Abstract
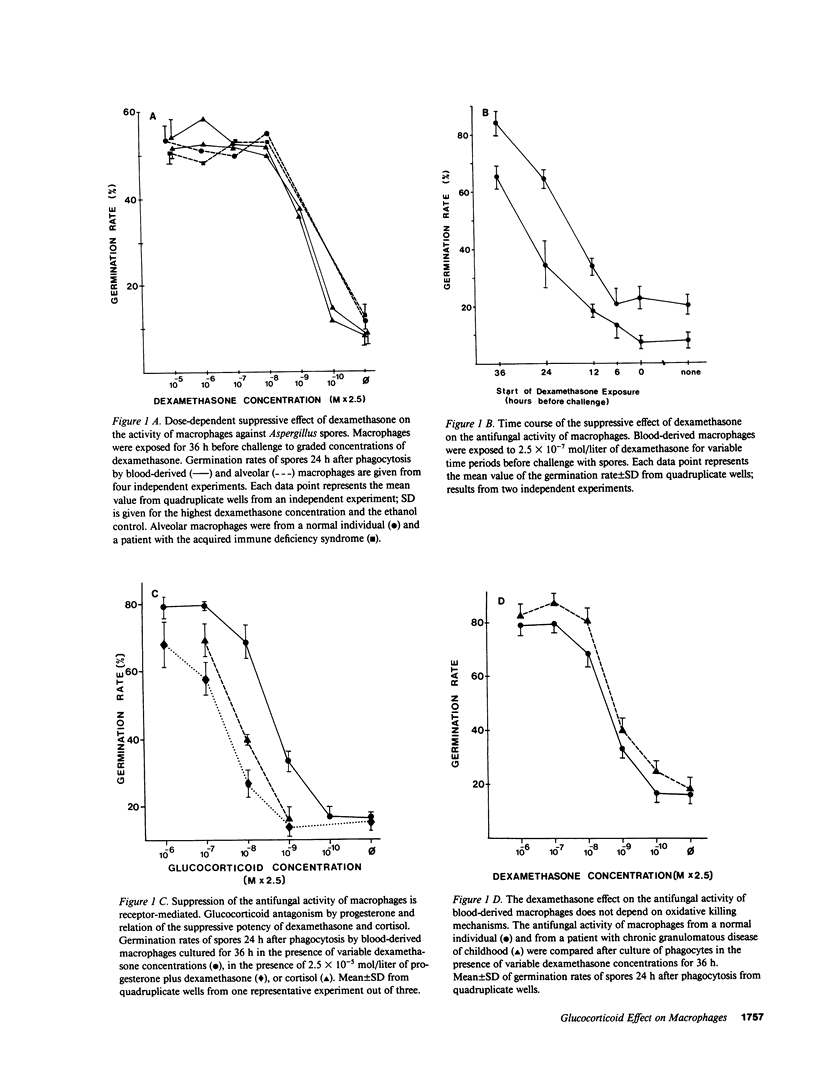
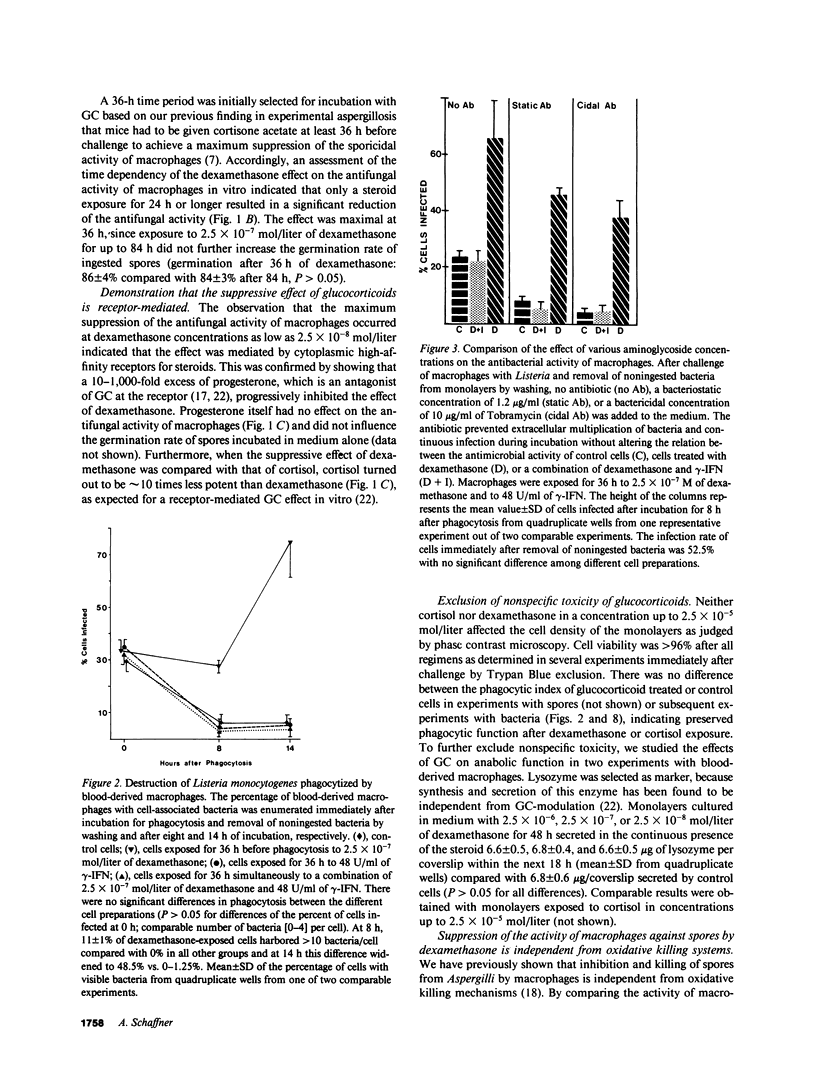
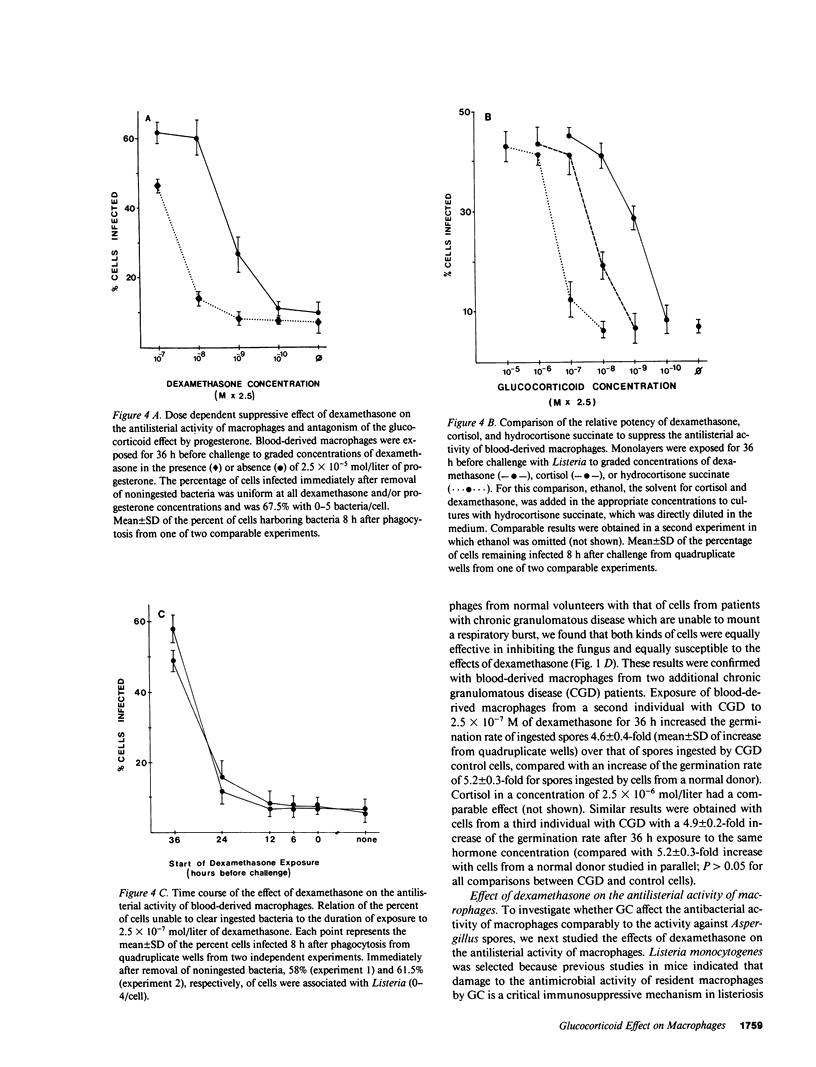
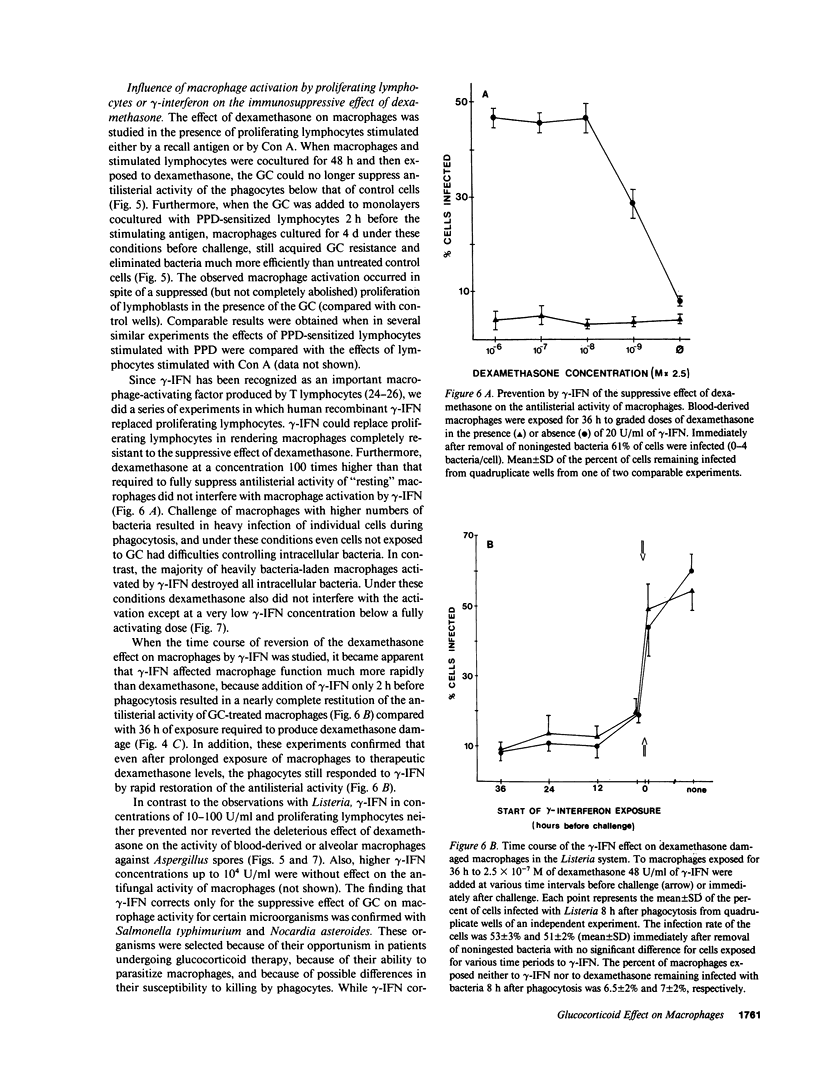
By exposing human blood-derived macrophages and alveolar macrophages in vitro to dexamethasone, we showed in these studies that glucocorticoids markedly suppress the antimicrobial activity of macrophages but not macrophage activation by lymphokines. As little as 2.5 X 10(-8) mol/liter of dexamethasone prevented macrophages from inhibiting germination of Aspergillus spores or from eliminating ingested bacteria such as Listeria, Nocardia, or Salmonella. Damage to macrophage function was inhibited by progesterone and appeared to be receptor-mediated. In accordance with in vivo observations, dexamethasone required 24-36 h to suppress antimicrobial activity. While glucocorticoids interfered with base-line activity of macrophages, dexamethasone concentrations comparable to drug levels in patients had no effect on macrophage activation. Proliferating lymphocytes and gamma-interferon thus increased the antimicrobial activity of phagocytes exposed to glucocorticoids over that of control cells. Macrophage activation and correction of the dexamethasone effect by gamma-interferon, however, was dependent on the pathogen. The lymphokine enhanced the antimicrobial activity of dexamethasone-treated macrophages against Listeria and Salmonella but not against Aspergillus or Nocardia. Dexamethasone-induced damage to the antimicrobial activity of human macrophages in vitro parallels