Abstract
Responsive magnetic resonance imaging (MRI) contrast agents, those that change their relaxivity according to environmental stimuli, have promise as next generation imaging probes in medicine. While several of these are known based on covalent modification of the contrast agents, fewer are known based on controlling non-covalent interactions. We demonstrate here accentuated relaxivity of a T1-shortening contrast agent, Gd-DOTP5− based on non-covalent, hydrogen bonding of Gd-DOTP5− with a novel fluorous amphiphile. By contrast to the phosphonate-containing Gd-DOTP5− system, the relaxivity of the analogous clinically approved contrast agent, Gd-DOTA− is unaffected by the same fluorous amphiphile under similar conditions.
Mechanistic studies show that placing the fluorous amphiphile in proximity of the gadolinium center in Gd-DOTP5− caused an increase in τm (bound-water residence lifetime or the inverse of water exchange rate, τm = 1/kex) and an increase in τR (rotational correlation time), with τR being the factor driving enhanced relaxivity. Further, these effects were not observed when Gd-DOTA− was treated with the same fluorous amphiphile. Thus, Gd-DOTP5− and Gd-DOTA− respond to the fluorous amphiphile differently, presumably because the former binds to the amphiphile with higher affinity. (DOTP = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraphosphonic acid; DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid).
Keywords: MRI Contrast, T1, Fluoroakyl Groups, Gadolinium, DOTP
Introduction
Magnetic resonance imaging (MRI) contrast agents are indispensable tools in the clinical use of MRI. [1] Commercially available gadolinium(III)-based T1-shortening contrast agents are widely used in clinical applications. [2] Although these clinically approved agents provide structural information, they lack the ability to provide functional information. To overcome this limitation a class of contrast agents known as adaptive (“smart”) contrast agents has emerged. [3] This new class of agents can adjust their contrast enhancement in response to specific stimuli, including enzymes,4] metal ions,5] light,6] anions,7] pH,8] temperature,9] dioxygen,10] various biomolecules,11] or even events that trigger self-assembly. [12] Some stimuli (e.g. enzymes) covalently modify the chemical structure of the contrast agents such that the changes in contrast behavior are irreversible.
Our groups are respectively interested in the use of non-covalent molecular interactions as the basis of adaptive imaging probes and understanding of the details of the mechanisms underlying the function of adaptive MRI contrast agents. [13–15] Along these lines, we report here the first use of fluoroalkyl moieties as tools to accentuate the T1 relaxivity of a gadolinium agent (Gd-DOTP5−), based on association with a fluoroalkylguanidinium (1) species (Figure 1 and Scheme 1). [16] We show that this interaction is specific to a phosphonate-functionalized agent, because the corresponding gadolinium-containing carboxylate analog (Gd-DOTA−) is not similarly affected. Further, we find that rather than restriction of water access to the metal center, the origin of the observed relaxivity enhancement is largely attributable to the rate of tumbling of the agent in solution, while modulation of the rate of water exchange at the agent’s metal center plays only a supporting role. This finding adds new insight to the possible utility of fluoroalkyl groups in the design of adaptive MRI agents.
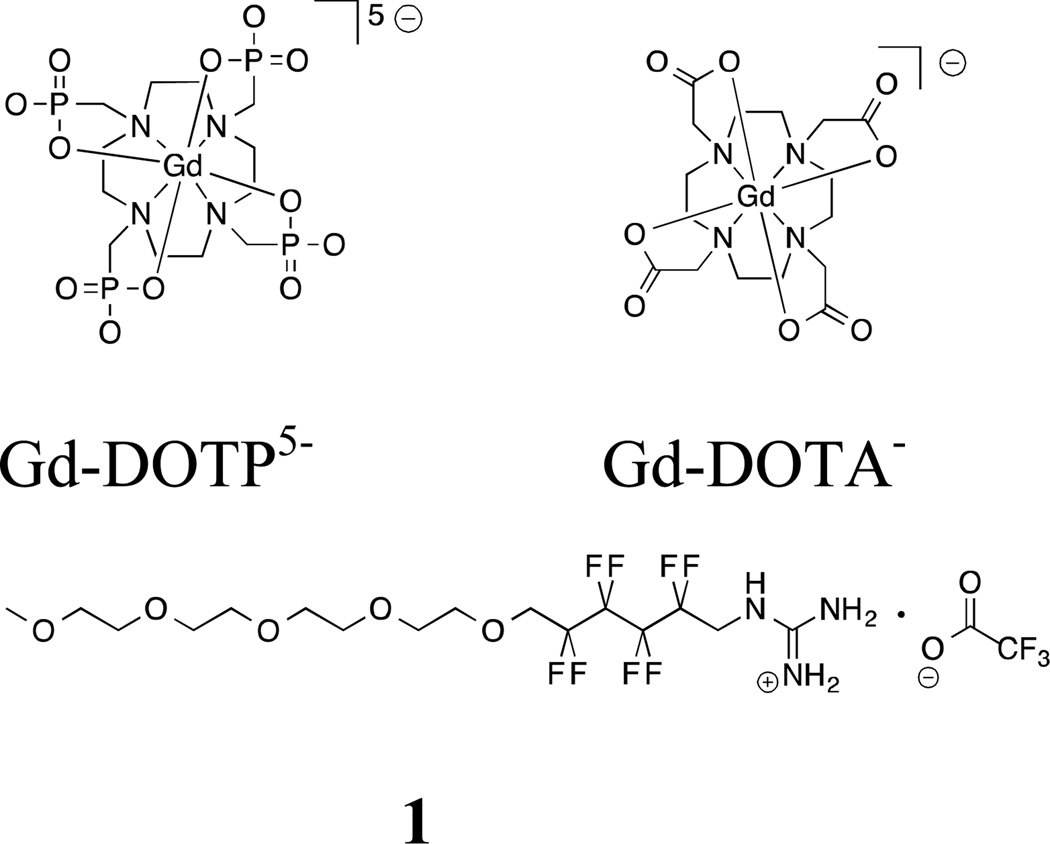
Figure 1.
Molecular structures of gadolinium-containing contrast agents Gd-DOTP5− and Gd-DOTA− and fluorous amphiphile (1).
