Abstract
As a strategy to increase the seed dormancy of soft white wheat, mutants with increased sensitivity to the plant hormone abscisic acid (ABA) were identified in mutagenized grain of soft white spring wheat “Zak”. Lack of seed dormancy is correlated with increased susceptibility to preharvest sprouting in wheat, especially those cultivars with white kernels. ABA induces seed dormancy during embryo maturation and inhibits the germination of mature grain. Three mutant lines called Zak ERA8, Zak ERA19A, and Zak ERA19B (Zak ENHANCED RESPONSE to ABA) were recovered based on failure to germinate on 5 µM ABA. All three mutants resulted in increased ABA sensitivity over a wide range of concentrations such that a phenotype can be detected at very low ABA concentrations. Wheat loses sensitivity to ABA inhibition of germination with extended periods of dry after-ripening. All three mutants recovered required more time to after-ripen sufficiently to germinate in the absence of ABA and to lose sensitivity to 5 µM ABA. However, an increase in ABA sensitivity could be detected after as long as 3 years of after-ripening using high ABA concentrations. The Zak ERA8 line showed the strongest phenotype and segregated as a single semi-dominant mutation. This mutation resulted in no obvious decrease in yield and is a good candidate gene for breeding preharvest sprouting tolerance.
Introduction
This study examines the hypothesis that seed dormancy can be increased in white wheat by selection for increased sensitivity to the plant hormone abscisic acid (ABA). It is particularly important to breed for the seed dormancy of white wheat cultivars because their tendency towards lower seed dormancy makes them more vulnerable to preharvest sprouting. Preharvest sprouting (PHS), the germination of grains when still on the mother plant, occurs when environmental conditions are cool and wet after grains reach physiological maturity but before harvest is completed (Bewley and Black 1994). PHS has been reported in many major wheat-growing areas globally. PHS is associated with physical characteristics of the spike (King 1984; King and Richards 1984; King and von Wettstein-Knowles 2000), insufficient seed dormancy at maturity (DePauw and McCaig 1991; Imtiaz et al. 2008; Fofana et al. 2009), and white seed coat color (Flintham 2000). In the United States, hard white wheat has gained popularity due to higher flour yields compared with red wheats and potential export opportunities to areas where white wheats are preferred. However, in some major US wheat-growing areas, such as the Great Plains, conditions conducive to PHS are common (Liu et al. 2008). Therefore, new sources of PHS resistance are desirable.
Dormant seeds do not germinate even under favorable conditions (reviewed by Finkelstein et al. 2008). The type and extent of dormancy exhibited by a seed lot is dependent on environmental conditions during seed development, germination conditions, and the genetic background. In wheat, two types of dormancy may be present: seed coat-imposed, in which the cutting or removal of the seed coat stimulates germination, and dormancy associated with the embryo, which is not responsive to seed coat removal. Wheat may have either or both types of dormancy (Schramm et al. 2010; reviewed by Bewley and Black 1994).
The notion that increased ABA sensitivity may lead to increased PHS tolerance is based in part on evidence showing that ABA biosynthesis is required to establish seed dormancy during embryo maturation in Arabidopsis and in part on evidence that higher ABA accumulation or sensitivity is linked to seed dormancy in cereals (Karssen et al. 1983; Walker-Simmons 1987; Barrero et al. 2009). ABA is required for the acquisition and maintenance of dormancy in seeds of wheat and other species (reviewed by Holdsworth et al. 2008). Reduced ABA sensitivity or biosynthesis is associated with decreased dormancy, whereas increased ABA sensitivity or accumulation is associated with increased seed dormancy (reviewed by Finkelstein et al. 2008).
In addition, mutants in several species with reduced ABA biosynthesis have seeds with reduced dormancy or premature germination, as well as impaired ability to control transpirational water loss from the leaves, resulting in a vegetative wilty phenotype (reviewed by Nambara and Marion-Poll 2005). In contrast, ABA-hypersensitive mutants in Arabidopsis such as enhanced response to ABA1 (era1) or ABA-hypersensitive1 (abh1) have increased ABA sensitivity during seed germination associated with increased seed dormancy and increased vegetative drought tolerance (Cutler et al. 1996; Hugouvieux et al. 2001).
Higher ABA accumulation and sensitivity is also correlated with seed dormancy in cereals. More dormant grain is highly sensitive to ABA, whereas after-ripened grain becomes less inhibited by ABA in germination as after-ripening proceeds (Walker-Simmons 1987; Morris et al. 1989; Warner et al. 2000; Gerjets et al. 2010). Grain after-ripened for very long periods of time may even become highly ABA insensitive (Morris et al. 1989; Schramm et al. 2010). ABA sensitivity and accumulation is associated with seed dormancy in barley, and after-ripening of barley grain results in reduced endogenous ABA levels as a result of increased ABA turnover through increased expression of the ABA 8′-hydroxylase catabolic enzyme (Millar et al. 2006; Barrero et al. 2009).
In wheat, red seed coat color is associated with stronger dormancy and higher levels of PHS tolerance than cultivars with white seed coat color, although some exceptions to this generality exist, with some white cultivars showing strong dormancy and resistance to PHS, and some red cultivars showing little dormancy or resistance to PHS (Morris et al. 1989; Torada and Amano 2002). White mutants derived from the single-gene red wheat Chinese Spring were found to have reduced dormancy in mature grains when compared with the wild-type (red) parent, but isolated embryos did not differ significantly in germination, rate of after-ripening, or ABA sensitivity from the wild-type parent (Warner et al. 2000). Similarly, a white mutant of the single-gene red wheat AUS1490 had a slight reduction in dormancy and ABA sensitivity, but absence of red grain color did not eliminate dormancy or ABA sensitivity (Himi et al. 2002).
It is clear that seed dormancy is desirable to aid in the prevention of PHS. However, prolonged dormancy may interfere with uniform seedling emergence and stand establishment in the field. Many studies have been carried out in attempts to elucidate the genetic control of dormancy and resistance to PHS in wheat. Single genes and QTLs affecting dormancy and PHS tolerance have been identified throughout the hexaploid wheat genome in various populations (Roy et al. 1999; Zanetti et al. 2000; Mares and Mrva 2001; Flintham et al. 2002; Groos et al. 2002; Fofana et al. 2009; Munkvold et al. 2009; Singh et al. 2010; Liu et al. 2011). Recently, the PHS tolerance QTL on chromosome 3A, Qphs.ocs-3A.1 was shown to co-localize with the wheat dormancy induction gene MFT-3A (Mother of FT and TFL1) (Nakamura et al. 2011). The lack of agreement in results of many QTL and mapping studies suggests that dormancy in wheat is complex and affected by many genes.
Mutant screens are another strategy that may contribute to our understanding of dormancy and PHS resistance in wheat. Relatively few such mutant screens have been reported in wheat or closely related species. Reduced seed dormancy and ABA insensitive mutants have been isolated in wheat (Kawakami et al. 1997; Warner et al. 2000; Rikiishi and Maekawa 2010; Schramm et al. 2012) and closely related species such as barley (Visser et al. 1996; Molina-Cano et al. 1999). ABA-insensitivity in red wheat is associated with reduced initial seed dormancy and rapid after-ripening (Schramm et al. 2012). While ABA-hypersensitive mutants isolated in red wheat show increased dormancy, these phenotypes are weak and transient (Kobayashi et al. 2008; Schramm et al. 2010). In this paper we describe the first isolation of mutants with increased ABA sensitivity in a cultivar with white kernels and low initial levels of dormancy. The objective of this work was to determine whether such mutants could be isolated in white wheat and if so, whether they would result in a strong increase in seed dormancy that may be useful in increasing PHS tolerance. The paper describes the characterization of the seed germination, dormancy, and ABA sensitivity of these mutants. In addition, genetic segregation of two mutants showed that they result in a semi-dominant mutation in a single gene.
Materials and methods
Plant materials
The soft white spring cultivars ‘Zak’ and ‘Alpowa’ (PI 566596) were released by Washington State University (Kidwell et al. 2002). The Triticum aestivum L. Chinese Spring germplasm used in these experiments were descendants of the J. Dvorak doubled haploid line Dv418 and obtained from Warner (2000). The original Chinese Spring was a landrace and the doubled haploid selection from it is presumed to be more homozygous and homogenous. The soft white winter cultivar ‘Brevor’ was obtained from M. K. Walker Simmons (1987).
