Abstract
Little is known about the effect of physical activity in early life on subsequent growth and regulation of inflammation. We previously reported that exposure of muscles in growing rats to IL-6 results in decreased muscle growth apparently due to a state of resistance to growth factors such IGF-I and that running exercise could ameliorate this growth defect. Herein we hypothesized that increased activity, for a brief period during neonatal life, would pattern the adult rat towards a less inflammatory phenotype. Neonatal rats were induced to move about their cage for brief periods from day 5 to day 15 postpartum. Additional groups were undisturbed controls (CON) and handled (HAND). Sub-groups of rats were sampled at 30 and 65 days of age. Relative to CON and HAND, neonatal exercise (EX) results in decreased circulating levels of TNFα, IL-6 and IL-1β in adulthood, primarily in male rats. In addition, adult male EX rats had lower body mass and increased skeletal muscle mass suggesting a leaner phenotype. The results of this study suggest that moderate increases in activity early in life can influence the adult toward a more healthy phenotype with regard to inflammatory mediators and relative muscle mass.
INTRODUCTION
The number of children with chronic inflammatory conditions is increasing rapidly. Whether in environmentally induced conditions, such as in childhood obesity, or the result of pediatric chronic lung disease like severe asthma or cystic fibrosis, chronic elevations of certain mediators (e.g., IL-6, C-reactive protein) in many childhood diseases are associated with relatively reduced lean tissue and muscle mass, reduced physical activity, and long term impairments of health (1–5). The precise mechanisms of the impairments in muscle mass are not known. However, work from this and other laboratories shows that physical activity influences muscle growth through the balance of growth factors [e.g., growth hormone and IGF-I] and stress/inflammatory mediators [e.g.,IL-6], which can inhibit the anabolic effects of growth mediators associated with the GH→IGF-I axis on skeletal muscle growth and development (6,7).
While emerging data indicate that regular exercise can ameliorate inflammation (8,9), very little is known about the effect of physical activity in early life on subsequent growth and regulation of inflammation. The inverse relationship between body and muscle mass and circulating levels of inflammatory cytokines is striking and has now been observed in a wide range of human subjects from premature babies (10) to elderly adults (11). We recently reported that the direct exposure of muscles in growing rats to IL-6 results in decreased muscle growth apparently due to a state of resistance to growth factors such IGF-I (11). The mechanistic link between proinflammatory cytokines and skeletal muscle growth factors probably lies in the activity of the SOCS (suppressors of cytokine signaling) family of proteins (13,14).
A growing number of investigations are providing support for the concept of “critical periods of growth” during which relatively short-term physiological perturbations early in life can lead to long term health consequences in adulthood, particularly with reference to body composition and obesity (15–19). While much attention has been paid to the long term impact of nutrition early in life on the development of conditions such as atherosclerosis (20), very little is known about how physical activity early in life could alter essential regulating mechanisms of body composition and impact muscle mass in adulthood. The purpose of the current study was to test the hypotheses that increased physical activity in the newborn period would alter growth of skeletal muscle and circulating inflammatory cytokines during adulthood. We used a protocol of increased physical activity during early postnatal development in the rat. The neonatal rat is useful in this context in that 1) readily simple means can be used to increase physical activity, and 2) the time interval for puberty and maturation is short. Consequently, the “adult” effects of early life perturbations, if they occur, can be measured in a reasonable period of time.
It is known that gentle handling of rat pups during the immediate postnatal period has profound effects on the stress responses of these animals later in life. As adults, rats handled during the neonatal period secrete less corticosterone and have a faster corticosterone return to baseline following stress (21,22). Moreover, Kruschinski et al. recently reported that increased handling of rats as neonates attenuated airway inflammation in experimentally induced asthma in the adult animals (23). In the 1950s, Weininger (24) found that gently handled rats gained weight more rapidly and were significantly larger than the nonhandled animals by 21 days of life. Moreover, the handled animals were more physically active. Despite these intriguing studies, very little is currently known about the interaction of physical activity in early life, subsequent growth, and the regulation of inflammatory mediators. In the current study, we tested our hypothesis using a novel protocol that enabled us to distinguish handling from increased physical activity in the neonatal period.
METHODS
All procedures were approved by the University of California Irvine Animal Care and Use Committee. Pregnant Sprague-Dawley dams were purchased (Chalres River. Wilmington, MA). The litters were randomized and the number of neonates normalized to 8 (4 males, 4 females) per dam using standard culling procedures on postpartum day 5 (25). Dams and litters were further randomized to three groups: Control (CON, n=32), Handled (HAND, n=32) and Exercised (EX, n=32). The CON group consisted of litters that were transported to the laboratory each treatment day but not disturbed in any other way. Pups from the HAND group were held by researchers for the same durations as the exercise treatment (see below).