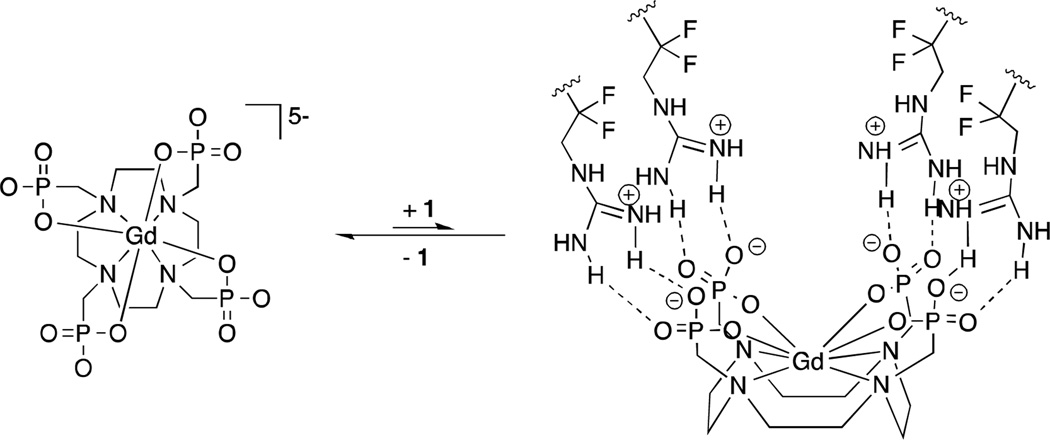
Scheme 1.
A possible interaction between Gd-DOTP5− and fluorous amphiphile 1.
Results
1. Relaxivity of Gd-DOTA− and Gd-DOTP5− Systems at 9.4 T
To determine if 1 would modulate the relaxivity of Gd-DOTP5−, we titrated an aqueous solution of Gd-DOTP5− with 0, 1, 2, 4, and 8 equiv. of 1, and we observed longitudinal relaxivity (r1) rising steadily from 3.04(16) to 3.86(22) mM−1 s−1 at 9.4 T (Table 1). We propose that the enhancement in r1 is due to association between Gd-DOTP5− and 1. Additionally, the greater charge of Gd-DOTP5− enables a stronger Columbic attraction with 1, although in water this is likely to be a minor contributor to their binding because of screening by water’s high dielectric constant. [17] The association of 1 with Gd-DOTP5− appears to be a rapid equilibrium, so we were not surprised to observe a continual increase in r1 beyond 4 equiv. of 1. Further, while we are unsure of the exact number of equivalents of 1 that are optically associated with Gd-DOTP5−, we suspect that in addition to the guanidinium-phosphate interaction, this assembly is stabilized by association of the fluorous groups of 1. We chose 1:4 Gd-DOTP5−:1 as the molar ratio for our subsequent experiments, because this ratio enables a 20% increase in r1 using only a moderate excess of 1. The pH of a sample containing 4 equiv. of 1 and 1 equiv. of Gd-DOTP5− was determined to be approximately 6.5. [18] To this solution was added a 37-fold excess of urea relative to 1 to reach a final concentration of 150 mM. Urea resembles guanidine in structure, but it has little effect on the r1 of Gd-DOTP5−. In the presence of an excess of urea we expected that 1 would be replaced by urea in the 1/Gd-DOTP5− adduct, restoring r1 to 3.04 mM−1 s−1. However, the r1 of the solution containing 1 and Gd-DOTP5− at a 4:1 molar ratio remains at 3.56(22) mM−1 s−1 after the addition of urea. The r1 of this solution was unaltered after subsequent sonication to break the hydrogen bonds (Table 2). Thus, these data suggest that 1 is not displaced by urea, and that association 1 and Gd-DOTP5− are not easily disrupted.
Table 1.
Relaxivity values of Gd-DOTP5− aqueous solution titrated with 1 at 9.4 T.
| equiv. of 1 | 0 | 1 | 2 | 4 | 8 |
|---|---|---|---|---|---|
| r1 (mM−1 s−1) | 3.04(16) | 3.22(23) | 3.52(19) | 3.67(30) | 3.86(22) |
Table 2.
Contrast agent relevant properties obtained for the Gd-DOTP5− System.
| Gd-DOTP5− | Gd-DOTP5− + 1 |
Gd-DOTP5− + 1 + urea |
Gd-DOTP5− + 1 + urea, after sonication |
|
|---|---|---|---|---|
| r1 (mM−1 s−1)a | 3.04(16) | 3.67(30) | 3.56(22) | 3.62(24) |
| r1 (mM−1 s−1)b | 3.17 | 3.65 | 3.75 | 3.75 |
| q | 0.1 | 0.1 | 0.1 | 0.1 |
| τm × 10−9 (s) | 0.80 | 1.1 | 1.8 | 1.9 |
| kex × 107 (s−1) | 13 | 9.4 | 5.5 | 5.2 |
| T1e × 10−8 (s) | 0.34 | 1.3 | 0.71 | 0.71 |
| T2e × 10−10 (s) | 2.94 | 2.97 | 2.96 | 2.96 |
| G | 1.97 | 1.96 | 1.96 | 1.96 |
| ΔHpp (G) | 227 | 225 | 225 | 225 |
| τR × 10−12 (s)c | 119 | 136 | 140 | 140 |
Acquired at 9.4 T, 25 °C.
Acquired at 1.4 T, 37 °C.
Assuming 56% second sphere and 44% outer sphere contribution. [32]
The above procedure was repeated using Gd-DOTA− with 4 equiv. of 1. We found that the r1 decreased from 2.96(6) to 2.69(21) mM−1 s−1 at 9.4 T after addition of 4 equiv. of 1 (Table 3); the addition of urea and sonication restored the r1 to 2.95(7) mM−1 s−1. Although the data imply that a non-covalent interaction occurred between 1 and Gd-DOTA−, which is disrupted by sonication, the observed error bars are too large to be conclusive.
Table 3.
Contrast agent relevant properties obtained for the Gd-DOTA− System.
| Gd-DOTA− | Gd-DOTA− + 1 |
Gd-DOTA− + 1 + urea |
Gd-DOTA− + 1 + urea, after sonication |
|
|---|---|---|---|---|
| r1 (mM−1 s−1)a | 2.97(6) | 2.69(21) | 2.67(18) | 2.95(7) |
| r1 (mM−1 s−1)b | 3.11 | 3.05 | 3.05 | 3.05 |
| Q | 0.9 | 0.9 | 0.9 | 0.9 |
| τm × 10−7 (s) | 2.4 | 2.7 | 1.7 | 1.3 |
| kex × 106 (s−1) | 4.2 | 3.7 | 5.9 | 7.7 |
| T1e × 10−7 (s) | 1.8 | 7.4 | 9.1 | 3.9 |
| T2e × 10−10 (s) | 1.44 | 1.49 | 2.68 | 1.09 |
| G | 1.98 | 1.99 | 1.98 | 1.98 |
| ΔHpp (G) | 460 | 444 | 247 | 60.6 |
| τR × 10−12 (s) | 58.7 | 57.7 | 58.5 | 58.6 |
Acquired at 9.4 T, 25 °C.