Germination assays
The term seed is used in reference to the wheat caryopsis or grain. Grain was harvested at physiological maturity (~ 10 % moisture) when the peduncle was yellow and then after-ripened at room temperature for 1 week (~7 % moisture) before use or storage at −20 °C to allow comparison of wild-type and mutant seed of uniform age in germination assays. Germination assays were performed using either whole or cut half-grains (also referred to as embryos) plated on a 9-cm petri dish lined with a single germination disc (Anchor steel blue germination blotter (SDB3.375), Anchor Paper Co., St. Paul MN) moistened with an aqueous solution of 6 mL of 5 mM MES buffer, pH 5.5 (2-[N-morpholino] ethane sulfonic acid, Sigma, St. Louis, MO) containing varying concentrations of either optically pure (+)-ABA or (+/−)-ABA. MES buffer without ABA was used as a negative control. (+)-ABA was obtained as a gift from S. Abrams (NRC, PBI58) and maintained as a 0.1-M stock in DMSO or methanol at −20 °C in the dark. (+/−)-ABA was obtained from Phytotechnology Laboratories. The plates were sealed with Parafilm to prevent evaporation, wrapped with foil, and incubated at 30 °C in the dark to be consistent with conditions previously used to characterize wheat grain ABA sensitivity (Walker-Simmons 1987). Germination was scored based on radicle emergence every 24 h for 5 days. Germination was expressed either as a percentage germinated or as a weighted germination index (GI) calculated over 5 days of scoring as (5 × gday1 + 4 × gday2… + 1 × gday5)/(5 − n) where g is the number of germinated seeds and n is the total number of viable seeds (Walker-Simmons 1987). Using this formula, the maximum possible GI is 1.0, and the speed of germination as well as number of germinated seeds are represented by a single value. If needed, the germination of ungerminated grains was stimulated by plating in a new petri dish containing a single germination disc moistened with 6 mL of 10 µM of GA3. Stocks of GA3 in ethanol were maintained at −20 °C in the dark. Unless otherwise noted, germination values represent means of three replicates of ten seeds each. If necessary, seeds were surface sterilized by soaking in 10 % bleach/ 0.01 % SDS with gentle shaking for 15 min and then rinsed thoroughly in sterile water before plating.
Mutagenesis and screening of wheat grain
A batch of 15,000 Zak grains were presoaked in 200 mL 50 mM phosphate buffer (pH 7.0) for 5 h. Grain was mutagenized in 200 mL 0.3 % (v/v) ethyl methane sulfate (EMS; Sigma Chemicals, St. Louis, MO) in 50 mM phosphate buffer (pH 7.0) in a sealed 2 L flask with shaking for 16 h at 22 °C. EMS was then neutralized with an equal volume of 10 % (w/v) sodium thiosulfate and washed ten times over a 5-h period with 30-min intervals of soaking in water. Mutagenized grain (M1) was grown in the field. A single head from each M1 plant was harvested. Four M2 grains from each of approximately 10,000 heads were screened for ABA hypersensitivity after 6 months of after-ripening by plating on 5 µM ABA as previously described.
Growth conditions
Plants were grown in the greenhouse except where otherwise stated with a photoperiod of 16 h at a light intensity of 400 µmol m−2 s−1 , with a daytime temperature of 21–24 °C, and a nighttime temperature of 15–18 °C. During winter months, supplemental light was provided with high-pressure sodium lamps. For the experiments to determine time to flowering and grain yield, plants were grown in a growth room with a 16-h photoperiod (22 °C day, 15 °C night) and light intensity of 400 µmol m−2 s−1 with a 1:1 ratio of metal halide: sodium lamps. Seeds or seedlings were planted into either 2.6- or 3 L pots, depending on the experiment, containing Sunshine LC 1 potting soil mixture (Sun Gro Horticulture). Pots were watered to saturation every 2 days. A nutrient solution (Peters Professional 20-10–20 Peat-Lite Special) was supplied once a week. Spikes were harvested at physiological maturity and seeds were hand threshed to avoid scarification of the seed coat. Seeds were allowed to dry at room temperature, and thereafter stored at −20 °C to maintain dormancy unless otherwise indicated. Heights were measured from the base of the plant to the top of the spike on the tallest tiller, excluding awns.
Seed of Alpowa, Zak, and Chinese Spring used for determination of length of time to after-ripen was obtained from plants grown in the field at Washington State University Spillman Farm located near Pullman, WA in 2005. Seed of Brevor used in the same experiment was grown at the USDA Central Ferry Research Station in Central Ferry, WA in 2002.
Data were analyzed using an analysis of variance, and tests of differences between the mutants and wild type were conducted using the Dunnett’s multiple comparison adjustment in Minitab software (version 16).
Results
Zak as a background for ABA-hypersensitive mutant isolation in wheat
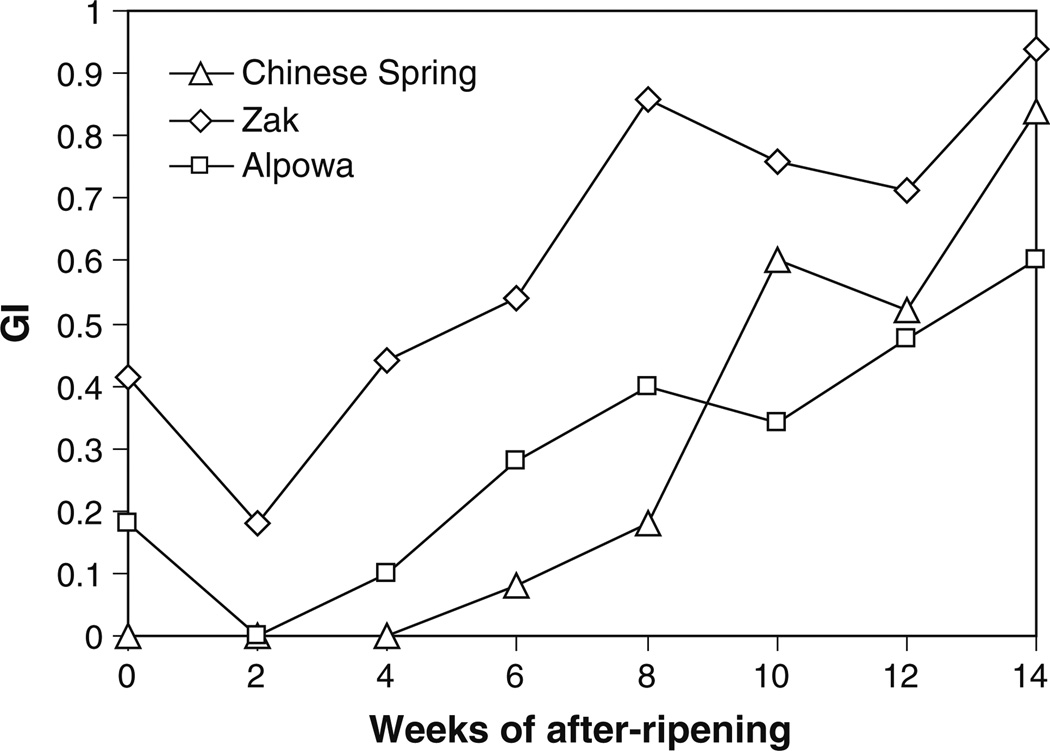
Previously isolated ABA-hypersensitive mutants in Chinese Spring, which has red seed coat color, proved difficult to characterize due to inconsistent embryo dormancy and the fact that the phenotype could only be scored within approximately a 2–6 week window (Schramm et al. 2010). Because white wheats generally have less embryo dormancy and require less time to after-ripen, a second mutant screen was undertaken to identify ABA-hypersensitive mutants directly in a white background. Two soft white spring wheat cultivars, Zak (Kidwell et al. 2002) and Alpowa (PI 566596), were considered as parents. A preliminary experiment was conducted to determine the length of time required for these two cultivars to after-ripen compared with Chinese Spring. A dormant control, the soft white winter cultivar Brevor (Walker-Simmons 1987), was included in the experiment. Zak seeds displayed less dormancy throughout the after-ripening time course than Chinese Spring seeds obtained from plants grown under the same conditions. Freshly harvested Zak seeds germinated more efficiently than Chinese Spring and required less time to after-ripen. Zak seeds achieved a GI of 0.8 in only 8 weeks, whereas Chinese Spring required 14 weeks to reach a comparable germination capacity (Fig. 1). The dormant control, Brevor, did not exceed a GI of 0.2 during this 14-week period, and needed 45 weeks to reach a GI of 0.73 (Online Resource 1). Alpowa seeds only reached a GI of 0.6 by week 14. Therefore, the less dormant of the two white-grained spring cultivars, Zak, was chosen for mutagenesis.
Fig. 1.