“Exercise” protocol
During the postpartum period, neonates in the EX treatment groups were removed from the dam and dispersed in a small (11″ × 7″) cage for 30 minutes once each day on postpartum days 5 through 10, then 45 minutes per day for the next 5 days (10 days total ending on day 15). In pilot studies these durations were found to be clearly submaximal. A small animal heating pad was placed under the treatment cage at one end creating a nesting area with slightly elevated temperature (~26°C). The activity protocol took advantage of the natural behavior of the rat pups to move to a warm spot in the cage and huddle together. During the exercise treatment period, the animals were repeatedly re-dispersed to promote crawling activity. Individual animals were tracked in order to achieve a uniform volume of crawling activity within and across groups.
Prior to each EX treatment, bedding from the home cage of the group was added to the treatment cage. In addition, research personnel handled the dam before touching the pups (HAND and EX).
Rats from all groups were weaned at 22 days postpartum.
Tissue Collection & Analysis
Subgroups of rats were sacrificed at 30 (equivalent to pre-pubertal humans) and 65 (~young adult humans) days postpartum (26). The rats were euthanized using Pentosol solution. Body mass was measured using a digital scale. (Sartorius BP310P, Bradford, MA). After induction of deep anesthesia, prior to cessation of breathing, blood was collected from the left ventricle via the diaphragm using a heparinized syringe. The ventricles, soleus, plantaris, medial gastrocnemius (MG) muscles were dissected free of connective tissue, weighed, snap frozen and stored at −80°C for later analysis.
Muscle Protein, Myofibrillar Protein and DNA
Plantaris muscle samples were homogenized in sucrose buffer and myofibrillar proteins quantitatively extracted from a known volume of the total homogenate by a modification of the original procedure described by Solaro et al. (27). Protein concentrations were determined using the Biorad Protein assay. Muscle total DNA concentration was determined using a fluorometric assay with DNA-specific fluorescent dye (28).
Plasma Cytokine Measurements Blood Sampling and Analysis
Aliquoted plasma was stored at −80c. Cytokine levels in plasma were measured using ELISA kits (R&D Systems, Minneapolis, MN) (Table 1).
Table 1.
Plasma Cytokine Assay Characteristics.
| Cytokine | IL-1β | TNF-α | IL-6 |
|---|---|---|---|
| Intra-assay CV% | 4.1–5.7 | 2.1–5.1 | 4.5–8.8 |
| Inter-assay CV% | 3.9–8.8 | 8.8–9.7 | 7.0–10.0 |
| Sensitivity pg/ml | ≤ 5.0 | ≤ 5.0 | 22 |
Statistical analysis
Values are reported as mean and standard error of the mean (SEM). Between group analysis was conducted using a One-Way ANOVA and Student post test (PRISM- Graphpad, La Jolla, CA). For all statistical tests the 0.05 level of confidence was accepted for statistical significance.
RESULTS
Growth Effects of Neonatal Exercise
The growth rates of each group are presented in Table 2. No differences in body mass were found at 30 days. The body mass of the EX rats was significantly lower than that of the CON and HAND groups in male adult (65 day) rats (Table 3). At 65 days, tibial length was not different between groups but had diverged by gender (male 37±0.3 vs. female 35±0.3 mm, p<0.0001).
Table 2.
Body Mass Growth Rate (Assessed By Exponential Fit)
| CON | HAND | Ex | ||
|---|---|---|---|---|
| Female | k | 0.0363±0.0015 | 0.0374±0.0014 | 0.0361±0.0012 |
| Fit (r2) | 0.96 | 0.96 | 0.97 | |
| Male | k | 0.0446±0.0017 | 0.0460±0.0012 | 0.0434±0.0016 |
| Fit (r2) | 0.98 | 0.99 | 0.97 |
k = rate constant
Table 3.
Effects of 10 Days of Neonatal Exercise on Body Mass.
| Body Mass | CON | HAND | EX |
|---|---|---|---|
| MALE 30 DAY | 95±3 | 95±2 | 101±1 |
| FEMALE 30DAY | 87±3 | 89±1 | 93±1 |
| MALE 65 DAY | 375±10 | 369±5 | 343±10*‡ |
| FEMALE 65DAY | 226±5 | 236±9 | 237±4 |
n = 8;
P<0.05 vs. CON;
P<0.05 vs. HAND
At 65 days of age, the relative mass (mg/g body weight) of leg muscles, such as the MG, was significantly greater in the male EX group (Table 4). Similar results were seen for soleus and plantaris muscle mass (data not shown).
Table 4.