Acquired at 1.4 T, 37 °C.
2. Relaxivity of Gd-DOTP5− and Gd-DOTA− Systems at 1.4 T
The value of r1 is generally dependent on field strength; therefore, we recorded the r1 values of both Gd-DOTP5− and Gd-DOTA− systems at low field strength (1.4 T). Relaxivity (r1) for the Gd-DOTP5− system increased from 3.17(2) to 3.65(0) mM−1 s−1 after 4 equiv. of 1 was added (Table 2). Additionally, 150 mM urea and sonication could not restore r1 to 3.17 mM−1 s−1, and r1 slightly increased to 3.75(1) mM−1 s−1. Thus, the pattern observed in the measured r1 results of the Gd-DOTP5− system at low field strength (1.4 T) was similar to that obtained at high field strength (9.4 T).
At 1.4 T, the r1 of Gd-DOTA− dropped slightly after the addition of 1, from 3.11(0) to 3.05(0) mM−1 s−1. Addition of urea and sonication did not affect r1, which remained at 3.05(0) mM−1 s−1 (Table 3). Because 1 has little or no effect on the r1 of Gd-DOTA− at low field strength (1.4 T), the results suggest that 1 does not strongly interact with Gd-DOTA−.
3. Luminescence-Decay Studies
Luminescence-decay measurements enable determination of the number of water molecules coordinated to the lanthanide center. The water coordination number (q) for gadolinium(III) complexes is calculated using Horrocks’s equation and luminescence-decay measurements obtained from corresponding Eu3+ or Tb3+ complexes. Eu3+ is considered as a better fit because it is only 0.9% different from Gd3+ in atomic radius. [19,20]
Our results show that despite sequential addition of 1, addition of urea, and sonication, q remained ca. 0.9 for the Gd-DOTA− system (Table 3). Similarly, q remained ca. 0.1 for the Gd-DOTP5− system (Table 2). Gd-DOTP5− is known to have a low q value and therefore, the observed relaxation enhancement is dependent on second- and outersphere contributions.[21–23] Overall, our luminescence-decay studies indicate that proximal fluorocarbon does not influence the number of coordinated water molecules in the inner sphere for Gd-DOTP5− or Gd-DOTA−.
4. Variable-Temperature (VT) 17O-NMR Studies
Water exchange rate (kex, reciprocal of the residency lifetime of coordinated water molecules, τm−1) is usually calculated from VT 17O-NMR data. [24] Merbach and coworkers extended this method to extract τm−1 for aqueous solutions of gadolinium(III)-containing chelates. [25] To obtain τm−1, NMR linewidth at half height of the 17O-labelled bulk water peak obtained from 1% 17O-enriched aqueous solutions of Y-DOTP5− and Gd-DOTP5− are fitted to Swift and Connick equation. [26] The water exchange rates obtained for GdDOTP5− and Gd-DOTA− systems are listed in Tables 2 and 3, respectively. For Gd-DOTP5− system, adding 4 equiv. of 1 to a solution of Gd-DOTP5− caused water exchange rate to slow down from 13 to 9.5 × 107 s−1. This decrease is likely caused by non-covalent interactions between 1 and Gd-DOTP5− that disrupts the accessibility of bulk water to the gadolinium(III) center. Addition of an excess of urea decreased the water exchange rate to 5.5 × 107 s−1 likely due to a hydrogen bond network formed between 1 that encapsulates Gd-DOTP5− and urea. It is likely that the hydrogen bond network between 1 and urea and the interactions between GdDOTP5− and 1 are not disrupted by sonication, because the water exchange rates before (5.5 × 107 s−1) and after sonication (5.2 × 107 s−1) are similar.
For the Gd-DOTA− system, the water exchange rate decreased with the addition of 1, from 4.2 to 3.7 × 107 s−1. Similar to what was observed with Gd-DOTP5−, 1 caused water exchange rate to slow for Gd-DOTA−, likely because of the interaction of 1 and Gd-DOTA−. After addition of urea and sonication, the water exchange rate increased to 7.7 × 107 s−1 suggesting that urea has likely displaced 1 from Gd-DOTA−, restoring water exchange at the metal center. Water exchange rate data obtained for Gd-DOTP5− and Gd-DOTA− systems suggest that the Gd-DOTP5− system has a stronger non-covalent interaction with 1 relative to the Gd-DOTA− system.
5. Data Obtained from Electron Paramagnetic Resonance (EPR) Spectroscopy
Peak-to-peak linewidth (ΔHpp) and electron g factor (g) were obtained from EPR spectra recorded for both of the Gd-DOTA− and Gd-DOTP5− systems. Gd-DOTA− (1 mM) displayed a well-defined line shape in the spectrum. ΔHpp and g extracted from the EPR spectra were combined with VT 17O-NMR and r1 data to estimate τR (Table 3) using the Solomon–Bloembergan–Morgan (SBM) equations (see supporting information for calculations).