Germination index (GI) over a 14-week after-ripening time course for Chinese Spring (hard red spring), Alpowa (soft white spring), and Zak (soft white spring). Freshly harvested seeds were obtained from plants grown in the field at Pullman, Washington in 2005. Ten to thirty whole seeds per genotype per time point were incubated for 5 days on 5 mM MES
Isolation and retests of Zak ABA-hypersensitive mutants
Approximately 40,000 M2 caryopses were screened for failure to germinate on 5 µM (+)-ABA after 6 months of after-ripening. Twelve putative mutants termed “ZakERA” (Zak ENHANCED RESPONSE TO ABA) were planted and advanced to the M3 generation for retesting. Intact M3 seeds after-ripened for 8 months were plated on 5 µM (+)-ABA. Individuals were considered to have a reproducible phenotype in the M3 generation if germination was less than or equal to 60 % at 48 h (Table 1). Seven mutants were advanced from the M3 retest to be retested in the M4 generation. Intact M4 seeds that had been after-ripened for 3 years were plated on 25 and 50 µM (+/−)-ABA. Three mutants showed reduced germination on one or both concentrations of ABA and were advanced once more to the M5 generation for a final retest. Intact M5 seeds of these three mutants were after-ripened for 2 months and plated on 5 µM (+)-ABA. All three mutants had germination of less than 60 % under these conditions, compared with Zak wild type, which had 90 % germination. These three mutants, Zak ERA8, Zak ERA19A, and Zak ERA19B passed all retests and were therefore chosen for more detailed characterization.
Table 1.
Germination of Zak ERA mutants and Zak wild type over three generations
| Genotype | M3a | M4b |
M5c | |
|---|---|---|---|---|
| 5 µM ABA | 25 µM ABA | 50 µM ABA | 5 µM ABA | |
| Zak | 73.3 | 87.5 | 93.3 | 90 |
| ERA8 | 0 | 70 | 53.3 | 35.4 |
| ERA19A | 36.7 | 56.7 | 53.3 | 51.1 |
| ERA19B | 60 | – | 51.7 | 57.8 |
| ERA26A | 33.3 | 90 | 93.3 | – |
| ERA3A | 33.3 | – | 86.7 | – |
| ERA26B | 60 | – | 90 | – |
| ERA33A | 33.3 | 100 | 91.7 | – |
M3 seed was tested after 8 months of after-ripening. Eight to 30 seeds were tested per genotype. Germination percentages after 48 h of incubation on 5 µM (+)-ABA in 5 mM MES are shown
M4 seed was tested after 3 years of after-ripening. Thirty to 60 seeds were tested per genotype. Germination percentages after 48 h of incubation on either 25 or 50 µM (+/−)-ABA in 5 mMMES are shown
M5 seed was tested after 2 months of after-ripening. Twenty to 65 seeds were tested per genotype. Germination percentages after 72 h of incubation on 5 µM (+)-ABA in 5 mM MES are shown
Characterization of ABA dose response in seed germination
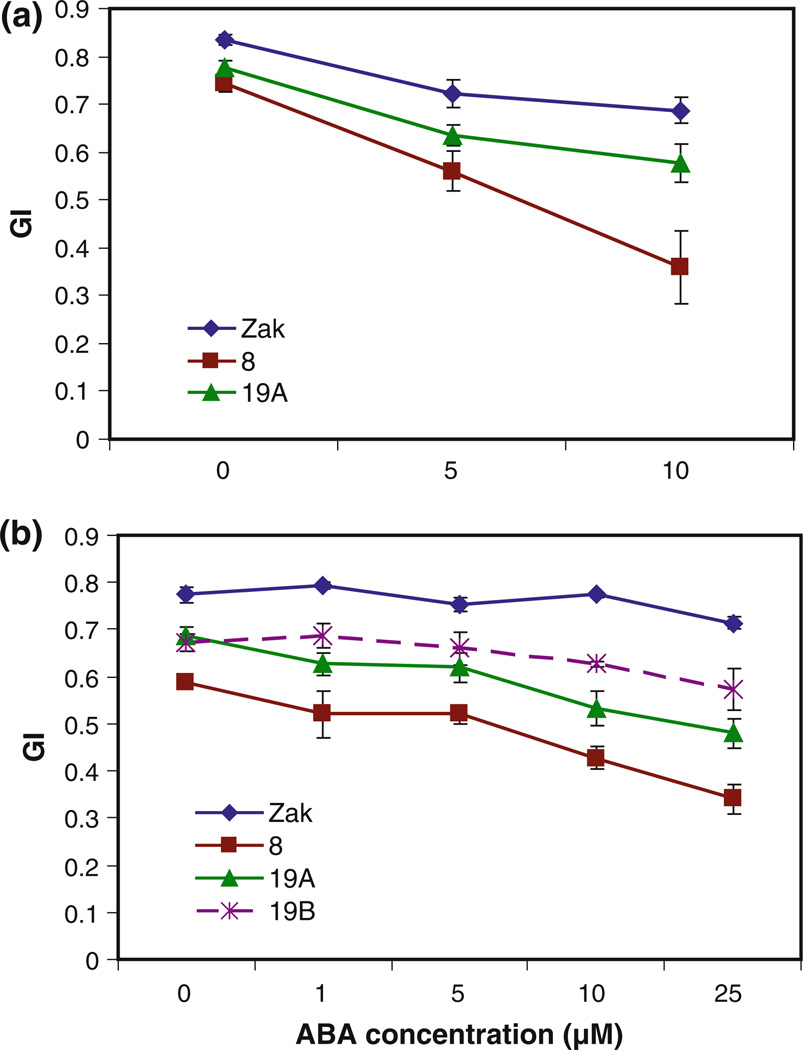
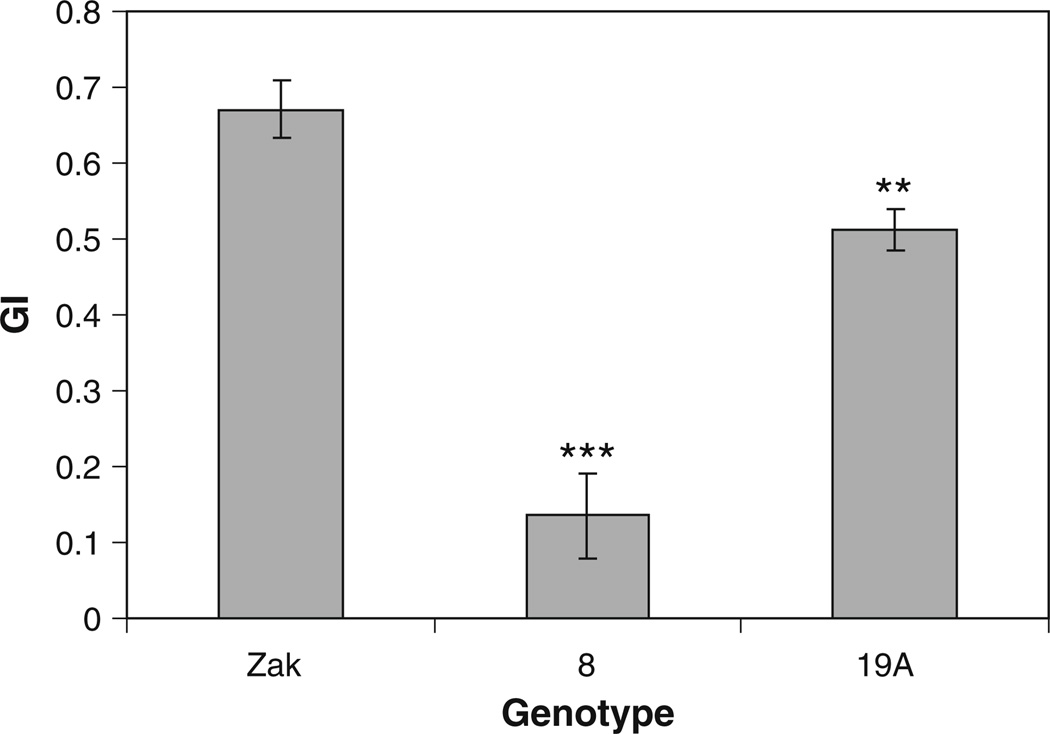
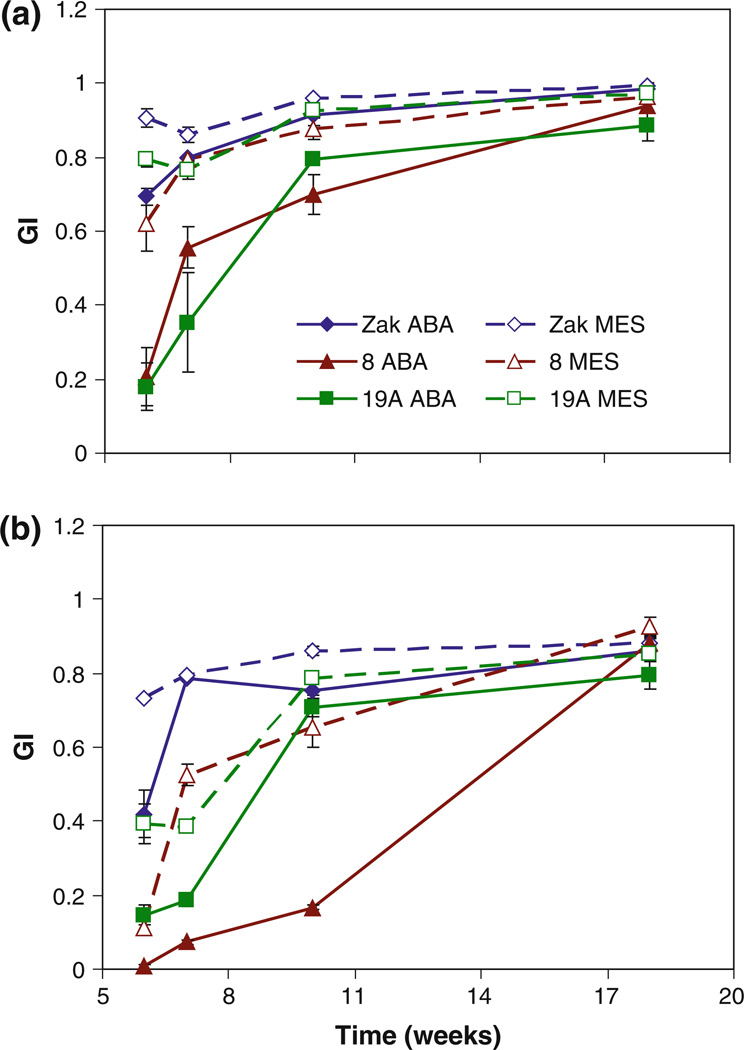
Zak ERA mutants and Zak wild type were plated on increasing concentrations of ABA to evaluate the germination response of mutants over a range of ABA concentrations. Germination in response to ABA of two independent lots of M5 seeds was monitored for 5 days in two experiments. In the first experiment, seeds of Zak ERA8, Zak ERA19A, and Zak wild type after-ripened for 3 weeks were tested. Half seeds from this seed lot were tested on three ABA concentrations (Fig. 2a for germination index; Online Resource 2 for percent germination), and intact seeds were tested without ABA to assess the dormancy level of the mutants compared with Zak (Fig. 3). Both mutants showed a reduction in germination at all ABA concentrations as half seeds (Fig. 2a, Online Resource 2), as well as in the absence of ABA as intact seeds (Fig. 3). The difference in germination index between mutants and Zak wild type was significant for both mutants at all ABA concentrations except for Zak ERA19A when treated with 10 µM ABA (Online Resource 3). In the second experiment, intact seeds of all three mutants were tested after 2 months of after-ripening on five ABA concentrations. Zak ERA8 and 19A again showed significant reductions in germination capacity compared with Zak at all concentrations (Fig. 2b, Online Resource 4). ZakER-A19B also had reduced germination at all ABA concentrations, although the difference between it and Zak was only significant at three of the five ABA concentrations (Online Resource 4). Zak ERA8, 19A, and 19B all had significantly reduced germination in the absence of ABA, indicating some increase in dormancy, particularly in Zak ERA8 (P <0.001 in both whole seed experiments; P < 0.01 as half seeds). In both experiments, Zak ERA8 exhibited the strongest response to ABA in germination, with germination index values significantly different from Zak at all ABA concentrations in both experiments (Online Resource 3 and 4).
Fig. 2.