Effects of 10 Days of Neonatal Exercise on M. Gastrocnemius Muscle Mass.
| Medial Gastrocnemius (mg/g body) |
CON | HAND | EX |
|---|---|---|---|
| MALE 30DAY | 1.9±0.03 | 1.9±0.03 | 1.9±0.06 |
| FEMALE 30DAY | 2.0±0.03 | 1.9±0.02 | 2.0±0.03 |
| MALE 65 DAY | 2.40±0.05 | 2.34±0.10 | 2.72±0.12*‡ |
| FEMALE 65DAY | 2.64±0.02 | 2.52±0.06 | 2.56±0.06 |
n = 8;
P<0.05 vs. CON;
P<0.05 vs. HAND
The plantaris muscle was analyzed for both total and myofibrillar protein composition (Table 5). At 30 and 65 days of age the total protein content of the plantaris muscle was greater in the male EX rats (8 and 11% respectively). Myofibrillar protein content was also significantly greater (15 and 20%) in the male EX rats. At 65 days, the myofibrillar protein content of female Ex rat muscle was also ~13% greater.
Table 5.
Effects of Neonatal 10 Days of Exercise on Muscle Protein
| Total Protein (mg) |
Myofibrillar Protein (mg) |
|||||
|---|---|---|---|---|---|---|
| CON | HAND | EX | CON | HAND | EX | |
| MALE 30 DAY | 14.9±0.9 | 14.7±0.5 | 16.2±0.6‡ | 7.6±0.4 | 7.5±0.2 | 8.7±0.3*‡ |
| FEMALE 30 DAY | 13.3±0.8 | 13.7±0.3 | 14.7±0.7 | 7.9±0.3 | 7.7±0.2 | 7.7±0.5 |
| MALE 65 DAY | 82±3 | 79±2 | 91±2*‡ | 43±3 | 44±1 | 51±2*‡ |
| FEMALE 65DAY | 57±2 | 58±1 | 61±2 | 30±1 | 31±1 | 34±1*‡ |
n = 8;
P<0.05 vs. CON;
P<0.05 vs. HAND
Pre-pubertal rats (30 days) that had been exposed to physical activity (EX) demonstrated significantly increased relative left ventricular mass (mg/g body) of ~11% compared to CON (Table 6). This difference was apparent for both genders. At 65 days of age, relative left ventricle mass was significantly greater (+12%) in EX males but not females.
Table 6.
Effects of 10 Days of Neonatal Exercise on Left Ventricle Muscle Mass.
| MG (mg/g body) | CON | HAND | EX |
|---|---|---|---|
| MALE 30DAY | 3.9±0.1 | 3.7±0.1 | 4.2±0.1*‡ |
| FEMALE 30DAY | 3.9±0.1 | 3.9±0.1 | 4.5±0.1*‡ |
| MALE 65 DAY | 2.6±0.1 | 2.7±0.1 | 2.9±0.1*‡ |
| FEMALE 65DAY | 2.9±0.0 | 3.0±0.1 | 3.1±0.0 |
No significant differences were found in DNA concentration at any time point.
Proinflammatory Cytokines
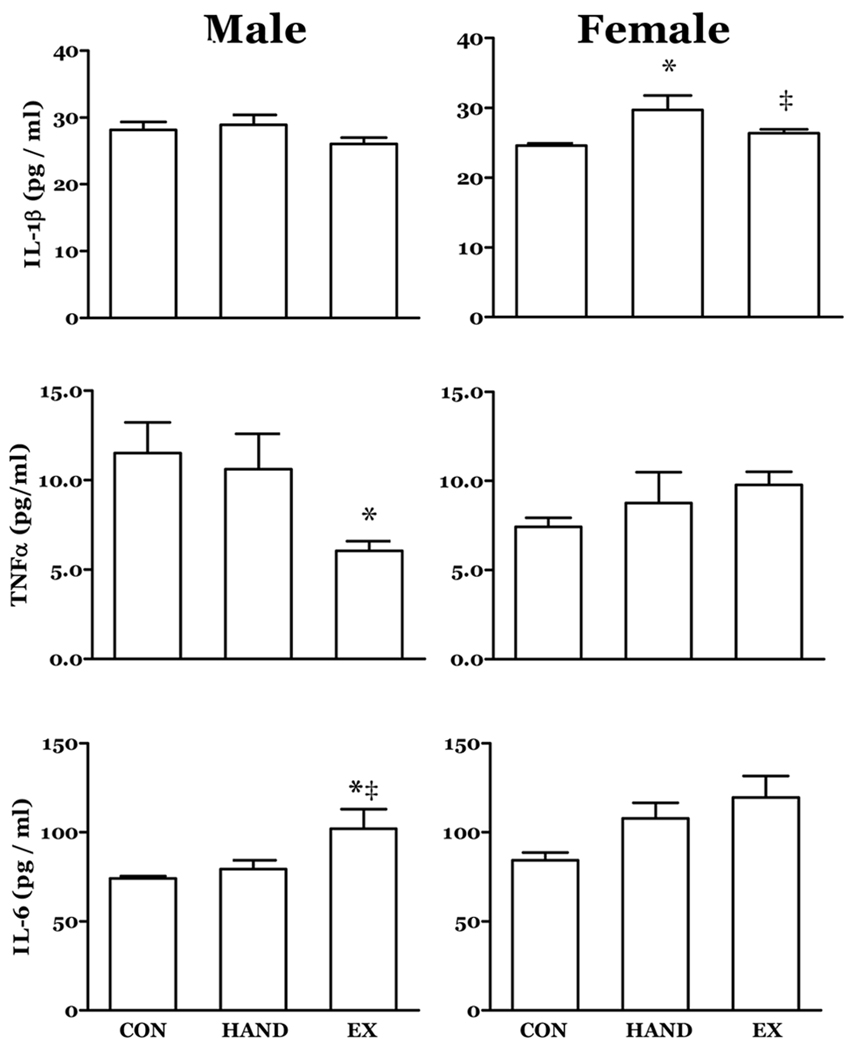
At 30 days of age the male EX rats had increased circulating levels of IL-6 while levels of TNFα were depressed (Fig. 1). In 30 day females, circulating IL-1β concentration was increased in rats that had been handled (HAND) compared to the CON and EX groups.