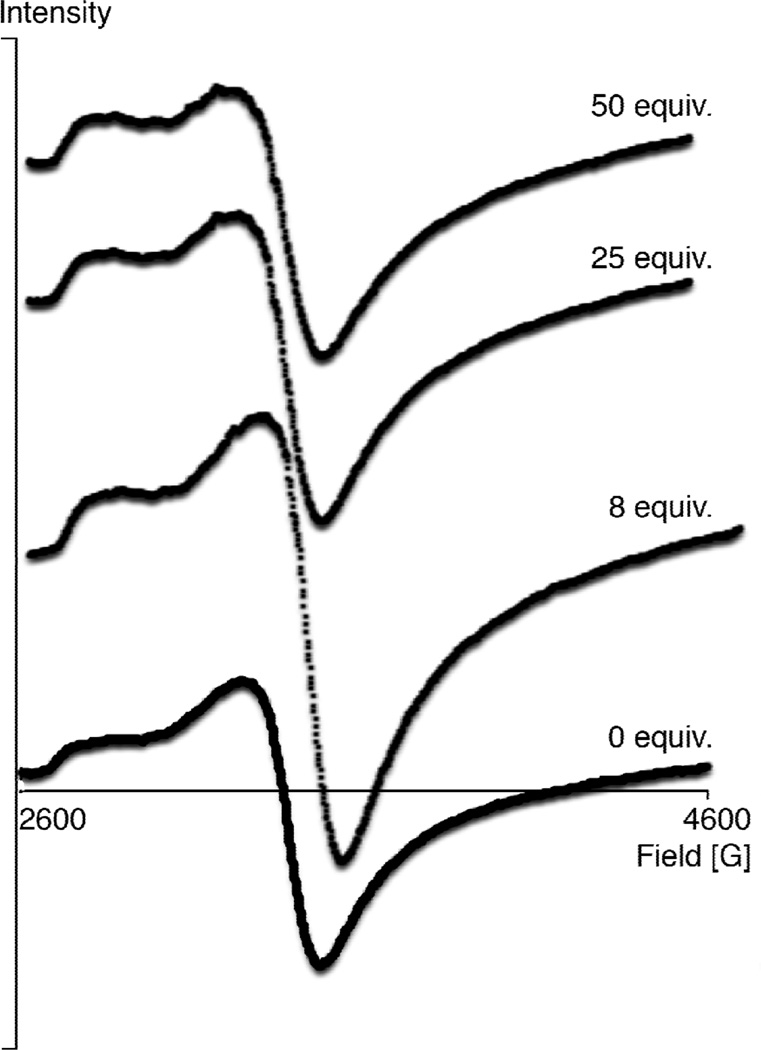
Reported Gd-DOTP5− EPR spectra were acquired at 25 °C in aqueous solution, and ΔHpp was shown to be independent of [Gd-DOTP5−] between 5 and 20 mM. [27] We found that it was convenient to extract ΔHpp from EDTA4−-buffered Gd-DOTP5− samples. [28] However, it is likely that EDTA4− and Gd-DOTP5− might compete for binding with 1 in solution. [29–31] To compensate for this competition arising from EDTA4−, we acquired EPR spectra with 8 or more equiv. of 1. If binding to 1 causes a change in ΔHpp, we expected to observe a gradually increasing ΔHpp as larger portions of 1 were added. To test our hypothesis, we titrated a Gd-DOTP5− aqueous solution with 0, 8, 25 and 50 equiv. of 1. All EPR spectra displayed the same ΔHpp irrespective of the amount of 1 used (Figure 2) suggesting that ΔHpp is not influenced by addition of 1 and the amount added.
Figure 2.
Stacked plot of EPR spectra of Gd-DOTP5− at 9.34 MHz, 25 °C.
With both τm and τR established, we can show that although τm was increased in the non-covalent adduct between 1 and Gd-DOTP5−, the larger τm is overwhelmed by slower molecular motion. Our mechanistic studies thus indicate that τR dominates in accentuating the r1 of Gd-DOTP5− in the presence of 1.
6. 19F NMR
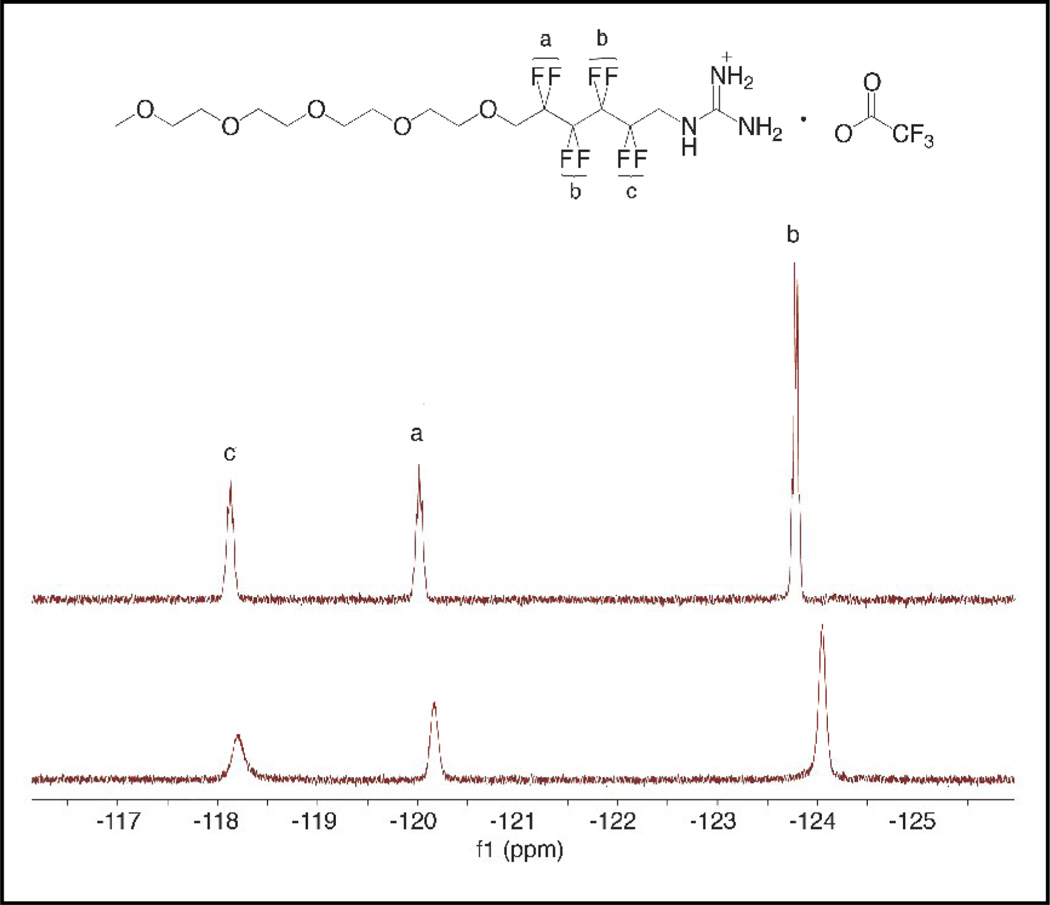
Because binding between the contrast agent and 1 will place 1’s fluorine atoms in close proximity to the metal center, the longitudinal relaxation time (T1) for the fluorine atoms in 1 is expected to decrease. The four different -CF2- groups in 1 are represented by three peaks in the 19F NMR spectrum. To unambiguously assign the fluorine atoms, a combination of 1D and 2D NMR techniques were used. [33] The 19F NMR assignment is shown in Figure 3.
Figure 3.
19F-NMR of an aqueous solution of 1 before (top) and after (bottom) the addition of Gd-DOTP5−.
Addition of 1 to a solution of Gd-DOTP5− caused a dramatic reduction in 19F T1 (Table 4) and slight changes in 19F chemical shift. All three peaks also broadened significantly (Figure 3). The percentage reduction in T1 before and after addition of Gd-DOTP5− for Fa, Fb, and Fc were 83.0, 90.3, and 95.2%, respectively. Based on the magnitude of reduction, the fluorine atoms from the closest to the furthest from the metal can be arranged in the order of Fc, Fb, and Fa. This order is in line with the model described in Scheme 1 and further supports the existence of an interaction between 1 and Gd-DOTP5−.
