The brain’s activity at rest changes dramatically as it develops. Resting oscillatory power within delta and theta bands, for example, decreases steadily between childhood and late adolescence (Gasser et al., 1988). Individual differences in resting EEG power have been linked to gray matter volume (Whitford et al., 2007). Thus, decreases in resting EEG power with age may reflect decreases in synaptic density due to synaptic pruning, which have been proposed to be linked to decreasing gray matter volume with age (Whitford et al., 2007).
Little is known, however, about how power within the higher-frequency gamma band (>30 Hz) at rest changes with age. Only a single study has examined resting gamma power over a wide range of ages, reporting an increase between ages 3 and 4 and a slight trend towards a decrease between ages 4 and 11 (Takano and Ogawa, 1998). Resting gamma has been linked to linguistic and attentional abilities in toddlers and young children (Benasich et al., 2008). As a result, examining the relationship between resting gamma and age could help elucidate the structural development of neural circuits underlying language and cognitive processing. Here, we aimed to define the developmental trajectory of resting gamma throughout childhood and into adulthood by collecting EEG data from subjects aged 3 to 38 while they were awake but not engaged in a task.
156 subjects from the greater Chicago area were tested. Subjects were recruited according to scholastic stages and divided accordingly: preschoolers (3 to 5 years old, n = 35, 18 females), school-aged children (6–13, n = 38, 20 females), adolescents (14–17, n = 56, 27 females) and adults (18–38, n = 27, 16 females). Informed consent (adults) or assent and parent consent (minors) were obtained for all testing procedures and subjects were monetarily compensated. Subjects/guardians reported no history of learning or neurological impairments.
Subjects were fitted with a 31-channel tin-electrode cap (Electrocap International, Eaton, OH, USA). In 3- to 5-year-old subjects only 19 channels were active to minimize cap application time. Blink-monitoring electrodes were placed on the superior and outer canthi of the left eye and reference electrodes were placed on the earlobes. Electrode impedance was kept below 10 kΩ. Subjects sat in a comfortable chair for three minutes in a lighted soundproof booth. Subjects were asked to keep their eyes open, to minimize their movements, and to fixate on a region of the wall in front of them (to limit eye movement). They were given no other task.
EEG activity was bandpass-filtered offline from 1 to 100 Hz (12-dB rolloff) to isolate the cortical contribution to the signal. Eye-blinks were removed via spatial filtering in NeuroScan Edit 4.3 (Compumedics, Charlotte, NC). The recording was segmented into 180 one-second non-overlapping epochs, the frequency spectrum of each epoch was calculated using a fast Fourier transform in Matlab (The Mathworks, Natick, MA, USA) and the 180 frequency spectra were averaged. Gamma power was calculated as 20*log10(x) where x is the mean amplitude from 31 to 50 Hz (following Benasich et al., 2008).
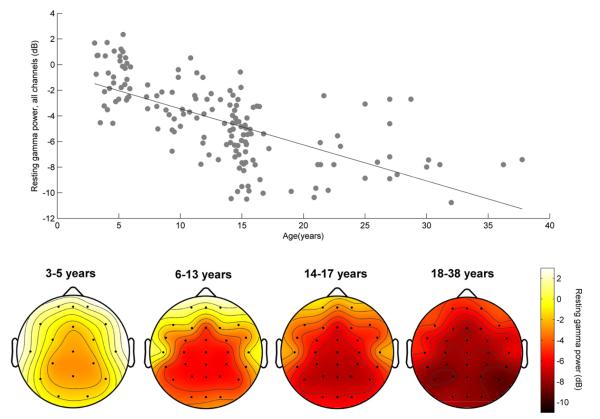
Resting gamma power is displayed topographically in Fig. 1 (bottom). Distribution of gamma power across channels was strikingly similar across the age groups, suggesting that topography of resting gamma power did not vary with age. Moreover, for each channel examined individually the correlation between resting gamma and age was negative and significant (all p < 1 × 10−7). In subsequent analyses, therefore, we examined the mean gamma power of all channels combined (excluding blink-monitoring electrodes). Fig. 1 (top) displays the relationship between age and resting gamma power. Older subjects showed less resting gamma than younger subjects (Pearson’s correlation; r = −0.65, p = 1.35 × 10−20).
Fig. 1.
Top: resting gamma power across all channels correlates with age. Bottom: the decrease in resting gamma with age occurs in every channel.
To confirm that older subjects had less resting gamma, we entered resting gamma power among the four age groups into an ANOVA, through which we observed a main effect of age (F(3,152) = 55.91, p < 1 × 10−20). Post-hoc independent samples t-tests revealed that each age group significantly differed from all other groups, with younger age groups demonstrating less resting gamma than older age groups (preschoolers/school-aged children: t = 6.15, p = 4.0 × 10−8; preschoolers/adolescents: t = 10.64, p = 1.5 × 10−17; preschoolers/adults: t = 11.91, p = 1.9 × 10−17; school-aged children/adolescents: t = 4.72, p = 8.3 × 10−6; school-aged children/adults: t = 6.6, p = 1.2 × 10−8 ; adolescents/adults: t = 2.46, p = 0.016). Thus, the decrease in gamma over time continues through early adulthood.