ABA dose response in germination of Zak ERA8, 19A, and 19B compared to Zak wild type. Two lots of M5 grain were tested as a cut seeds after 3 weeks of after-ripening and b intact seeds after 2 months of after-ripening. Seeds were treated with varying concentrations of (+)-ABA in MES or MES in the absence of ABA and scored daily for 5 days for the calculation of germination index (GI). Four to fifteen replicates of ten seeds each were tested in (a), and three replicates of ten seeds each were tested in (b). Error bars represent standard errors
Fig. 3.
Germination index (GI) of intact M5 seeds of Zak ERA8 and Zak ERA19A compared to Zak wild type following 3 weeks of after-ripening. Four to fifteen replicates of ten seeds each were incubated for 5 days on 5 mM MES. Error bars represent standard errors. Significance determined by analysis of variance using Dunnett’s multiple comparison adjustment, with asterisk signifying P < 0.05, **P <0.01, and ***P <0.001
Mutation segregation analysis
Two mutants, Zak ERA19B and 8, were backcrossed to Zak wild type to examine genetic segregation. In all experiments, the parent plants were advanced with crosses to compare germination of both parents with progeny. A germination time course was used to determine when the F2 seeds were after-ripened to the point where the mutant and wild-type parental controls showed the greatest difference in percent germination.
If the ABA-hypersensitive phenotype were completely penetrant, then we would be able to find a condition such that the F2 populations would show 25 % germination if the trait is dominant (−/− and +/− seeds give 0 % germination, +/+ 100 % germination), but 75 % germination if the trait is recessive (−/− seeds give 0 % germination, +/+ and +/− 100 % germination). However, these hypotheses are based on parental germination of 0 % for the mutant and 100 % for wild-type seed. As the mutant-wild type comparison only rarely approaches this situation, the actual parental germination behavior in any given experiment was used to estimate F2 expected germination percentages for the purposes of Chi-square tests. Since the parental plants were grown, harvested, and after-ripened side-by-side with F1 plants, we expect these parental controls to be indicative of the behavior of the wild-type and mutant seeds segregating in the F2 population.
F2 seed obtained from six independent F1 plants derived from a cross between Zak ERA19B and Zak wild type after-ripened for 3 months and treated with 2 µM ABA showed 75.4 % germination, whereas Zak ERA19B and Zak wild-type lines descended from the plants used in the original cross showed 51.7 and 96.7 % germination, respectively (Table 2). The Zak ERA19B F2 population would be expected to show 62.9 % germination if dominant (¾ of the seeds are −/− or +/− giving 51.7 % germination, and ¼ of the seeds are +/+ giving 96.7 % germination) and 85.4 % germination if recessive based on parental germination behavior. The observed F2 population gave 75.4 % germination suggesting that ZakERA19B results from a partially dominant (also called semi-dominant or additive) mutation in a single gene (χ2 = 0.408). However, the weaker the mutant phenotype, the closer the values expected for dominant and recessive F2 segregation, and the more difficult it is to distinguish between the two. In light of this, further experiments will need to be undertaken to confirm that this mutation is in fact semi-dominant, since there is a fairly small margin separating the three possible outcomes in the case of Zak ERA19B.
Table 2.
F2 segregation analysis of two Zak ERA mutants
| Genotype | na | Genb | Germ (%)c | χ2 |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|
| 3:1 | 1:3 | 1:2:1 | 3:1 | 1:3 | 1:2:1 | ||||
| ERA19B/Zak | 540 | F2 | 75.4 | 35.9 | 43.8 | 0.4 | <0.001 | <0.001 | 0.523 |
| ERA19B parent | 60 | M6 | 51.7 | ||||||
| Zak parent | 90 | 96.7 | |||||||
| F2 expected % germination | 62.9 | 85.4 | 74.2 | ||||||
| ERA8/Zak | 362 | F2 | 48.3 | 98.3 | 102.9 | 0.0 | <0.001 | <0.001 | 0.832 |
| ERA8 parent | 90 | M6 | 2.2 | ||||||
| Zak parent | 90 | 95.6 | |||||||
| F2 expected % germination | 25.6 | 72.3 | 48.9 | ||||||
Number of seeds tested for germination
Generation of seeds tested
Percent germination after 96 h (both experiments)
In the case of Zak ERA8, however, parental germination showed much greater separation and therefore less uncertainty in evaluation of segregating populations. Segregation analysis was performed using F2 seeds derived from six independent F1 plants. In the F2 experiment, the Zak ERA8 mutant parent (−/−) control showed 2.2 % germination, whereas the wild type (+/+) showed 95.6 % germination (Table 2). Therefore, we would expect the Zak ERA8 F2 population to show 25.6 % germination if dominant (¾ of the seeds are −/− or +/− giving 2.2 % germination, and ¼ of the seeds are +/+ giving 95.6 % germination), and 72.25 % germination if recessive (¾ of the seed are +/+ or +/− giving 95.6 % germination, and ¼ of the seed are −/− giving 2.2 % germination). The observed is 48.3 % germination which is intermediate between the two. This percentage is very close to the expected percentage (48.9 %; χ2 = 0.045) for a semi-dominant trait.
