological study, without long-term follow-up, and the results should be verified by more studies with larger samples. Because of the epidemiological nature of our investigation, the possibility of residual confounding cannot be completely eliminated. Some diseases, such as anemia, could influence HbA1c test results, but we did not detect blood cell classification. Moreover, HbA1c cannot reflect changes in plasma glucose levels in the short term; thus, diagnosing diabetes by HbA1c alone may miss patients with a diabetes course of <3 months.
Nevertheless, despite the controversies concerning its practical application, HbA1c appears to be a reliable tool for detecting undiagnosed diabetes and prediabetes. HbA1c could be used as a single diagnosing test to detect newly diagnosed diabetes and prediabetes in community-based Chinese adults 40 years of age or older in Shandong Province, China. An HbA1c threshold of 6.3% was highly valuable for detecting undiagnosed diabetes, and 5.9% was relatively weakly valuable for detecting prediabetes.
Acknowledgments
The present study was supported by grants from the Chinese Society of Endocrinology, the National Natural Science Foundation of China (number 81100617), the Medical and Health Science and Technology Development Projects of Shandong Province (number 2011HD005), the National Science and Technology Support Plan (number 2009BAI80B04), the Natural Science Foundation of Shandong Province (number ZR2012HM014), the International Science and Technology Projects of Shandong Province (numbers 2010GHZ20201 and 2012GGE27126), the Business Plan of Jinan Students Studying Abroad (number 20110407), and the special scientific research fund of clinical medicine of the Chinese Medical Association (number 12030420342).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yang WY, Lu JM, Weng JP, et al. : Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Cadwell BL, Cheng YJ, et al. : Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 2004;27:2806–2812 [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Klein R, Welborn TA, et al. : Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 1992;15:815–819 [DOI] [PubMed] [Google Scholar]
- 4.Pan XR, Li GW, Hu YH, et al. : Effect of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, et al. : Prevention of type 2 diabetes mellitus by change in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Crainiceanu CM, Brancati FL, et al. : Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007;167:1545–1551 [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Buse JB, Davidson MB, et al. : Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association: Standards of medical care in diabetes: 2010. Diabetes Care 2010;33(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Expert Committee: International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning G; Reaction Study Group: Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes 2012;4:172–173 [DOI] [PubMed] [Google Scholar]
- 11.Bi Y, Lu J, Wang W, et al. : Cohort profile: Risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes 2014;6:147–157 [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Bi Y, Wang T, et al. : The relationship between insulin-sensitive obesity and cardiovascular diseases in a Chinese population: Results of the REACTION study. Int J Cardiol 2014;172:388–394 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. www.staff.ncl.ac.uk/philip.home/who_dmg.pdf (accessed March12, 2013)
- 14.Selvin E, Steffes MW, Zhu H, et al. : Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao Y, Ma X, Li H, et al. : Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ 2010;340:c2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagami T, Tominaga M, Nishimura R, et al. : Is the measurement of glycated hemoglobin A1c alone an efficient diagnosing test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract 2007;76:251–256 [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Kim GW, Lee MY, et al. : Role of HbA1c in the diagnosing of diabetes mellitus in a Korean rural community. Diabetes Metab J 2012;36:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajat C, Harrison O, Al Siksek Z: Diagnostic testing for diabetes using HbA1c in the Abu Dhabi population: Weqaya: the Abu Dhabi cardiovascular screening program. Diabetes Care 2011;34:2400–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou XH, Ji LN, Luo YY, et al. : Performance of HbA1c for detecting newly diagnosed diabetes and pre-diabetes in Chinese communities living in Beijing. Diabet Med 2009;26:1262–1268 [DOI] [PubMed] [Google Scholar]
- 20.Helanza Y, Hara S, Arase Y, et al. : HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378:147–155 [DOI] [PubMed] [Google Scholar]