Table 4.
19F T1 values before and after addition of Gd-DOTP5−.
| T1 (ms) | Fa | Fb | Fc |
|---|---|---|---|
| 1 only | 417 | 507 | 443 |
| 1 + Gd-DOTP5− | 70.8 | 49.2 | 24.1 |
| reduction (%) | 83.0 | 90.3 | 95.2 |
For the Gd-DOTA− system, similar T1-shortening and upfield chemical shifts were observed for an aqueous solution of 1. Similar to the Gd-DOTP5− system Fc displayed the greatest reduction in T1. The percentages reduction before and after addition of Gd-DOTA− for Fa, Fb, and Fc were 53.4, 59.6, and 70.1%, respectively (Table 5).
Table 5.
19F T1 values before and after addition of Gd-DOTA−.
| T1 (ms) | Fa | Fb | Fc |
|---|---|---|---|
| 1 only | 474 | 527 | 501 |
| 1 + Gd-DOTA− | 221 | 213 | 150 |
| reduction (%) | 53.4 | 59.6 | 70.1 |
Because Fa was affected to a greater extent in the Gd-DOTP5− system relative to the Gd-DOTA− system, the interaction between Gd-DOTA− and 1 appears less intimate than that between 1 and Gd-DOTP5−. [34,35]
Discussion
The SBM equations predict that inner-sphere relaxivity is influenced by four factors of the gadolinium(III)-containing complex: the number of bound water molecules (q), the water exchange rate (kex, the reciprocal of τm), the rotational correlation time (τR), and the electron-spin relaxation times of the gadolinium(III) center (T1e and T2e). Adjusting these four factors enables optimization of molecular relaxivity in predictable ways, however, T1e and T2e are difficult to manipulate experimentally. While q and τR are expected to affect r1 in a linear fashion, the influence of τm is a less simple relationship. Typically, it is desirable to modulate τm to be as close as possible to the theoretically predicted optimal value of a given field strength. At a clinically relevant field strength of 1.4 T, the calculated optimal τm is ca. 10−8 s for small molecule MRI contrast agents, and 10−10 s1 for macromolecular contrast agents.6b Strategies such as adding steric bulk at the site of water coordination, manipulating the mechanism of water exchange, changing the charge of the gadolinium(III) complex, and derivitizing the metal’s chelating ligand enable τm modulation.[36,37] In fact, τm of most small molecule contrast agents in clinical use lie in the range of 1–4 × 10−6 s and are considered slower than the optimal value. [38]
Gd-DOTP5− is a known outer-sphere relaxation agent, and the magnitude of its relaxivity is comparable to that of Gd-DOTA−. Outer-sphere relaxation is a process where the longitudinal relaxation of protons bound in the second and outer coordination sphere is accelerated by the metal center to effect relaxivity. In this work, we show that the r1 of Gd-DOTP5− is accentuated via formation of a non-covalent adduct with 1, a cationic fluorocarbon-based amphiphile. To our knowledge, this is the third case where Ln-DOTP5− complexes were employed in a non-covalent adduct to affect relaxivity. In this study we utilized a fluorous amphiphile that was placed proximally to the metal center. Amphiphile 1 enhances the r1 of Gd-DOTP5− at both low (1.4 T) and high (9.4 T) field strengths. Because an excess of urea cannot reverse the change caused by 1, the interaction between 1 and Gd-DOTP5− is apparently robust. Previous studies show that the relaxivity of Gd-DOTP5− can be enhanced by the addition of meglumine or hexacyclen at proper pH. This effect is attributed to the large number of water molecules “sandwiched” between the ion pair, due to formation of an extensive hydrogen bond network. We suspect that a similar extensive hydrogen bond network is not formed between Gd-DOTP5− and guanidinium or urea, because we did not observe relaxivity enhancement in the presence of 150 mM of urea. We think this is because unlike meglumine or hexacyclen, urea or guanidine does not have functional groups capable of hydrogen bond formation distributed evenly along the backbone. Our system is unique, because we show here for the first time that although a fluoralkyl group can be brought into the proximity of a T1-shortening agent, it does not restrict water coordination, or even water exchange, at the metal center to an extent that is meaningful in controlling the agent’s T1 relaxivity. Rather, the role of the fluroralkyl group as a relaxivity-modulating agent is based in its ability to restrict molecular tumbling of the agent, and thus increase its τr.
Luminescence-decay studies indicate that for Gd-DOTP5−, q remains unchanged in the presence or absence of 1, and VT 17O-NMR data demonstrate that water exchange rate is decreased after addition of 1. This observation suggests that the non-covalent interaction between Gd-DOTP5− and 1 inhibits the accessibility of bulk water to the gadolinium(III) center resulting in slower water exchange rates. This finding will be helpful for designing future contrast agents with slow water exchange rates.
Because slower water exchange rates negatively affect the value of r1 while q remains constant, the origin of accentuated r1 involves factors other than τm and q. Based on the τR values calculated for the Gd-DOTP5− system, non-covalent interaction with 1 causes slower molecular motion (lengthened τR) of Gd-DOTP5−. Analogously, amphiphile 1 is capable of forming a macromolecular assembly with methyl phosphonic acid, akin to the τR elongation described in this study. The influence of τR predominates that of τm, giving a net result of accentuated r1. Furthermore, 19F T1 NMR evidence supports a model where a strong interaction occurs between the guanidinium head of 1 and phosphonate groups in Gd-DOTP5−.
For the carboxylate analog, Gd-DOTA−, its r1 was not affected in a way similar to Gd-DOTP5−. At high field strength (9.4 T) 1 induced attenuated relaxivity of Gd-DOTA that is restored with the addition of urea and sonication. The relaxivity of Gd-DOTA− was found to be unresponsive to 1 at low field strengths (1.4 T). As indicated by luminescence-decay studies, interaction with 1 does not change the number of bound water molecules on the gadolinium(III) center of Gd-DOTA−. The τm of Gd-DOTA− was increased after addition of 1 likely due to 1 interacting with Gd-DOTA− resulting in hindering of water accessibility to the gadolinium(III) center. However, treatment with urea and sonication caused τm to decrease to a value lower than before the addition of 1, for unknown reasons. Based on the calculated τR values, addition of 1 did not affect the molecular motion of Gd-DOTA−, indicating that no adduct was formed. Overall, our findings on q, τm, and τR are consistent with the relaxivity measurement for the Gd-DOTA− system. The percentage of 19F T1 reduction in the Gd-DOTA− system was far less than it was in the Gd-DOTP5− system, suggesting that 1 associates only weakly or transiently with Gd-DOTA−.