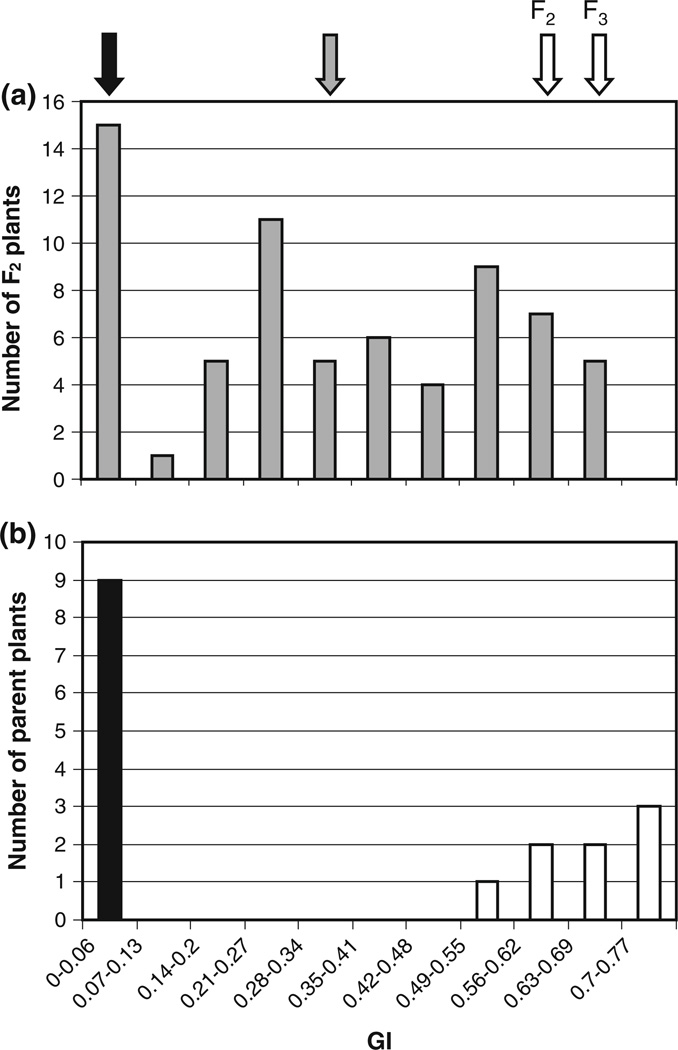
Sixty-eight F2 seedlings from across the range of germination in the Zak ERA8 F2 experiment were randomly advanced, and F3 seed harvested from F2 individuals for further testing. Conditions were the same as those used in the F2 experiment, with intact seeds after-ripened for 6 weeks treated with 1 µM ABA. Parental germination percentages at 96 h were similar to those in the F2 experiment (0 % for Zak ERA8 and 98.3 % for Zak in F3 experiment, compared to 2.2 and 95.6 % in F2 (Table 2)), as was the overall mean of the F3 population when compared with the overall F2 mean (51.8 vs. 48.3 % (Table 2)). However, GI was chosen to assess the F3 population and for comparison between the F2 and F3 because it better reflected the large differences in the speed of germination across these populations and between the parents. The GI range of F2 plants (as determined by each plant’s F3 progeny) was 0–0.68 (Fig. 4a), while the Zak parent ranged from 0.53 to 0.72 and the mutant parents all resulted in a GI of 0 (Fig. 4b). Therefore, the segregating population encompassed the same range represented by the parental ranges. The resulting distribution is roughly tri-modal, with peaks corresponding to each parent and an additional peak intermediate to both parents (Fig. 4a). The mean GIs of F1 and F2 plants (F2 and F3 seeds) were 0.29 and 0.32, respectively, suggesting that the mutation is exhibiting partial dominance since the overall population means are consistently intermediate to the parental means. This is in agreement with the conclusion reached in the F2 segregation analysis described in Table 2. With partial dominance, F2 plants should follow a segregation ratio of 1 homozygous mutant: 2 heterozygotes: 1 homozygous wild type. If parental GI ranges and overall F3 germination behavior are used to assign F2 plants to these categories, then 16 plants appear to be homozygous mutant (GI = 0–0.11), 35 appear to be heterozygous (GI = 0.15–0.5), and 17 appear to be homozygous wild type (GI = 0.52–0.68) (χ2 = 0.088, P>0.90).
Fig. 4.
Segregation analysis of Zak ERA8 and comparison of F2 and F3 populations. Germination index (GI) of a F2 plants based on germination of F3 seeds derived from individual F2 plants and b parental Zak ERA8 (black bar) and Zak wild type (white bars) parent lines from the same experiment. Black arrow indicates mean GI for Zak ERA8 parent lines in F2 and F3 experiments (0.02 and 0, respectively). White arrows indicate means for Zak wild type in the F2 and F3 experiments (0.62 and 0.65, respectively). Gray arrow indicates overall population means for both F2 and F3 seeds (0.29 and 0.32, respectively). Intact seeds after-ripened for 6 weeks were treated with 1 µM (+)-ABA and scored over 5 days in both the F2 and F3 experiments
Determination of length of time to after-ripen for Zak mutants
Zak ERA8 and 19A have reduced germination in the absence of ABA (Figs. 2b, 3). To determine if these mutations cause an increase in the time required to after-ripen, and to examine changes in ABA sensitivity over time, mutants and Zak wild type were monitored for germination in the presence and absence of ABA over an 18-week period of dry after-ripening. This experiment allows us to test if the observed increases in dormancy and ABA sensitivity in these mutants can be overcome by after-ripening, and to determine if the increased dormancy in these mutants is temporary.
Germination of both embryo half (Fig. 5a) and intact (Fig. 5b) seeds was examined in this experiment. Germination of embryo half seeds is expected to be more efficient than germination of intact seeds, both with and without ABA, especially when seeds are less after-ripened. As half seeds, the two mutants show only a slight reduction in germination at 6 weeks of after-ripening compared with Zak wild type in the absence of ABA, a difference that rapidly disappears as after-ripening proceeds (Fig. 5a). However, treatment of cut seeds with 5 µM ABA reduces germination of both mutants at the first two time points examined, weeks 6 and 7. In particular, at week 6, both mutants have significantly reduced germination compared with Zak wild type (P < 0.01 for both, Online Resource 5). This difference is also quickly reduced with further after-ripening.
Fig. 5.
Germination index (GI) of Zak ERA mutants and wild type over an after-ripening time course. Seeds of Zak ERA8, Zak ERA19A (M6 generation), and Zak wild type were plated as a cut and b whole seeds over an 18-week after-ripening period on either 5 µM (+)-ABA in MES or MES buffer without any hormone added. Error bars represent standard errors of three replicates of 10 seeds each
As observed in prior experiments (Figs. 2b, 3), both mutants have significantly reduced germination as whole seeds even in the absence of ABA (Fig. 5b, Online Resource 5), although the difference between the mutants and wild type is much reduced by 10 weeks of after-ripening. As with cut seeds, treatment with 5 µM ABA inhibits germination of both mutants more than it does the wild type. This inhibition disappears for Zak ERA19A by 10 weeks in both cut and whole seeds. For whole seeds of Zak ERA8, however, inhibition of germination by ABA persists at least to 10 weeks of after-ripening. By week 18, 5 µM ABA is no longer enough to inhibit germination of either mutant, either as cut or whole seeds (Fig. 5a, b), but sensitivity to higher concentrations of ABA after longer durations of after-ripening (3 years) has been observed for both mutants in other experiments (Table 1).
Because this experiment examined seeds already partially after-ripened, germination behavior of half seeds of Zak ERA8 and Zak from a different seed lot only after-ripened for 10 days was also tested. In previous experiments, half seeds of Zak ERA8 incubated in the absence of ABA showed little difference from Zak wild type (Figs. 2a, 5a). However, seeds of Zak ERA8 after-ripened for 10 days only reached 1.7 % germination (GI = 0.01) after 5 days of incubation on 5 mM MES without ABA, compared with Zak, which reached 86.7 % germination (GI = 0.54). Therefore, Zak ERA8 appears to have embryo dormancy that persists for at least 10 days after maturity.
Characterization of final plant height, yield, and flowering time for Zak mutants
Zak mutants were grown in the greenhouse to determine final plant height relative to Zak wild-type control plants. Zak ERA19A was divided into two distinct subtypes for analysis. Of the four original M3 plants that gave rise to this mutant, descendants of one plant are consistently shorter. Therefore, the progeny of that plant will be referred to as the dwarf subtype of 19A. Plants descended from the other three M3 plants will be referred to as the tall subtype of 19A. Only the dwarf subtype of Zak ERA19A was significantly shorter than Zak wild type (P < 0.001). The remaining mutants appear to not have a major effect on plant height (Table 3).
Table 3.
