Abstract
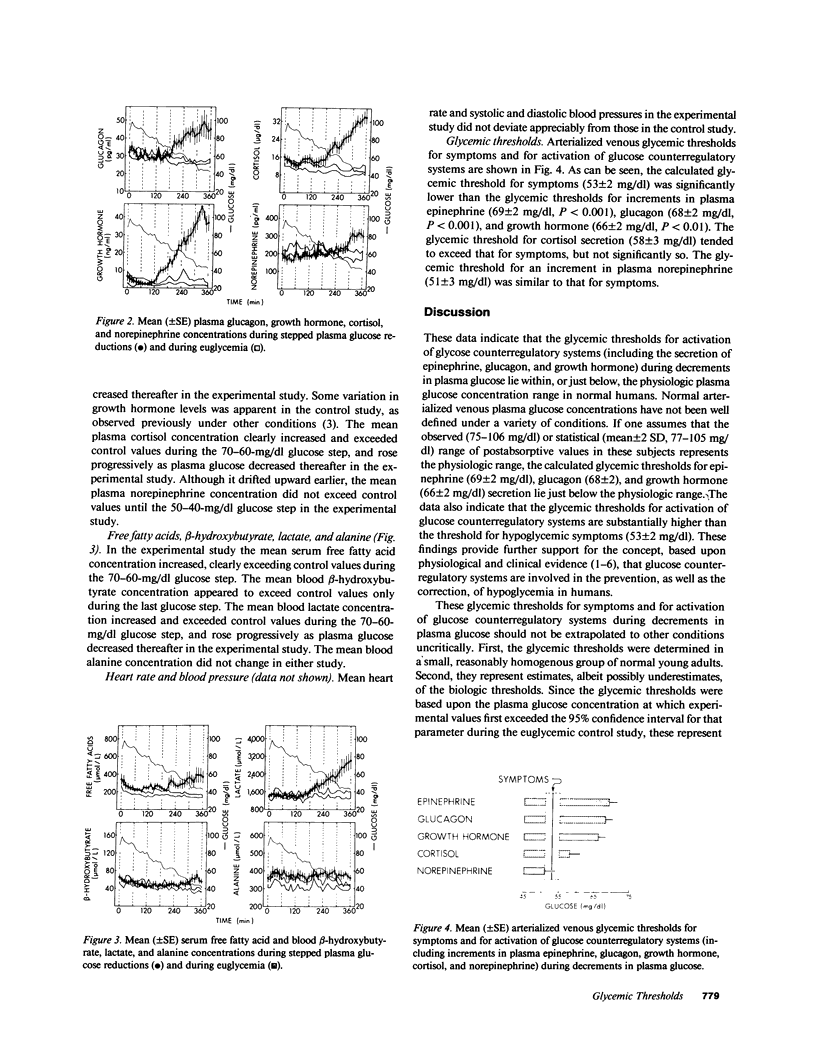
To define glycemic thresholds for activation of glucose counterregulatory systems and for symptoms of hypoglycemia, we measured these during stepped reductions in the plasma glucose concentration (in six 10-mg/dl hourly steps) from 90 to 40 mg/dl under hyperinsulinemic clamp conditions, and compared these with the same measurements during euglycemia (90 mg/dl) under the same conditions over 6 h in 10 normal humans. Arterialized venous plasma glucose concentrations were used to calculate glycemic thresholds of 69 +/- 2 mg/dl for epinephrine secretion, 68 +/- 2 mg/dl for glucagon secretion, 66 +/- 2 mg/dl for growth hormone secretion, and 58 +/- 3 mg/dl for cortisol secretion. In contrast, the glycemic threshold for symptoms was 53 +/- 2 mg/dl, significantly lower than the thresholds for epinephrine (P less than 0.001), glucagon (P less than 0.001), and growth hormone (P less than 0.01) secretion. Thus, the glycemic thresholds for activation of glucose counterregulatory systems during decrements in plasma glucose lie within or just below the physiologic plasma glucose concentration range, and are substantially higher than the threshold for hypoglycemic symptoms in normal humans. These findings provide further support for the concept that glucose counterregulatory systems are involved in the prevention, as well as the correction, of hypoglycemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. A., Roddie I. C. The role of circulating catecholamines in sweat production in man. J Physiol. 1972 Dec;227(3):801–814. doi: 10.1113/jphysiol.1972.sp010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M. A., Clutter W. E., Skor D., Shah S. D., Gingerich R. P., Parvin C. A., Cryer P. E. Enhanced glycemic responsiveness to epinephrine in insulin-dependent diabetes mellitus is the result of the inability to secrete insulin. Augmented insulin secretion normally limits the glycemic, but not the lipolytic or ketogenic, response to epinephrine in humans. J Clin Invest. 1985 Jun;75(6):1842–1851. doi: 10.1172/JCI111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G., De Feo P., Perriello G., De Cosmo S., Ventura M., Campbell P., Brunetti P., Gerich J. E. Role of hepatic autoregulation in defense against hypoglycemia in humans. J Clin Invest. 1985 May;75(5):1623–1631. doi: 10.1172/JCI111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke W. L., Santiago J. V., Thomas L., Ben-Galim E., Haymond M. W., Cryer P. E. Adrenergic mechanisms in recovery from hypoglycemia in man: adrenergic blockade. Am J Physiol. 1979 Feb;236(2):E147–E152. doi: 10.1152/ajpendo.1979.236.2.E147. [DOI] [PubMed] [Google Scholar]
- Corrall R. J., Frier B. M., Davidson N. M., Hopkins W. M., French E. B. Cholinergic manifestations of the acute autonomic reaction to hypoglycaemia in man. Clin Sci (Lond) 1983 Jan;64(1):49–53. doi: 10.1042/cs0640049. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Gerich J. E. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med. 1985 Jul 25;313(4):232–241. doi: 10.1056/NEJM198507253130405. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., White N. H., Santiago J. V. The relevance of glucose counterregulatory systems to patients with insulin-dependent diabetes mellitus. Endocr Rev. 1986 May;7(2):131–139. doi: 10.1210/edrv-7-2-131. [DOI] [PubMed] [Google Scholar]
- Doberne L., Greenfield M. S., Schulz B., Reaven G. M. Enhanced glucose utilization during prolonged glucose clamp studies. Diabetes. 1981 Oct;30(10):829–835. doi: 10.2337/diab.30.10.829. [DOI] [PubMed] [Google Scholar]