Figure 1. The effects of prior neonatal activity on selected circulating cytokines at post-partum day 30.
Rat neonates experienced increased physical activity (EX), handling (HAND) or no treatment (CON) from post-partum day 5 through 15. A subgroup was sampled at day 30. The neonatal activity treatment resulted in lower circulating levels of TNFα and higher IL-6 in 30 day males. Plasma levels of IL-1β were elevated in HAND rats relative to CON and EX. n = 8; *, P<0.05 vs. CON; ‡, P<0.05 vs. HAND.
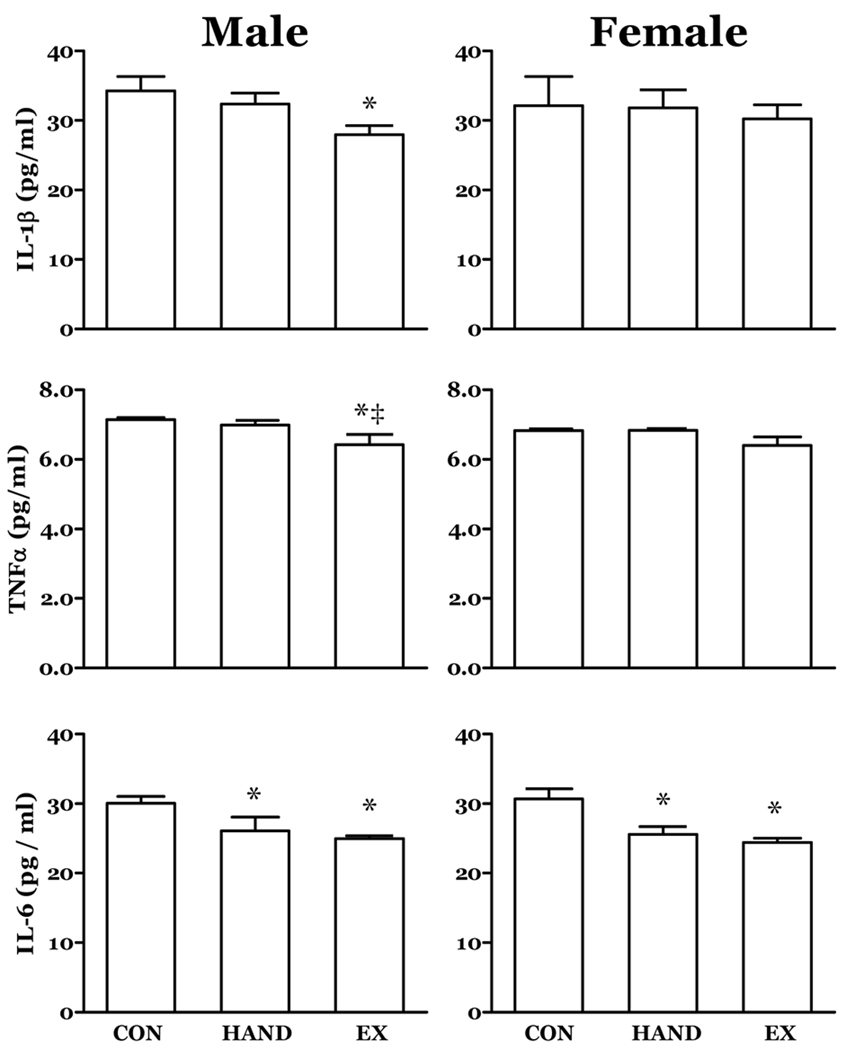
In adult rats (65 Days), both the HAND and EX groups had lower circulating levels of IL-6 that were similar in both genders (Fig. 2). In male adult rats in the EX group, circulating concentrations of both IL-1β and TNF-α were reduced (Fig. 2).