Resting gamma power across all channels decreased with age and this decrease continued into adulthood. Although we cannot determine whether this age-related decrease persists or stabilizes within early adulthood, further studies, preferably with longitudinal designs, should determine whether gamma stabilizes or continues to decline into adulthood. The steady decrease we observed in resting gamma follows the same trajectory as gray matter volume, which decreases from age four through adulthood (Whitford et al., 2007). Changes in resting gamma may, therefore, reflect decreases in synaptic density linked to synaptic pruning. Resting gamma relates to linguistic and attentional abilities (Benasich et al., 2008). As a result, our finding that young adults have less resting gamma than adolescents may reflect structural development within brain circuits underlying language and cognitive processing that continues between adolescence and young adulthood, such as decreases in gray matter volume within frontal cortex (Sowell et al., 1999).
There are other interpretations of our findings that must be considered. For example, these results could reflect a developmental trajectory for electromyographic (EMG) activity from cranial muscle contractions, which contribute to increased scalp-recorded gamma power (Shackman et al., 2009). Because the influence of EMG on EEG is far greater in peripheral versus central channels (Whitham et al., 2008), with little to no effect on gamma in the 31–50 Hz range for central electrodes, EMG-contributions to the age–gamma relationship would be lessened or non-existent in central channels. In contrast, we found a significant age–gamma relationship at every channel—and, as the topographic plots at the bottom of Fig. 1 show, the amount of decrease in resting gamma is relatively constant across channel locations. While EMG contamination could contribute to age–gamma relationships in peripheral channels, the global pattern of our results cannot be entirely accounted for by changes in muscle activity with development.
Another consideration is the developmental variation in skull thickness, which increases with age (Epstein, 1974) and varies inversely with EEG amplitudes (Frodl et al., 2001). The relationship between skull thickness and resting oscillatory EEG power is very slight, however, with researchers reporting a correlation coefficient of −0.2, an effect only trending towards significance (Hagemann et al., 2008). The relationship reported here between age and gamma reveals a much stronger effect (r = −0.65). While individual differences in skull thickness could influence the relationship between resting gamma and age, other factors, such as those related to maturation, must be at play .
Despite the strength of the relationship between resting gamma and age, there was substantial variability within each age range. Because resting gamma continues to decline from early childhood through young adulthood, can easily be collected on subjects of any age, and takes only 3 min to collect, resting gamma may be a useful measure of individual differences in neural maturational rate over a variety of developmental stages. A longitudinal assessment of resting gamma power over development could strengthen the link between resting gamma and neural maturation. Given connections between resting gamma and linguistic and attentional skills (Benasich et al., 2008), resting gamma may index the development of neural circuits underlying language processing.
Contributor Information
Adam Tierney, Auditory Neuroscience Laboratory, Northwestern University, Evanston, IL, USA; Department of Communication Sciences, Northwestern University, Evanston, IL, USA.
Dana L. Strait, Auditory Neuroscience Laboratory, Northwestern University, Evanston, IL, USA Institute for Neuroscience, Northwestern University, Chicago, IL, USA.
Samantha O’Connell, Auditory Neuroscience Laboratory, Northwestern University, Evanston, IL, USA.
Nina Kraus, Auditory Neuroscience Laboratory, Northwestern University, Evanston, IL, USA; Department of Communication Sciences, Northwestern University, Evanston, IL, USA; Institute for Neuroscience, Northwestern University, Chicago, IL, USA; Department of Neurobiology and Physiology, Northwestern University, Evanston, IL, USA; Department of Otolaryngology, Northwestern University, Chicago, IL, USA.
References
- Benasich A, Gou Z, Choudhury N, Harris K. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res. 2008;195:215–22. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. Phrenoblysis: special brain and mind growth periods. I. Human brain and skull development. Dev Psychobiol. 1974;7:207–16. doi: 10.1002/dev.420070304. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Müller D, Leinsinger G, Juckel G, Hahn K. The effect of the skull on event-related P300. Clin Neurophysiol. 2001;112:1773–6. doi: 10.1016/s1388-2457(01)00587-9. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol. 1988;69:91–9. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Walter C, Naumann E. Skull thickness and magnitude of EEG alpha activity. Clin Neurophysiol. 2008;119:1271–80. doi: 10.1016/j.clinph.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Shackman A, McMenamin B, Slagter H, Maxwell J, Greischar L, Davidson R. Electromyogenic artifacts and electroencephalographic inferences. Brain Topogr. 2009;22:7–12. doi: 10.1007/s10548-009-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E, Thompson P, Holmes C, Jernigan T, Toga A. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Takano T, Ogawa T. Characterization of developmental changes in EEG gamma-band activity during childhood using the autoregressive model. Acta Paediatr Jpn. 1998;40:446–52. doi: 10.1111/j.1442-200x.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Whitford T, Rennie C, Grieve S, Clark C, Gordon E, Williams L. Brain maturation in adolescence. concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–37. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham E, Lewis T, Pope K, Fitzgibbon S, Clark C, Loveless S, et al. Thinking activates EMG in scalp electrical recordings. Clin Neurophysiol. 2008;119:1166–75. doi: 10.1016/j.clinph.2008.01.024. [DOI] [PubMed] [Google Scholar]