Final plant heights of M4 Zak ERA mutants and Zak wild type
| Genotype | Height (cm) | Ratioa | P valueb | nc |
|---|---|---|---|---|
| Zak | 78.1 | – | – | 11 |
| ERA8 | 77.5 | 1.0 | NS | 24 |
| ERA19A (tall) | 80.8 | 1.0 | NS | 29 |
| ERA19A (dwarf) | 68.6 | 0.9 | <0.001 | 7 |
| ERA19B | 76.7 | 1.08 | NS | 21 |
NS not significant
Height of each mutant relative to Zak wild type
P values refer to significance determined by analysis of variance using the Dunnett’s multiple comparison adjustment
Number of plants
To determine if the mutation affecting height in the dwarf subtype of Zak ERA19A affects germination, M6 seeds of both tall and dwarf Zak ERA19A plants were tested for response to ABA in germination. M5 plants were grouped according to the M3 plant it was derived from. In this experiment, M3 plants 1 and 2 (19A.1 and 19A.2) represent the tall subtype, and plant 3 (19A.3) represents the dwarf subtype. When half seeds after-ripened for 5 weeks were treated with 5 µM ABA, both tall and dwarf plants had similar germination after 48 h of imbibition, and all were more inhibited by ABA in germination than Zak wild type (Online Resource 6). No correlation exists between height and germination capacity in this population. Therefore, it is likely that the mutation causing dwarfism in the dwarf subtype of Zak ERA19A is independent of the mutation causing ABA hypersensitivity in germination, and appears to have no effect on germination, as dwarf plants show no additional ABA sensitivity compared to taller Zak ERA19A plants.
Mutants were also evaluated in two growth room experiments for altered flowering time and yield compared with Zak wild type. M5 plants were evaluated for flowering time in the first experiment. In this experiment, all three mutants showed a significant delay in the length of time from planting until the first head emerged of approximately 2–3 days (Table 4). Mutants were grown in a separate experiment to determine if grain yield was affected. Zak ERA19A and 19B both had significantly reduced grain yield compared with Zak wild type (Table 5), while Zak ERA8 did not differ significantly from Zak wild type. However, the Zak ERA19A and 19B plants evaluated were M6 plants that had not yet been backcrossed to Zak wild type, so these plants may have had additional mutations that negatively impacted yield.
Table 4.
Time required to flower for M5 Zak ERA mutants and Zak wild type grown in the greenhouse
| Genotype | Days to first head emergencea | P valueb | nc |
|---|---|---|---|
| Zak | 50.7 | – | 32 |
| ERA8 | 52.7 | <0.001 | 36 |
| ERA19A | 53.6 | <0.001 | 30 |
| ERA19B | 53.3 | <0.05 | 8 |
Number of days from planting until the first head was fully emerged
P values refer to significance determined by analysis of variance using the Dunnett’s multiple comparison adjustment
Number of plants scored for head emergence
Table 5.
Grain yield in grams per plant of ZakERA mutants and Zak wild type plants grown in a growth room
| Genotype | Grain yield (g)a | P valueb | nc | Generation |
|---|---|---|---|---|
| Zak | 22.5 | – | 31 | – |
| ERA8 | 21.8 | NS | 30 | BC1F3 |
| ERA19A | 14.9 | <0.001 | 30 | M6 |
| ERA19B | 16.8 | <0.001 | 29 | M6 |
Per plant
P values refer to significance determined by analysis of variance using the Dunnett’s multiple comparison adjustment
Number of plants
Discussion
Isolation of ZakERA mutants with increased dormancy and sensitivity to ABA during seed germination
This paper describes the isolation of ABA-hypersensitive mutants in soft white spring Zak. This approach was undertaken to determine if ABA-hypersensitive mutations could be used to increase the seed dormancy of white wheat, because higher seed dormancy is associated with higher preharvest sprouting tolerance (DePauw and McCaig 1991; Gerjets et al. 2010). Wheat varieties with red kernel color tend to have higher grain dormancy than those with white kernels (Flintham 2000; Warner et al. 2000). The increased ABA sensitivity in Zak ERA lines appears to result in increased grain dormancy in soft white kernels of Zak (Figs. 2, 3). This is consistent with previous research showing that high grain dormancy in the soft white winter ‘Brevor’ is correlated with higher ABA sensitivity (Walker-Simmons 1987). The fact that soft white wheat mutants with increased ABA sensitivity have higher grain dormancy, while hard red mutants with decreased ABA sensitivity have lower grain dormancy suggests that ABA control of grain dormancy is independent of kernel/ testa pigmentation (Fig. 3; Rikiishi and Maekawa 2010; Schramm et al. 2012). The higher dormancy of red wheat is associated with higher ABA sensitivity, suggesting that red testa color and ABA signaling act in parallel and have synergistic effects on seed dormancy (Himi et al. 2002; Schramm et al. 2012). Unlike dicots, the cereals wheat and barley gradually lose ABA sensitivity with after-ripening (Millar et al. 2006; Barrero et al. 2009; Schramm et al. 2010). The increased ABA sensitivity in Zak ERA mutants slows but does not eliminate the loss of ABA sensitivity with after-ripening (Fig. 5). However, all three Zak ERA mutants retained the some ability to respond to ABA after 3 years of dry after-ripening, albeit at high ABA concentrations (Table 1). Future research will need to further examine the mechanisms by which cereals lose ABA sensitivity with after-ripening.
Previous work suggested that Triticum aestivum is essentially an “ABA-insensitive mutant” due to the mis-splicing of the B3 transcription factor TaVP1 (VIVIPA-ROUS1) transcript (McKibbin et al. 2002). Loss of function mutations in the maize and Arabidopsis homologues, ZmVP1 and ABI3, respectively, result in an ABA-insensitive phenotype (reviewed by Finkelstein et al. 2008). If all wheat lacked VP1 function, then ABA-hypersensitive mutants of wheat would essentially be suppressors of the lack of vp1 function. Although subsequent research has found cultivar-dependent variation in the accumulation of correctly spliced TaVP1 transcript (Yang et al. 2007; Utsugi et al. 2008), TaVP1 mis-splicing provides a possible explanation for the observation that some wheat varieties only respond to ABA concentrations higher than physiological levels (25–50 µM ABA, Nyachiro et al. 2002a). Seeds requiring such high ABA concentrations to block germination would be regarded as ABA insensitive in other plant species. The fact that some wheat cultivars respond to physiologically relevant ABA concentrations, 1–5 µM, indicates that wheat is not innately “ABA insensitive” (Fig. 3; Walker-Simmons 1987; Schramm et al. 2010, 2012; Rikiishi and Maekawa 2010).
Genetic analysis of the Zak ERA mutants
This study and others have proven that it is possible to recover ABA signaling mutants in allohexaploid wheat via a forward genetics approach (Kawakami et al. 1997; Kobayashi et al. 2008; Rikiishi and Maekawa 2010; Schramm et al. 2010, 2012). Crosses of Zak ERA8 and Zak ERA19B to wild-type Zak were used to reduce the number of background mutations and to perform segregation analysis. Analysis of the F2 generation following backcross of Zak ERA19B to wild type suggested that this mutant may be semi-dominant (Table 2). However, it was difficult to reach a strong conclusion due to the fact that the Zak ERA19B phenotype is variable and weak. In contrast, Zak ERA8 shows good penetrance and was clearly defined as a semi-dominant trait in the F2 generation. When F2 segregation analysis is performed using F3 progeny to represent F2 individuals, then the F2 plants appear to segregate 1:2:1 (Fig. 4), confirming the interpretation that Zak ERA8 is a semi-dominant mutation. All of the ABA response mutants isolated in wheat thus far are either dominant or semi-dominant, suggesting it may only be possible to recover loss of function ABA signaling mutations in wheat by combining recessive mutations on all three homoeologues recovered by reverse genetics (i.e. TILLING, see Slade et al. 2005; Uauy et al. 2009).
The ABA-hypersensitive mutants isolated in this study may represent mutations in two or three genes. Zak ERA19A and Zak ERA19B are derived from the same M1 plant and could potentially be two isolates of the same allele. Zak ERA8 is an independent mutation event derived from another M1 plant. Dose–response analysis showed that although all three lines are significantly more sensitive to ABA than wild-type Zak, Zak ERA8 has the strongest phenotype (Fig. 2). After 3 weeks of after-ripening, Zak ERA8 appeared much more dormant than wild type when germinated as whole grain in the absence of ABA (Fig. 3). However, when the grains were cut, Zak ERA8 germinated nearly as efficiently as wild type (Fig. 2a). This indicates that the dormancy in the Zak ERA8 mutant is seed coat-dependent at this stage of after-ripening. However, Zak ERA8 does exhibit embryo dormancy at very early stages of after-ripening (10 days past physiological maturity). Zak ERA19A is less dormant than Zak ERA8, but more dormant than wild-type Zak (Figs. 2, 5). The increase in dormancy levels in Zak ERA19A is both less extreme and less consistent between seed lots than that of Zak ERA8.