observations that glucocorticoids render laboratory animals susceptible to listeriosis and aspergillosis by damaging resident macrophages. Suppression of macrophage antimicrobial activity should thus be considered when treating patients with glucocorticoids; its prevention by gamma-interferon might be beneficial for some but not all pathogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Schafer L. A., Olin D. B., Eickhoff T. C. Infectious risk factors in the immunosuppressed host. Am J Med. 1973 Apr;54(4):453–460. doi: 10.1016/0002-9343(73)90041-7. [DOI] [PubMed] [Google Scholar]
- Ballard P. L. Delivery and transport of glucocorticoids to target cells. Monogr Endocrinol. 1979;12:25–48. doi: 10.1007/978-3-642-81265-1_2. [DOI] [PubMed] [Google Scholar]
- Baumann G., Rappaport G., Lemarchand-Béraud T., Felber J. P. Free cortisol index: a rapid and simple estimation of free cortisol in human plasma. J Clin Endocrinol Metab. 1975 Mar;40(3):462–469. doi: 10.1210/jcem-40-3-462. [DOI] [PubMed] [Google Scholar]
- Blackwood L. L., Pennington J. E. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982 Dec;126(6):1045–1049. doi: 10.1164/arrd.1982.126.6.1045. [DOI] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Gillis S., Smith K. A., Munck A. Glucocorticoids and immune responses. Arthritis Rheum. 1979 Nov;22(11):1246–1256. doi: 10.1002/art.1780221112. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Immunosuppressive and anti-inflammatory effects of glucocorticoids. Monogr Endocrinol. 