Conclusions
Phosphonate-bearing Gd-DOTP5− forms a non-covalent adduct with fluorous amphiphile 1, which leads to slower molecular tumbling and enhanced relaxivity of the contrast agent. Interstingly, 1’s fluoroalkyl group does not cause a sufficient change in water exchange at the metal center to account for the observed relaxivity modulation. In contrast, Gd-DOTA− only weakly associates with 1 and its r1 is not changed significantly. We also found that this non-covalent interaction between Gd-DOTP5− and 1 causes an increase in τm. However, based on the results obtained, τR is likely the dominant factor increasing in r1. Because phosphonates are prevalent in biological environments and important biochemical functional groups, we envision that our approach to modulate τR through phosphonate-guanidinium non-covalent interactions could provide a basis for non-covalently introducing fluorous groups into future molecular probes for MRI.
Experimental
1. General Procedures
All air- and water sensitive procedures were carried out using standard Schlenk techniques. Deuterated NMR solvents were purchased from Cambridge Isotope Laboratories (MA) and used as received. Hexahydrates of GdCl3, YCl3, and EuCl3 were obtained from Alfa Aesar (REacton, Ward Hill, MA). Cyclen and DOTA were purchased from Strem Chemicals (Newburyport, MA). All other organic solvents and bulk inorganic reagents were purchased from EM Science (Gibbstown, NJ) and used as received, except where indicated. Distilled water was purchased from Arrowhead (Louisville, KY). Dionized water was generated from a PURELAB Ultra Mk2 water purifier (Elga). 1H T1 measurements were acquired on a Varian 400MR NMR Spectrometer at 9.4 T, or on a Bruker mq 60 NMR Analyzer at 1.4 T. 19F-NMR spectra were acquired on a Varian VNMRS 500 NMR Spectrometer at 11.7 T. Chemical shifts were referenced to residual solvent (1H) or C6F6 (19F).
2. Na5[Gd(DOTP)]∙9H2O
H8DOTP was prepared according to a literature procedure. [39] Na5[Gd(DOTP)]∙9H2O was prepared according to a modified literature procedure.24 To an aqueous solution (27.4 mL) containing H8DOTP (150 mg, 0.274 mmol) heated to 80 °C, at pH = ca. 8–9, an aqueous solution of GdCl3∙6H2O (97 mg, 0.26 mmol in 13 mL H2O) was slowly added at a speed of 20 sec/drop. The pH was maintained at ca. 8–9. After the addition was finished, the solution was allowed to stand for 12 h at ambient temperature. The solution was then lyophilized to obtain a white powder. The white powder was dissolved in MeOH, and H2O was added dropwise to the MeOH solution to obtain a white precipitate. The resulting white precipitate was washed with a mixture of MeOH and H2O (9:1 v/v) and filtered over a fine fritted funnel. The precipitate was dried under reduced pressure to obtain a white powder (277.8 mg). The white powder was dissolved in H2O and recrystallized by slow diffusion in isopropanol and lyophilized as translucent, square-shaped solids. Yield: 195.3 mg, 77.1%.
Elemental analysis: calc’d C, 14.79; H, 4.34; N, 5.75; Na, 11.79; found C, 14.75; H, 4.02; N, 5.36; Na, 11.88. MALDI MS for [Gd(DOTP)]5− m/z: [H6Gd(DOTA)]+ calc’d 704.0, found 703.9; [H5NaGd(DOTA)]+ calc’d 726.0, found 725.8; [H4Na2Gd(DOTA)]+ calc’d 748.0, found 747.8 g/mol.
3. Synthesis of Na[Gd(DOTA)]∙4H2O
Na[Gd-DOTA]∙4H2O was prepared according to modified literature procedures. [40] DOTA (24 mg, 0.060 mmol) was dissolved in distilled Arrowhead water (3 mL). GdCl3∙6H2O (22 mg, 0.060 mmol) was added to the solution of ligand while stirring. After the addition of GdCl3, the pH of the resulting reaction mixture was 2.12. The pH of the resulting reaction mixture was adjusted to be between 5.5 and 7.0 by adding NaOH (1 M), and thus the reaction was monitored until the pH remained constant for > 1 h. Upon equilibration the pH of the reaction mixture was adjusted with 1 M NaOH to a final pH ≥ 11, and a white precipitate was observed. The precipitate was removed using a 0.45 µm PTFE filter and the filtrate was lyophilized to dryness. The dried solid was purified via reverse phase column chromatography using H2O and MeOH as solvents. Na[Gd-DOTA]∙4H2O was produced as a white solid (21.3 mg) in 54% yield.
Elemental analysis: calc’d C, 29.44; H, 4.94; N, 8.58; found C, 29.73; H, 4.46, N, 8.41. MALDI MS for [Gd(DOTA)]− m/z: [H2Gd(DOTA)]+ calc’d 560.1, found 560.0; [HNaGd(DOTA)]+ calc’d 582.1, found 582.0; [Na2Gd(DOTA)]+ calc’d 604.1, found 603.9; [Na2Gd(DOTA)(H2O)]+ calc’d 622.1, found 621.9 g/mol.
4. Synthesis of 1
Compound 1 was synthesized according to a literature procedure.
5. Relaxivity (r1) Measurements at 9.4 T
A stock solution of the contrast agent (Gd-DOTP5− or Gd-DOTA−) at 100 mM was prepared by weighing out the appropriate amount of the contrast agent into a tared 1 dram vial and dissolving it in appropriate amount of distilled H2O. This stock solution was diluted to make 0.25, 0.5, 0.75, and 1 mM stock solutions. To prepare a solution of 1, a methanol solution of 1 was dispensed into a tared 1 dram vial. After removal of methanol under reduced pressure, 1 was redissolved in distilled H2O to a 100 mM solution. An aliquot of 1 solution corresponding to 4 equiv. of the contrast agent (100 µL, 0.25 mM) was added to a 0.5 dram vial and lyophilized. 1 was then rinsed out of the 0.5 dram vial using 100 µL of 0.25 mM contrast agent solution and added to an external 3 mm coaxial insert NMR tube.
Subsequently, the 3 mm coaxial inserts containing 0.5, 0.75, and 1 mM of contrast agent solution and the corresponding 4 equiv. of 1 were prepared. The coaxial inserts were placed in 5 mm NMR tubes containing D2O for T1 measurements, and the 1H T1 measurements were taken on a 400 MR NMR instrument using inversion recovery method. 1H T1 relaxation times obtained from these samples were given by VnmrJ program. The 1/T1 (s−1) values were plotted against the concentration of the contrast agent (mM) to obtain r1.