- Fagius J., Niklasson F., Berne C. Sympathetic outflow in human muscle nerves increases during hypoglycemia. Diabetes. 1986 Oct;35(10):1124–1129. doi: 10.2337/diab.35.10.1124. [DOI] [PubMed] [Google Scholar]
- Farmer R. W., Pierce C. E. Plasma cortisol determination: radioimmunoassay and competitive protein binding compared. Clin Chem. 1974 Apr;20(4):411–414. [PubMed] [Google Scholar]
- Foster K. G., Ginsburg J., Weiner J. S. Role of circulating catecholamines in human eccrine sweat gland control. Clin Sci. 1970 Dec;39(6):823–832. doi: 10.1042/cs0390823. [DOI] [PubMed] [Google Scholar]
- Gerich J., Davis J., Lorenzi M., Rizza R., Bohannon N., Karam J., Lewis S., Kaplan R., Schultz T., Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979 Apr;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton S. M., Morgan L. M., Tredger J. A., Cramb R., Marks V. Insulin and C-peptide levels after oral and intravenous glucose. Contribution of enteroinsular axis to insulin secretion. Diabetes. 1986 May;35(5):612–616. doi: 10.2337/diab.35.5.612. [DOI] [PubMed] [Google Scholar]
- Hansen I., Firth R., Haymond M., Cryer P., Rizza R. The role of autoregulation of the hepatic glucose production in man. Response to a physiologic decrement in plasma glucose. Diabetes. 1986 Feb;35(2):186–191. doi: 10.2337/diab.35.2.186. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Orth D. N., Jackson R. V., DeCherney G. S., DeBold C. R., Alexander A. N., Island D. P., Rivier J., Rivier C., Spiess J., Vale W. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. J Clin Invest. 1983 Mar;71(3):587–595. doi: 10.1172/JCI110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. G., Clutter W. E., Berk M. A., Shah S. D., Cryer P. E. Epinephrine supports the postabsorptive plasma glucose concentration and prevents hypoglycemia when glucagon secretion is deficient in man. J Clin Invest. 1984 Feb;73(2):405–411. doi: 10.1172/JCI111226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Shah S. D., Clutter W. E., Cryer P. E. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med. 1985 Dec;106(6):624–629. [PubMed] [Google Scholar]
- Shah S. D., Tse T. F., Clutter W. E., Cryer P. E. The human sympathochromaffin system. Am J Physiol. 1984 Sep;247(3 Pt 1):E380–E384. doi: 10.1152/ajpendo.1984.247.3.E380. [DOI] [PubMed] [Google Scholar]
- Tse T. F., Clutter W. E., Shah S. D., Cryer P. E. Mechanisms of postprandial glucose counterregulation in man. Physiologic roles of glucagon and epinephrine vis-a-vis insulin in the prevention of hypoglycemia late after glucose ingestion. J Clin Invest. 1983 Jul;72(1):278–286. doi: 10.1172/JCI110967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse T. F., Clutter W. E., Shah S. D., Miller J. P., Cryer P. E. Neuroendocrine responses to glucose ingestion in man. Specificity, temporal relationships, and quantitative aspects. J Clin Invest. 1983 Jul;72(1):270–277. doi: 10.1172/JCI110966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Nakai Y., Koh T., Tsujii S., Imura H. Plasma adrenocorticotropin and cortisol responses to intravenous injection of corticotropin-releasing factor in the morning and evening. J Clin Endocrinol Metab. 1983 Oct;57(4):869–871. doi: 10.1210/jcem-57-4-869. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Sundlöf G., Eriksson B. M., Dominiak P., Grobecker H., Lindblad L. E. Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand. 1981 Jan;111(1):69–73. doi: 10.1111/j.1748-1716.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- White N. H., Skor D. A., Cryer P. E., Levandoski L. A., Bier D. M., Santiago J. V. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983 Mar 3;308(9):485–491. doi: 10.1056/NEJM198303033080903. [DOI] [PubMed] [Google Scholar]