Figure 2. The effects of prior neonatal activity on selected circulating cytokines at post-partum day 65.
Rat neonates experienced increased physical activity (EX), handling (HAND) or no treatment (CON) from post-partum day 5 through 15. A subgroup was sampled at day 65. In males, the neonatal activity treatment resulted in lower circulating levels of IL-6, TNFα and IL-1β at 60 days of age. Plasma levels of IL-6 were also lower in 65 day EX females. Both male and female HAND rats demonstrated IL-6 levels that were lower than CON. n = 8; *, P<0.05 vs. CON; ‡, P<0.05 vs. HAND.
DISCUSSION
We found that a modest increase in physical activity in the neonatal period had significant effects on muscle mass and circulating inflammatory cytokines in adulthood. Early life exercise, but not handling alone, led to significant increases in skeletal muscle mass and myofibrillar protein in adulthood. The muscle effects occurred in both males and females, but were more pronounced in the males. The increase in muscle mass and protein was not accompanied by increased body weight, indeed, the male rats that had exercised as neonates weighed significantly less as adults (Table 3). Bone length was not affected by the early-in-life exercise, suggesting that the lower body weights did not result from a global catabolic effect of early exercise. Left ventricular mass was greater in adult rats that had exercised as neonates. It is important to note that all programmed exercise ceased at post-partum day 15. Therefore, the data from the pre-pubertal and adult animals represents time points either 15 or 50 days after the last programmed exercise. This suggests that between-group differences represent relatively long lived effects.
Although statistically significant, the magnitude of the effects on muscle mass and myofibrillar protein appear to be modest. Changes of this magnitude in the newborn period are deceptively small relative to potential long-term consequences, (29–31) perhaps due to “reprogramming”. For example, Stettler et al.(32) observed in American formula-fed subjects that for every 100g increase in weight gain during the first week of infancy there was a 28% increase in the risk of adult obesity. We speculate that the increase in muscle myofibrillar protein that we observed in the adult rats that had exercised as neonates reflects a broad, heretofore undiscovered set of biological mechanisms that likely affect more than just muscle size.
A unique feature of this study was the specific measurement of myofibrillar protein content in addition to the gross mass of specific muscle groups. The measurement of muscle mass can be confounded by a number of factors including intramuscular fat, which can be influenced by specific genetic factors during growth (33), and connective tissue, which can be affected by physical activity (34). Myofibrillar protein content accurately reflects the pool of functional proteins that account for the ability of muscles to perform work (35). Although relatively poorly studied in the neonatal period, changes in myofibrillar elements can occur leading to improved function, without concomitant increases in overall muscle size (36). Our finding of increased myofibrillar protein content in adult rats indicates that a profound, developmentally sensitive and functionally consequential reprogramming event had occurred earlier in life as a result of the neonatal exercise.
Increased cardiac work (pressure load) is a well-established cause of increased heart muscle. Far less is known about the impact of physical activity on cardiac dimensions in children, and reports are contradictory. For example, while increased cardiac work secondary to obesity can alter cardiac dimensions in children (37), Dencker et al. (38) found no association between physical activity in children and cardiac dimensions. Our study is the first to show an effect of neonatal exercise on subsequent cardiac size, an effect that was observed at 30 days in both males and females and at 60 days in males. The physiological and functional impact of this adaptation is as yet unknown. However, it is assumed that increased oxygen demand resulted in increased cardiac output, e.g., flow work, rather than pressure work, leading to functional hypertrophy.
These data add to a small but growing number of studies suggesting that early life physical activity might benefit body composition throughout the lifespan. For example, Levin and coworkers (39) have selectively bred rats to manifest a diet-induced obesity (DIO) phenotype. These investigators found that only three weeks of exercise early in life was sufficient to prevent DIO rats from becoming obese for up to ten weeks once the exercise was terminated. Similar effects were not observed in adult DIO rats. Finally, in marked contrast to early-in-life exercise, early-in-life caloric restriction led to DIO animals that were more obese as adults once allowed to eat ad libitum.
Gender dimorphism in physiological and muscle responses to exercise and training are well-described (40), and we found gender differences in the adult response to neonatal exercise. Interestingly, others have reported that the body composition of female rats is much less sensitive to perturbations, such as running exercise or cardiac cachexia, than that of males (41,42). Colom et al. (43) recently demonstrated that gastrocnemius muscle in female rats had higher mitochondrial DNA and protein contents, as well as oxidative and phosphorylative enzymatic machinery, which could explain the higher facility of females to adapt to altered metabolic energy situations. Perhaps, if this gender-related metabolic advantage exists in the skeletal muscle of the neonatal rat, then the perturbation imposed by early-life exercise was mitigated in the females thereby muting any long-term adaptive or reprogramming processes. In addition, we designed the experiment to find changes in muscle properties in the late to postpubertal maturational stages. It remains possible that some combination of the pubertal sex steroids--estradiols and testosterone, which are known to influence skeletal muscle differently (44) with the, as yet, undefined signal from neonatal exercise led to an attenuated effect on muscle size in the adult female rats.