Genetic background and testa color may impact the ABA-hypersensitive phenotype
The white-kernelled Zak ERA mutants show both a stronger and more persistent ABA-hypersensitive germination phenotype than those previously characterized in red wheat backgrounds (Schramm et al. 2010; Kobayashi et al. 2008). We suspect this is due to differences in the genetic background, but cannot rule out that it is due to differences in the nature of the ABA-hypersensitive mutants. ABA-hypersensitive mutants in Chinese Spring termed Warm (Wheat ABA-Responsive Mutant) were isolated based on the failure to germinate on 5 µM ABA after long dry after-ripening (Schramm et al. 2010). Because of prolonged dormancy in the Chinese Spring background, scoring of the Warm phenotype could only be done when the seeds had after-ripened sufficiently to allow germination of wild type (4–8 weeks), but before mutants lost ABA sensitivity (10–18 weeks). The duration of after-ripening required to reach this point varied from seed lot to seed lot. In contrast, Zak ERA mutants show a difference in germination on lower concentrations of ABA compared with wild type within 3 weeks of dry after-ripening (Fig. 2a), and Zak ERA8 maintains a pronounced difference on 5 µM ABA until at least 10 weeks of dry after-ripening (Fig. 5b).
The Zak ERA mutants not only have a lower baseline level of dormancy contributed by the parent background, but also appear to have more consistency between seed lots in terms of level of dormancy. The Warm mutants were associated with increased dormancy and ABA hypersensitivity, but also were difficult to score due to inconsistency in dormancy between seed lots and the short and variable period during which it is possible to see the difference between mutant and wild type. Chinese Spring appears to have both embryo and seed-coat imposed dormancy which may persist several weeks after physiological maturity, whereas Zak appears to have primarily seed coat-imposed dormancy that subsided by 3 weeks of after-ripening (Fig. 3). This difference in the nature of the parent seed dormancy likely had an impact on the phenotype of the ABA-hypersensitive mutants. For example, the two strongest Warm mutants, Warm1 and Warm4, display embryo dormancy such that cutting the seed coat can only partly rescue germination at 6 weeks of after-ripening, reaching a final germination of 43 and 53 %, respectively (Schramm et al. 2010). In contrast, the germination of Zak ERA mutants can be fully rescued (95–100 % germination) by cutting the seed coat within three to 6 weeks of after-ripening (Figs. 2a, 5a), depending on the specific seed lot. Zak ERA8 has the strongest ABA-hypersensitive phenotype, but is still able to germinate fairly efficiently in the absence of ABA within approximately 2 months of after-ripening (Figs. 2b, 5b). Thus, Zak ERA8 and Zak ERA19A have primarily seed coat-based dormancy, whereas the Warm1 and Warm4 mutants have some embryo dormancy contributing to their inconsistent germination behavior.
It is interesting that, at least in this case, the red testa is associated with greater embryo dormancy as opposed to greater seed coat-imposed dormancy. One explanation for the fact that ABA-hypersensitive mutants in Chinese Spring are more difficult to score than Zak ERA mutants may be that Chinese Spring has a single gene providing red testa color whereas Zak has a white testa. The red pigmentation results in higher seed dormancy, but it also appears to result in variability in the degree of dormancy. This variability results in variability in ABA sensitivity since ABA sensitivity is tightly linked to degree of dormancy in wheat (Morris et al. 1989; Walker-Simmons 1987). An alternative explanation may be that only stronger mutations can be recovered in a white testa background, making it more likely that stronger mutants will be isolated by screening mutagenized white than red wheats. Future work will need to explore the interaction between testa color and ABA response either by determining whether the expressivity of Warm1 and Warm4 improves when crossed into a white background or by determining if the Zak ERA ABA-hypersensitive phenotypes are more difficult to score in a red background.
Molecular mechanisms for increasing ABA sensitivity
An apparent increase in sensitivity to applied ABA can result from three molecular mechanisms: a loss-of-function mutation resulting in decreased ABA turnover, a loss-of-function mutation in a negative regulator of ABA response, and a gain-of-function mutation in a positive regulator of ABA response (Cutler et al. 1996; Ghassemian et al. 2000). For example, reduced expression or function of the ABA catabolic gene, ABA 8′-hydroxylase, (CYP707A), in Arabidopsis or in barley results in increased seed dormancy as a result of increased endogenous ABA accumulation (Millar et al. 2006; Okamoto et al. 2006). Loss-of-function mutations in negative regulators of ABA signaling, such as ERA1, ABH1, and ABI1, result in increased sensitivity to ABA in Arabidopsis seed germination (Cutler et al. 1996; Gosti et al. 1999; Hugouvieux et al. 2001). The alleles identified in this study are unusual in that ABA hypersensitivity appears to result from gain-of-function mutations. The only dominant Arabidopsis mutant with ABA-hypersensitive seed germination is hat1 (harmattan tolerant1), a mutation conferring resistance to hot, dry air (Yan et al. 2006). Another possibility is that gain-of-function mutations in positive regulators of ABA signaling such as the ABA receptor PYR/RCAR, the protein kinase PKABA1, or the ABF (ABA BINDING FACTOR) transcriptional activator might result in an ABA-hypersensitive phenotype (Gómez-Cadenas et al. 1999; Johnson et al. 2002; Park et al. 2009).
Utility of ABA response mutants in wheat
Analysis of ABA response mutants of wheat offers both the opportunity to elucidate the hormonal mechanisms controlling seed dormancy and to breed preharvest sprouting tolerant wheat. Control of ABA and gibberellin (GA) signaling alleles hold the promise of developing wheat plants that have sufficient dormancy to avoid sprouting but that after-ripen quickly enough to avoid problems with emergence. The Zak ERA8 allele recovered in this study is an excellent candidate for this purpose since it after-ripens sufficiently to show efficient germination at 30 °C within 6–7 weeks of after-ripening (Fig. 5b). Since higher temperatures tend to result in higher grain dormancy, germination efficiency may be even higher in locations where soil temperatures are lower at planting (Nyachiro et al. 2002b). Since spring wheat grain is stored over the winter, Zak ERA8 should be sufficiently after-ripened to germinate well for spring planting. This is unlike the sprouting-resistant cultivar Brevor which requires 6 months of after-ripening to germinate efficiently (Online Resource 1). Future research will develop a molecular marker for ERA8 to facilitate breeding this allele into spring wheat. This allele could be particularly useful to breeding broadly adapted hard white wheat varieties that are PHS tolerant in a wider range of environments.
Acknowledgments
Thanks are due to S. Abrams for providing (+)-ABA, to R. Parveen and E. Getzin for expert assistance, and to K. Garland-Campbell for advice and assistance. The authors wish to thank members of the Campbell and Steber labs for helpful comments on the research and manuscript. This work was funded by an NIH protein biotechnology training grant (to ECS), by the Washington Grain Alliance (to CMS), and by USDA CSREES grant 2005-01099 (to C.M.S.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00122-012-2018-0) contains supplementary material, which is available to authorized users.
Contributor Information
Elizabeth C. Schramm, Department of Crop and Soil Sciences, Washington State, University, Pullman, WA 99164-6420, USA Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA.
Sven K. Nelson, Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA
Kimberlee K. Kidwell, Department of Crop and Soil Sciences, Washington State, University, Pullman, WA 99164-6420, USA
Camille M. Steber, Department of Crop and Soil Sciences, Washington State, University, Pullman, WA 99164-6420, USA, csteber@wsu.edu, URL: http://public.wsu.edu/~csteber/ Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA; USDA-ARS, Wheat Genetics, Physiology, Biochemistry, and Quality Unit, Pullman, WA 99164-6420, USA.