1979;12:449–465. doi: 10.1007/978-3-642-81265-1_24. [DOI] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Krick J. A., Remington J. S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980 Sep;142(3):432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- Ginzler E., Diamond H., Kaplan D., Weiner M., Schlesinger M., Seleznick M. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum. 1978 Jan-Feb;21(1):37–44. doi: 10.1002/art.1780210107. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Tucker W. S., Jr Opportunistic infections in endogenous Cushing's syndrome. Ann Intern Med. 1984 Sep;101(3):334–338. doi: 10.7326/0003-4819-101-3-334. [DOI] [PubMed] [Google Scholar]
- Gustafson T. L., Schaffner W., Lavely G. B., Stratton C. W., Johnson H. K., Hutcheson R. H., Jr Invasive aspergillosis in renal transplant recipients: correlation with corticosteroid therapy. J Infect Dis. 1983 Aug;148(2):230–238. doi: 10.1093/infdis/148.2.230. [DOI] [PubMed] [Google Scholar]
- Masur H., Murray H. W., Jones T. C. Effect of hydrocortisone on macrophage response to lymphokine. Infect Immun. 1982 Feb;35(2):709–714. doi: 10.1128/iai.35.2.709-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena T., Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985 Feb;75(2):624–631. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984 Sep;74(3):771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The action of cortisone acetate on cell-mediated immunity to infection. Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971 Dec 1;134(6):1485–1500. doi: 10.1084/jem.134.