A key finding of this study was the effect of neonatal exercise on levels of circulating inflammatory mediators later in life. In addition to their catabolic properties, stress and inflammatory mediators like IL-6, TNF-α, and IL-1β are all involved in atherosclerosis and increased risk for cardiovascular disease (45–47). Not surprisingly, most research focused on neonatal programming during critical periods of growth are centered on the pediatric origins of adult disease and target abnormal early life events [e.g., small for gestational age, prematurely born infants being predisposed to adult obesity and cardiovascular disease (48–50)]. Ours is one of the first studies to examine perturbations during critical developmental periods in otherwise healthy newborns. First, we found that both handling and exercise during the neonatal period led to reduced circulating levels of IL-6 in both male and female adult rats. Although we did not record the impact of either handling or exercise during the neonatal period on subsequent patterns of physical activity, the data from Weininger (24) cited early suggested that handling in and of itself leads to increased physical activity which, perhaps, was a common stimulus for the subsequent changes in circulating cytokines observed later in life.
We also found evidence of gender dimorphism in the effect of neonatal interventions on inflammatory cytokine profiles later in life. In males, but not in females, the early exercise intervention influenced TNF-α and IL-1β in the adult animals. Gender dimorphism in inflammatory mediator responses to a variety of perturbations is now well-established (51,52), and most authors attribute these differences, in part, to the varied effects of the sex steroids on immune responsive cells. However, the mechanisms responsible for the gender dimorphism in the late-effect cytokine profiles in our study remain elusive.
In general, the regulation of circulating levels of inflammatory mediators like IL-6 has undergone substantial rethinking in recent years. For example, the discovery that IL-6 is produced by muscle tissue and not solely immune cells and that exercising muscle is responsible for the increase in IL-6 observed with exercise (53,54) has led to the novel idea that muscle itself plays a role in endocrine and inflammatory regulation. We did find significant changes in myofibrillar protein in adult muscles consequent to exercise early in life. This leads to the intriguing hypothesis that skeletal muscle itself plays a more active role in the regulation of circulating inflammatory cytokines than previously imagined.
There are a number of potential limitations to this study. Differences in maternal care could contribute to lasting effects seen in adult animals. To minimize this effect, each group in the current study consisted of litters reared by four different dams which would be expected to randomly distribute any differences in maternal behaviors. Analysis of growth rates by litter did not indicate any maternal bias (data not shown).
There are also reports of gender specific preferences in maternal care (e.g., 55). However, this is not a universal finding (56). In murine species, effects of gender differences in care appear to be primarily confined to later behavioral differences (e.g. 57) but not necessarily physiological development (58). In the current study, the gender specific growth rates through weaning was very similar indicating that this was not a confounding factor (data not shown).
The estrous phase of the female rats was not controlled and could have affected the plasma cytokine results.
In summary, the unique finding of this experiment was that a relatively brief intervention that modestly increased physical activity in a critical period of neonatal development in the rat led to changes in skeletal muscle and heart mass and circulating inflammatory cytokine profiles in the adult animals. The changes were influenced by gender. The observed increases in muscle mass and myofibrillar protein were not accompanied by increases in body weight, findings consistent with previous studies of exercise intervention in rats indicating that early-in-life exercise mitigates adult onset obesity. In addition, through mechanisms that are still unclear, the early-in-life exercise was associated with a reduction in inflammatory cytokines that are known to antagonize skeletal muscle growth and contribute to the development of cardiovascular disease. These studies provide experimental models to elucidate novel mechanisms of long term effects of exercise in early, critical periods of growth. Such research could complement clinical studies targeting the role of exercise in newborns (59,60) and children as adjunctive or preventive therapy both in health (e.g., to prevent obesity) or to attenuate the deleterious effects of chronic inflammatory diseases of childhood.
Acknowledgments
Funding for this project was provided by the National Institutes of Health, P01HD048721.