References
- Barrero J, Talbot MJ, White RG, Jacobsen JV, Gubler F. Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Phys. 2009;150:1006–1021. doi: 10.1104/pp.109.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. New York: Plenum Press; 1994. [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- DePauw RM, McCaig TN. Components of variation, heritabilities and correlations of indices of sprouting tolerance and seed dormancy in Triticum spp. Euphytica. 1991;52:221–229. [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Flintham JE. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci Res. 2000;10:43–50. [Google Scholar]
- Flintham JE, Adlam R, Bassoi M, Holdsworth M, Gale M. Mapping genes for resistance to sprouting damage in wheat. Euphytica. 2002;126:39–45. [Google Scholar]
- Fofana B, Humphreys DG, Rasul G, Cloutier S, Brûlé-Babel A, Woods S, Lukow OM, Somers DJ. Mapping quantative trait loci controlling pre-harvest sprouting resistance in a red × white seeded spring wheat cross. Euphytica. 2009;165:509–521. [Google Scholar]
- Gerjets T, Scholefield D, Foulkes MJ, Lenton JR, Holdsworth MJ. An analysis of dormancy, ABA responsiveness, after-ripening and pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.) caryopses. J Exp Bot. 2010;61(2):597–607. doi: 10.1093/jxb/erp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho T-hD, Walker-Simmons MK. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. P Natl Acad Sci USA. 1999;96:1767–1772. doi: 10.1073/pnas.96.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell. 1999;11:1897–1909. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos C, Gay G, Perretant M-R, Gervais L, Bernard M, Dedryver F, Charmet G. Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor Appl Genet. 2002;104:39–47. doi: 10.1007/s001220200004. [DOI] [PubMed] [Google Scholar]
- Himi E, Mares DJ, Yanagisawa A, Noda K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J Exp Bot. 2002;53(374):1569–1574. doi: 10.1093/jxb/erf005. [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Imtiaz M, Ogbonnaya FC, Oman J, van Ginkel M. Characterization of quantitative trait loci controlling genetic variation for preharvest sprouting in synthetic backcross-derived wheat lines. Genetics. 2008;178:1725–1736. doi: 10.1534/genetics.107.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RR, Wagner RL, Verhey SD, Walker-Simmons MK. The abscisic acid-responsive kinase PKABA1 interacts with a seed-specific abscisic acid response element-binding factor, TaABF, and phosphorylates TaABF peptide sequences. Plant Phys. 2002;130:837–846. doi: 10.1104/pp.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen CM, der Brinkhorst-van Swan DLC, Breekland AE, Koornneef M. Induction of dormancy during seed development by endogenous abscisic acid: studies in abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta. 1983;157:158–165. doi: 10.1007/BF00393650. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Miyake Y, Noda K. ABA insensitivity and low ABA levels during seed development of non-dormant wheat mutants. J Exp Bot. 1997;48(312):1415–1421. [Google Scholar]
- Kidwell KK, Shelton GB, DeMacon VL, Morris CF, Engle DA, Burns JW, Line RF, Konzak CF, Hatchett JH. Registration of ‘Zak’ wheat. Crop Sci. 2002;42:661–662. [Google Scholar]
- King RW. Water uptake in relation to pre-harvest sprouting damage in wheat: grain characteristics. Aust J Agr Res. 1984;35:337–345. [Google Scholar]
- King RW, Richards RA. Water uptake in relation to pre-harvest sprouting damage in wheat: ear characteristics. Aust J Agr Res. 1984;35:327–336. [Google Scholar]
- King RW, von Wettstein-Knowles P. Epicuticular waxes and regulation of ear wetting and pre-harvest sprouting in barley and wheat. Euphytica. 2000;112:157–166. [Google Scholar]
- Kobayashi F, Takumi S, Nakamura C. Increased freezing tolerance in an ABA-hypersensitive mutant of common wheat. J Plant Physiol. 2008;165:224–232. doi: 10.1016/j.jplph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Liu S, Cai S, Graybosch R, Chen C, Bai G. Quantitative trait loci for resistance to pre-harvest sprouting in US hard white winter wheat Rio Blanco. Theor Appl Genet. 2008;117:691–699. doi: 10.1007/s00122-008-0810-7. [DOI] [PubMed] [Google Scholar]
- Liu S, Bai G, Cai S, Chen C. Dissection of genetic components of preharvest sprouting resistance in white wheat. Mol Breeding. 2011;27:511–523. [Google Scholar]
- Mares DJ, Mrva K. Mapping quantitative trait loci associated with variation in grain dormancy in Australian wheat. Aust J Agr Res. 2001;52:1257–1265. [Google Scholar]
- McKibbin RS, Wilkinson MD, Bailey PC, Flintham JE, Andrew LM, Lazzeri PA, Gale MD, Lenton JR, Holdsworth MJ. Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. P Natl Acad Sci USA. 2002;99:10203–10208. doi: 10.1073/pnas.152318599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- Molina-Cano JL, Sopena A, Swanston JS, Casas AM, Moralejo MA, Ubieto A, Lara I, Pérez-Vendrell AM, Romagosa I. A mutant induced in the malting barley cv Triumph with reduced dormancy and ABA response. Theor Appl Genet. 1999;98:347–355. [Google Scholar]
- Morris CF, Moffatt JM, Sears RG, Paulsen GM. Seed dormancy and responses of caryopses, embryos, and calli to abscisic acid in wheat. Plant Physiol. 1989;90:643–647. doi: 10.1104/pp.90.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkvold JD, Tanaka J, Benscher D, Sorrells ME. Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor Appl Genet. 2009;119(7):1223–1235. doi: 10.1007/s00122-009-1123-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Abe F, Kawahigashi H, Nakazono K, Tagiri A, Matsumoto T, Utsugi S, Ogawa T, Handa H, Ishida H, Mori M, Kawaura K, Ogihara Y, Miura H. A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell. 2011;23:3215–3229. doi: 10.1105/tpc.111.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Nyachiro JM, Clarke FR, DePauw RM, Knox RE, Armstrong KC. The effects of cis-trans ABA on embryo germination and seed dormancy in wheat. Euphytica. 2002a;126:129–133. [Google Scholar]
- Nyachiro JM, Clarke FR, DePauw RM, Knox RE, Armstrong KC. Temperature effects on seed germination and expression of seed dormancy in wheat. Euphytica. 2002b;126:123–127. [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensible for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow T-fF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu J-K, Schroeder JI, Volkman BF, Cutler SR. Abscisic acid inhibits type 2C protein phosphatases via the PYR/ PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikiishi K, Maekawa M. Characterization of a novel wheat (Triticum aestivum L.) mutant with reduced seed dormancy. J Cereal Sci. 2010;51:292–298. [Google Scholar]
- Roy JK, Prasad M, Varshney RK, Balyan HS, Blake TK, Dhaliwal HS, Singh H, Edwards KJ, Gupta PK. Identification of a microsatellite on chromosomes 6B and a STS on 7D of bread wheat showing an association with preharvest sprouting tolerance. Theor Appl Genet. 1999;99:336–340. [Google Scholar]
- Schramm EC, Abellera JC, Strader LC, Campbell KG, Steber CM. Isolation of ABA-responsive mutants in allohexaploid bread wheat (Triticum aestivum L.): drawing connections to grain dormancy, preharvest sprouting, and drought tolerance. Plant Sci. 2010;179:620–629. [Google Scholar]
- Schramm EC, Nelson SK, Steber CM. Wheat ABA-insensitive mutants result in reduced grain dormancy. Euphytica. 2012;188:35–49. doi: 10.1007/s10681-012-0669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Matus-Cádiz M, Båga M, Hucl P, Chibbar RN. Identification of genomic regions associated with seed dormancy in white-grained wheat. Euphytica. 2010;174:391–408. [Google Scholar]
- Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D. A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol. 2005;23:75–81. doi: 10.1038/nbt1043. [DOI] [PubMed] [Google Scholar]
- Torada A, Amano Y. Effect of seed coat color on seed dormancy in different environments. Euphytica. 2002;126:99–105. [Google Scholar]
- Uauy C, Paraiso F, Colasuonno P, Tran RK, Tsai H, Berardi S, Comai L, Dubcovsky J. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 2009;9:115. doi: 10.1186/1471-2229-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi S, Nakamura S, Noda K, Maekawa M. Structural and functional properties of Viviparous1 genes in dormant wheat. Genes Genet Syst. 2008;83:153–166. doi: 10.1266/ggs.83.153. [DOI] [PubMed] [Google Scholar]
- Visser K, Vissers APA, Cagirgan MI, Kijne JW, Wang M. Rapid germination of a barley mutant is correlated with a rapid turnover of abscisic acid outside the embryo. Plant Physiol. 1996;111:1127–1133. doi: 10.1104/pp.111.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 1987;84:61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Kudrna DA, Spaeth SC, Jones SS. Dormancy in white-grain mutants of Chinese Spring wheat (Triticum aestivum L.) Seed Sci Res. 2000;10:51–60. [Google Scholar]
- Yan C, Shen H, Li Q, He Z. A novel ABA-supersensitive mutant in Arabidopsis defines a genetic locus that confers tolerance to xerothermic stress. Planta. 2006;224:889–899. doi: 10.1007/s00425-006-0272-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma YZ, Xu ZS, Chen X, He ZH, Yu Z, Wilkinson M, Jones HD, Shewry PR, Xia LQ. Isolation and characterization of Viviparous-1 genes in wheat cultivars with distinct ABA sensitivity and pre-harvest sprouting tolerance. J Exp Bot. 2007;58:2863–2871. doi: 10.1093/jxb/erm073. [DOI] [PubMed] [Google Scholar]
- Zanetti S, Winzeler M, Keller B, Messmer M. Genetic analysis of pre-harvest sprouting in a wheat × spelt cross. Crop Sci. 2000;40:1406–1417. [Google Scholar]