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W. Steroid hormone action in eucaryotic cells. J Clin Invest. 1984 Aug;74(2):307–312. doi: 10.1172/JCI111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. E., WYNGAARDEN J. B., GUERRA S. L., BRODIE B. B., BUNIM J. J. The physiological disposition and metabolic fate of hydrocortisone in man. J Clin Invest. 1955 Dec;34(12):1779–1794. doi: 10.1172/JCI103233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponec M. Glucocorticoids and cultured human skin cells: specific intracellular binding and structure-activity relationships. Br J Dermatol. 1982 Nov;107 (Suppl 23):24–29. doi: 10.1111/j.1365-2133.1982.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Balcerzak S. P., Sagone A. L., LoBuglio A. F. Effects of corticosteroids on human monocyte function. J Clin Invest. 1974 Dec;54(6):1337–1343. doi: 10.1172/JCI107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Webel M. L., Ritts R. E., Jr, Taswell H. F., Danadio J. V., Jr, Woods J. E. Cellular immunity after intravenous administration of methylprednisolone. J Lab Clin Med. 1974 Mar;83(3):383–392. [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Foley R., Munck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J Exp Med. 1978 Jun 1;147(6):1684–1694. doi: 10.1084/jem.147.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwet T. L., Thompson J., Furth R. Effect of glucocorticosteroids on the phagocytosis and intracellular killing by peritoneal macrophages. Infect Immun. 1975 Oct;12(4):699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