Abbreviations
- CON
Control
- DIO
Diet-induced obesity
- EX
Exercised
- HAND
Handled
- MG
Medial Gastrocnemius
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bechtold S, Alberer M, Arenz T, Putzker S, Filipiak-Pittroff B, Schwarz HP, Koletzko S. Reduced muscle mass and bone size in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:216–225. doi: 10.1002/ibd.21021. [DOI] [PubMed] [Google Scholar]
- 2.Bechtold S, Dalla PR, Schwarz HP, Simon D. Effects of growth hormone treatment in juvenile idiopathic arthritis: bone and body composition. Horm Res. 2009;72:60–64. doi: 10.1159/000229766. [DOI] [PubMed] [Google Scholar]
- 3.Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle Size and Cardiorespiratory Response to Exercise in Cystic Fibrosis. Am J Respir Crit Care Med. 2000;162:1823–1827. doi: 10.1164/ajrccm.162.5.2003057. [DOI] [PubMed] [Google Scholar]
- 4.Ralt D. The muscle--fat duel or why obese children are taller? BMC Pediatr. 2006;6:33. doi: 10.1186/1471-2431-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahlkvist S, Pedersen S. Fitness, daily activity and body composition in children with newly diagnosed, untreated asthma. Allergy. 2009;64:1649–1655. doi: 10.1111/j.1398-9995.2009.02081.x. [DOI] [PubMed] [Google Scholar]
- 6.Adams GR. Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- 7.Nystrom G, Pruznak A, Huber D, Frost RA, Lang CH. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism. 2009;58:787–797. doi: 10.1016/j.metabol.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann Transplant. 2005;10:43–48. [PubMed] [Google Scholar]
- 10.Ahmad I, Zaldivar F, Iwanaga K, Koeppel R, Grochow D, Nemet D, Waffarn F, Eliakim A, Leu SY, Cooper DM. Inflammatory and growth mediators in growing preterm infants. J Pediatr Endocrinol Metab. 2007;20:387–396. doi: 10.1515/jpem.2007.20.3.387. [DOI] [PubMed] [Google Scholar]
- 11.Grounds MD. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19–24. doi: 10.1023/a:1015234709314. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 13.Pass C, MacRae VE, Ahmed SF, Farquharson C. Inflammatory cytokines and the GH/IGF-I axis: novel actions on bone growth. Cell Biochem Funct. 2009;27:119–127. doi: 10.1002/cbf.1551. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 16.Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;119(Suppl 35):159–184. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 17.Gicquel C, El-Osta A, Le BY. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22:1–16. doi: 10.1016/j.beem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, Muhlhausler BS. Early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;646:71–81. doi: 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- 19.Nesterenko TH, Aly H. Fetal and neonatal programming: evidence and clinical implications. Am J Perinatol. 2009;26:191–198. doi: 10.1055/s-0028-1103027. [DOI] [PubMed] [Google Scholar]
- 20.Singhal A. Early nutrition and long-term cardiovascular health. Nutr Rev. 2006;64:S44–S49. doi: 10.1301/nr.2006.may.s44-s49. [DOI] [PubMed] [Google Scholar]
- 21.Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 22.Meaney MJ, Mitchell JB, Aitken DH, Bodnoff SR, Iny LJ, Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- 23.Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, Grote K, Jacobs R, Stephan M, Pabst R, von Hörsten S. Postnatal life events affect the severity of asthmatic airway inflammation in the adult. rat. J Immunol. 2008;180:3919–3925. doi: 10.4049/jimmunol.180.6.3919. [DOI] [PubMed] [Google Scholar]
- 24.Weininger O. The effects of early experience on behavior and growth characteristics. J Comp Physiol Psychol. 1956;49:1–9. doi: 10.1037/h0045334. [DOI] [PubMed] [Google Scholar]
- 25.Agnish ND, Keller KA. Rationale for culling of rodent litters. Fundam Appl Toxicol. 1997;38:2–6. doi: 10.1006/faat.1997.2318. [DOI] [PubMed] [Google Scholar]
- 26.Evans AM. Age at puberty and first litter size in early and late paired rats. Biol Reprod. 1986;34:322–326. doi: 10.1095/biolreprod34.2.322. [DOI] [PubMed] [Google Scholar]
- 27.Solaro RJ, Pang DC, Briggs FN. Purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971;245:259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- 28.Labarca C, Paigen K. DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 29.McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, Muhlhausler BS. Early origins of later obesity. Adv Exp Med Biol. 2009;646:71–81. doi: 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- 30.Morris MJ, Velkoska E, Cole TJ. Central and peripheral contributions to obesity-associated hypertension: impact of early overnourishment. Exp Physiol. 2005;90:697–702. doi: 10.1113/expphysiol.2005.030783. [DOI] [PubMed] [Google Scholar]
- 31.Ong KK, Emmett PM, Noble S, Ness A, Dunger DB. Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics. 2006;117:e503–e508. doi: 10.1542/peds.2005-1668. [DOI] [PubMed] [Google Scholar]