6. T1 Measurements at 1.4 T
The Gd content was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (see part 10 of the Experimental section). Relaxation times (T1) were obtained using a Bruker mq 60 NMR Analyzer (1.4 T) at 37 °C. The measurements were triplicated using three independently prepared samples. Relaxivities were obtained from the slopes of the linear plots of 1/T1 versus Gd3+ concentration.
7. Luminescence-Decay Measurements
Luminescence-decay measurements were acquired using a HORIBA Jobin Yvon Fluoromax-4 spectrofluorometer in decay by delay scan mode using the phosphorescence lifetime setting. Excitation and emission wavelengths of 393 and 596 nm were used, respectively, while the other parameters were kept constant: excitation and emission slit widths (5 nm), flash count (100), initial delay (0.01 ms), maximum delay (2 ms for solutions in H2O and 8 ms for solutions in D2O), and delay increment (0.01 ms). All measurements were repeated using three independently prepared samples. The number of coordinated water molecules, q, was determined using the method developed by Horrocks and coworkers.8
8. Variable-Temperature (VT) 17O-NMR Measurements
Variable-temperature 17O-NMR measurements of Gd-DOTP5− (25 mM) and Gd-DOTA− (4.4 mM) systems and their diamagnetic Y3+ analogues, Y-DOTP5− (25 mM) and Y-DOTA− (4.4 mM) systems in 1% 17O-enriched water starting from 10% 17O-enriched water (Cambridge Isotope Laboratories, Inc.) were carried out on a Varian-500S (9.4 T) spectrometer. Line widths at half height of the bulk water peaks were measured at 15, 20, 30, 40, 50, 60, and 70 °C. A/ħ and ΔE were fixed to −3.8 × 10−6 rad/s and 2.5 × 10−11 J/mol, respectively, for the Gd-DOTP5− and Gd-DOTA− systems. Water coordination numbers, q, were set to the values obtained from luminescence-decay measurements. The least-squares fits of the 17O-NMR relaxation data were calculated using Origin software (8.0951 B951) following a previously published procedure16b,c to obtain the water exchange rates.
9. Electron Paramagnetic Resonance (EPR) Spectroscopy
Gd-DOTP5− EPR spectra were acquired at 25 °C on a Bruker EMX instrument at 9.34 T, using 25 mM solutions. The sample was prepared by dissolving the appropriate amount of solid Na5[Gd(DOTP)]∙9H2O and 1 equiv of Na4EDTA in HPLC-grade H2O to make [Gd3+] = 25 mM. The solution was heated at reflux for 16 h and cooled to ambient temperature before use. An aqueous solution of 1 was prepared, and the amount corresponding to 8 equiv. of 1 relative to Gd-DOTP5− (delivered as a 20 µL 25 mM solution) was dispensed into a 0.5 dram vial. The vial was lyophilized to remove the solvent, and the residual 1 formed a thin membrane on the wall of the vial. The membrane was rinsed with 20 µL of Gd-DOTP5− solution (25 mM) and transferred into a borosilicate glass tube (0.6 I.D. × 0.84 O.D.). Similar procedures were used to prepare 25 and 50 equiv samples.
EPR measurements of the Gd-DOTA− systems (1 mM) were performed on a Bruker EMX X-band spectrometer. From the EPR spectra, the electronic Landé g factor, gL, peak-to-peak line width, ΔHpp, and transverse electronic relaxation rates, 1/T2e, were obtained according to a previously reported method.1b
10. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP–OES)
ICP–OES measurements were performed on a HORIBA Jobin Yvon ULTIMA spectrometer. Concentrations of Gd3+ in samples used for T1 measurements, Gd3+ and Y3+ in samples used for variable-temperature 17O-NMR measurements, and Gd3+ in samples used for EPR spectroscopy were verified by ICP–OES. Samples for ICP–OES were diluted with nitric acid (2% v/v, aqueous), and standards were prepared by serial dilution of Gd, Eu, and Y standards (High-Purity Standards).
Supplementary Material
Highlights.
-
-
The first use of fluoroalkyl moieties to accentuate the relaxivity of a gadolinium agent
-
-
This effect above is specific to a phosphonate-bearing Gd agent
-
-
The origin of the effect primarily involves the agent’s solution tumbling rate
Acknowledgements
This work is sponsored by the USC Ming Hsieh Institute, the Donald and Delia Baxter foundation, and a Schaap Faculty Scholar Award from Wayne State University (M.J.A.). We are grateful to the NSF (DBI-0821671, CHE-0840366) and NIH (S10 RR25432) for their sponsorship of NMR spectrometers. The authors acknowledge the Lumigen Instrument Center at Wayne State University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a González G, Powell DH, Tissières V, Merbach AE. J. Phys. Chem. 1994;98:53–59. [Google Scholar]; b Powell HP, Ni Dhubhghail OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J. Am. Chem. Soc. 1996;118:9333–9346. [Google Scholar]
- 2.Merbach AS, Helm L, Tóth É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. 2nd ed. New York: John Wiley & Sons; 2013. [Google Scholar]
- 3.a. Contrast Agents I: Magnetic Resonance Imaging, Krause W. Topics in Current Chemistry. New York: Springer-Verlag; 2002. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. Aime S, Botta M, Fasano M, Terreno E. Chem. Soc. Rev. 1998;27:19–29. Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Anal. Chem. 2012;84:6278–6287. doi: 10.1021/ac300527z.
- 4.a Moats, Fraser RA, Meade SE, Angew TJ. Chem. Int. Ed. 1997;36:726–728. [Google Scholar]; b Stavila V, Allali M, Canaple L, Stortz Y, Franc C, Marin P, Beuf O, Dufay O, Samarut J, Janier M, Hasserodt J. New J. Chem. 2008;32:428–435. [Google Scholar]
- 5.Que EL, Gianolio E, Baker SL, Wong AP, Aime S, Chang CJ. J. Am. Chem. Soc. 2009;131:8527–8536. doi: 10.1021/ja900884j. [DOI] [PubMed] [Google Scholar]
- 6.a Tu T, Louie AY. Chem. Commun. 2007:1131–1133. [Google Scholar]; b Osborne E, Jarett BR, Tu C, Louie AY. J. Am. Chem. Soc. 2010;137:5934–5935. doi: 10.1021/ja100254m. [DOI] [PubMed] [Google Scholar]
- 7.Dorazio SJ, Tsitovich PB, Gardina SA, Morrow JR. J. Inorg. Biochem. 2012;117:212–219. doi: 10.1016/j.jinorgbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 8.a Zhang S, Wu K, Sherry AD. Angew. Chem. Int. Ed. 1999;38:3192. [PubMed] [Google Scholar]; b Raghunand N, Howison C, Sherry AD, Zhang S, Gillies RJ. Magn. Reson. Med. 2003;49:249–257. doi: 10.1002/mrm.10347. [DOI] [PubMed] [Google Scholar]
- 9.Fossheim SL, Il’-yasov KA, Hennig J. A. Bjornerud Acad. Radiol. 2000;7:1107–1115. doi: 10.1016/s1076-6332(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 10.Aime S, Botta M, Gianolio E, Terreno E. Angew. Chem. Int. Ed. 2000;39:747–750. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Lu Y. Chem. Commun. 2011;47:4998–5000. doi: 10.1039/c1cc10161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li V, Ghang Y-J, Hooley RJ, Williams TJ. Chem. Commun. 2014;50:1375–1377. doi: 10.1039/c3cc48389d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li V, Chang AY, Williams TJ. Tetrahedron. 2013;69:7741–7745. doi: 10.1016/j.tet.2013.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For examples see a. note Siriwardena-Mahanama B, Allen MJ. Dalton. Trans. 2013;42:6724–6727. doi: 10.1039/c3dt50885d.