- 32.Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, Ziegler EE, Strom BL. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–1903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 33.Fan B, Du ZQ, Rothschild MF. Fat mass and obesity-associated gene is associated with intramuscular fat content and growth rate in the pig. Anim Biotechnol. 2009;20:58–70. doi: 10.1080/10495390902800792. [DOI] [PubMed] [Google Scholar]
- 34.Willems ME, Miller GR, Stauber FD. Stauber WT Effects of repeated lengthening contractions on skeletal muscle adaptations in female rats. J Physiol Sci. 2010;60:143–150. doi: 10.1007/s12576-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gokhin DS, Ward SR, Bremner SN, Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J Exp Biol. 2008;211:837–843. doi: 10.1242/jeb.014340. [DOI] [PubMed] [Google Scholar]
- 37.Peralta-Huertas J, Livingstone K, Banach A, Klentrou P, O'Leary D. Differences in left ventricular mass between overweight and normal-weight preadolescent children. Appl Physiol Nutr Metab. 2008;33:1172–1180. doi: 10.1139/H08-082. [DOI] [PubMed] [Google Scholar]
- 38.Dencker M, Thorsson O, Karlsson MK, Linden C, Wollmer P, Andersen LB. Objectively measured daily physical activity related to cardiac size in young children. Scand J Med Sci Sports. 2009;19:664–668. doi: 10.1111/j.1600-0838.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 39.Levin BE. Epigenetic influences on food intake and physical activity level: review of animal studies. Obesity (Silver Spring) 2008;16:S51–S54. doi: 10.1038/oby.2008.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galassetti P, Neill AR, Tate D, Ertl AC, Wasserman DH, Davis SN. Sexual dimorphism in counterregulatory responses to hypoglycemia after antecedent exercise. J Clin Endocrinol Metab. 2001;86:3516–3524. doi: 10.1210/jcem.86.8.7720. [DOI] [PubMed] [Google Scholar]
- 41.Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. Daily exercise reduces fat, protein and body mass in male but not female rats. Physiol Behav. 1997;62:105–111. doi: 10.1016/s0031-9384(97)00148-0. [DOI] [PubMed] [Google Scholar]
- 42.Palus S, Akashi Y, vonHaehling S, Anker SD, Springer J. Influence of age and sex on disease development in a novel animal model of cardiac cachexia. Int J Cardiol. 2009;133:388–393. doi: 10.1016/j.ijcard.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 43.Colom B, Alcolea MP, Valle A, Oliver J, Roca P, Garcia-Palmer FJ. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities than males. Cell Physiol Biochem. 2007;19:205–212. doi: 10.1159/000099208. [DOI] [PubMed] [Google Scholar]
- 44.Paroo Z, Dipchand ES, Noble EG. Estrogen attenuates postexercise HSP70 expression in skeletal muscle. Am J Physiol Cell Physiol. 2002;282:C245–C251. doi: 10.1152/ajpcell.00336.2001. [DOI] [PubMed] [Google Scholar]
- 45.Grundtman C, Hollan I, Forre OT, Saatvedt K, Mikkelsen K, Lundberg IE. Cardiovascular disease in patients with inflammatory rheumatic disease is associated with up-regulation of markers of inflammation in cardiac microvessels and cardiomyocytes. Arthritis Rheum. 2010;62:667–673. doi: 10.1002/art.27264. [DOI] [PubMed] [Google Scholar]
- 46.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 47.Zee RY, Glynn RJ, Cheng S, Steiner L, Rose L, Ridker PM. An evaluation of candidate genes of inflammation and thrombosis in relation to the risk of venous thromboembolism: The Women's Genome Health Study. Circ Cardiovasc Genet. 2009;2:57–62. doi: 10.1161/CIRCGENETICS.108.801969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. Adv Exp Med Biol. 2009;646:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 49.Singhal A. The early origins of atherosclerosis. Adv Exp Med Biol. 2009;646:51–58. doi: 10.1007/978-1-4020-9173-5_5. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends Endocrinol Metab. 2008;19:146–152. doi: 10.1016/j.tem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Diodato MD, Knoferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–169. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- 52.Edwards KM, Burns VE, Ring C, Carroll D. Individual differences in the interleukin-6 response to maximal and submaximal exercise tasks. J Sports Sci. 2006;24:855–862. doi: 10.1080/02640410500245645. [DOI] [PubMed] [Google Scholar]
- 53.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release. Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528:157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 56.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 57.Moore CL, Power KL. Variation in maternal care and individual differences in play, exploration, and grooming of juvenile Norway rat offspring. Dev Psychobiol. 1992;25:165–182. doi: 10.1002/dev.420250303. [DOI] [PubMed] [Google Scholar]
- 58.Alleva E, Caprioli A, Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam. J Comp Psychol. 1989;103:83–87. doi: 10.1037/0735-7036.103.1.83. [DOI] [PubMed] [Google Scholar]
- 59.Eliakim A, Nemet D. Osteopenia of prematurity - the role of exercise in prevention and treatment. Pediatr Endocrinol Rev. 2005;2:675–682. [PubMed] [Google Scholar]
- 60.Moyer-Mileur LJ, Brunstetter V, McNaught TP, Gill G, Chan GM. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000;106:1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]