- 15.a Pereira GA, Peters JA, Terreno E, Castelli Delli, Aime DS, Laurent S, Elst S, Vander L, Muller RN, Geraldes CFGC. Eur. J. Inorg. Chem. 2012:2087–2098. [Google Scholar]; b Aime, Botta S, Garino M, Crich E, Giovenzana SG, Pagliarin G, Palmisano R, Sisti G. M. Chem. Eur. J. 2000;6:2609–2617. doi: 10.1002/1521-3765(20000717)6:14<2609::aid-chem2609>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Boz E, Sirkis A, Chang A, Williams TJ. J. Fluor. Chem. 2012;135:665–669. [Google Scholar]
- 17.a Schug KA, Lindner W. Chem. Rev. 2005;105:67–113. doi: 10.1021/cr040603j. [DOI] [PubMed] [Google Scholar]; b Luo R, David L, Hung H, Devaney J, Gilson MK. J. Phys. Chem. B. 1999;103:727. [Google Scholar]
- 18.Gd-DOTP5− is prepared at pH = 8~9.
- 19.Supkowski RM, Horrocks WD., Jr Inorg. Chim. Acta. 2002;340:44–48. [Google Scholar]
- 20.Dissanyayake P, Mei Y, Allen MJ. ACS Catal. 2011;1:1203–1212. [Google Scholar]
- 21.Literature reports on this value are scattered and have high error. See Geraldes CFGC, Brown RD, III, Cacheris WP, Koenig SH, Sherry AD, Spiller M. Magn. Reson. Med. 1989;9:94–104. doi: 10.1002/mrm.1910090111. Anelli PL, Balzani V, Prodi L, Uggeri F. Gazz. Chim. Ital. 1991;121:359–364.
- 22.Aime S, Botta M, Terreno E, Anelli PL, Uggeri F. Magn. Reson. Med. 1993;30:583–591. doi: 10.1002/mrm.1910300509. [DOI] [PubMed] [Google Scholar]
- 23.Avecilla F, Peter JA, Geraldes Eur. J. Inorg. Chem. 2003:4179–4186. C. F. G. C. [Google Scholar]
- 24.Swift TJ, Connick RE. J. Chem. Phys. 1962;37:307–320. [Google Scholar]
- 25.Micskei K, Helm L, Brücher E, Merbach AE. Inorg. Chem. 1993;32:3844–3805. [Google Scholar]
- 26.a Urbanczyk-Pearson LM, Femia FJ, Simith J, Parigi G, Duimstra JA, Eckerman AL, Lucinat C, Meade TJ. Inorg. Chem. 2008;47:56–68. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]; b Garcia J, Neelavalli J, Haacke EM, Allen MJ. Chem. Commun. 2011;47:12858–12860. doi: 10.1039/c1cc15219j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira GA, Ball L, Sherry AD, Peter JA, Geraldes CFGC. Helv. Chim. Acta. 2009;92:2532–2551. [Google Scholar]
- 28.EDTA4− is unlikely to decomplex Gd3+ from Gd-DOTP5− due to the large differences in stability constants log(KML), however, EDTA4− is negatively charged and is capable of binding to 1. See Ref.[6]; Sherry AD, Ren J, Huskens J, Brücher E, Tóth É, Geraldes CFCG, Castro MMCA, Cacheris WP. Inorg. Chem. 1996;35:4604–4612.
- 29.Dietrich B, Fyles DL, Fyles TM, Lehn J-M. Helv. Chim. Acta. 1979;62:2763–2767. [Google Scholar]
- 30.Antonov VK. Chemistry of Proteolysis. New York: Springer-Verlag; 1993. [Google Scholar]
- 31.Blanco JLJ, Bootello P, Benito JM, Mellet CO, Fernandez JMG. J. Org. Chem. 2006;71:5136–5143. doi: 10.1021/jo060360q. [DOI] [PubMed] [Google Scholar]
- 32.Botta M. Eur. J. Inorg. Chem. 2000:399–407. [Google Scholar]
- 33.For an unambiguous assignment of 19F NMR and related 2-D heteronuclei correlation experiments (e.g. 19F-13C gHMBC), please refer to the SI.
- 34.Berquist TH. MRI of the Musculoskeletal System. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 39–41. [Google Scholar]
- 35.Zech SG, Eldredge HB, Lowe MP, Caravan P. Inorg. Chem. 2007;46:3576–3584. doi: 10.1021/ic070011u. [DOI] [PubMed] [Google Scholar]
- 36.Doble DMJ, Botta M, Wang J, Aime S, Barge A, Raymond KN. J. Am. Chem. Soc. 2001;123:10758–10759. doi: 10.1021/ja011085m. [DOI] [PubMed] [Google Scholar]
- 37.For a reviews on τm modulation see: Siriwardena-Mahanama BN, Allen MJ. Molecules. 2013;18:9352–9381. doi: 10.3390/molecules18089352. Sherry AD, Wu Y. Curr. Opin. Chem. Biol. 2013;288:2914–2922. doi: 10.1016/j.cbpa.2012.12.012.
- 38.Jászberényi Z, Sour A, Tóth É, Benmelouka M, Merbach AE. Dalton Trans. 2005:2713. doi: 10.1039/b506702b. [DOI] [PubMed] [Google Scholar]
- 39.Sherry AD, Malloy CR, Jeffrey MH, Cacheris WP, Geraldes CFGC, Magn J. Reson. 1988;76:528–533. [Google Scholar]
- 40.Averill DJ, Garcia J, Siriwardena-Mahanama BN, Vithanarachchi SM, Allen MJ. J. Vis. Exp. 2011:53. doi: 10.3791/2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.