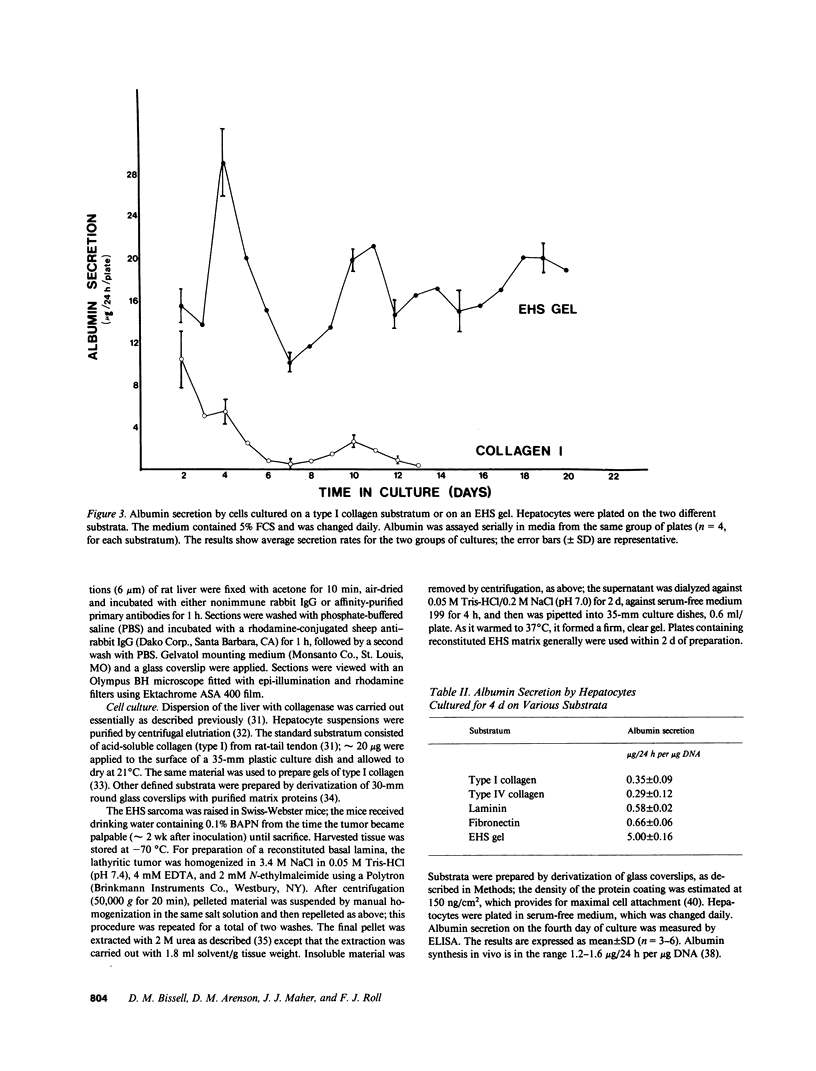
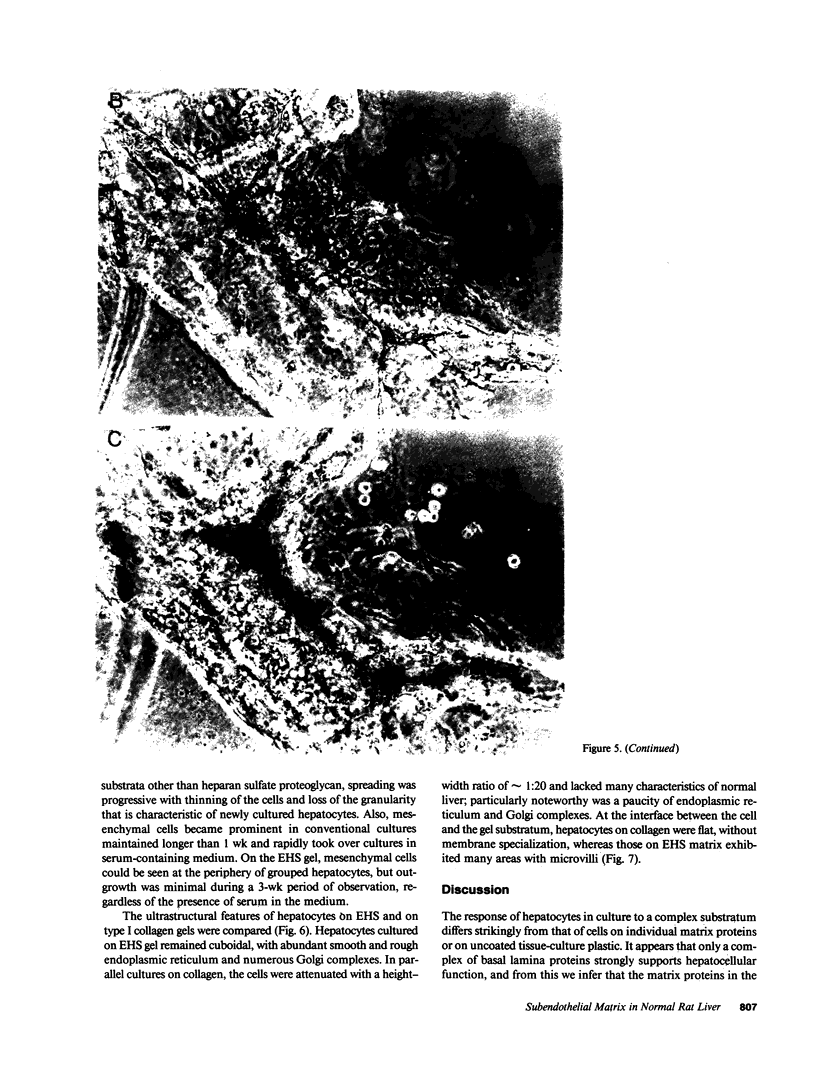
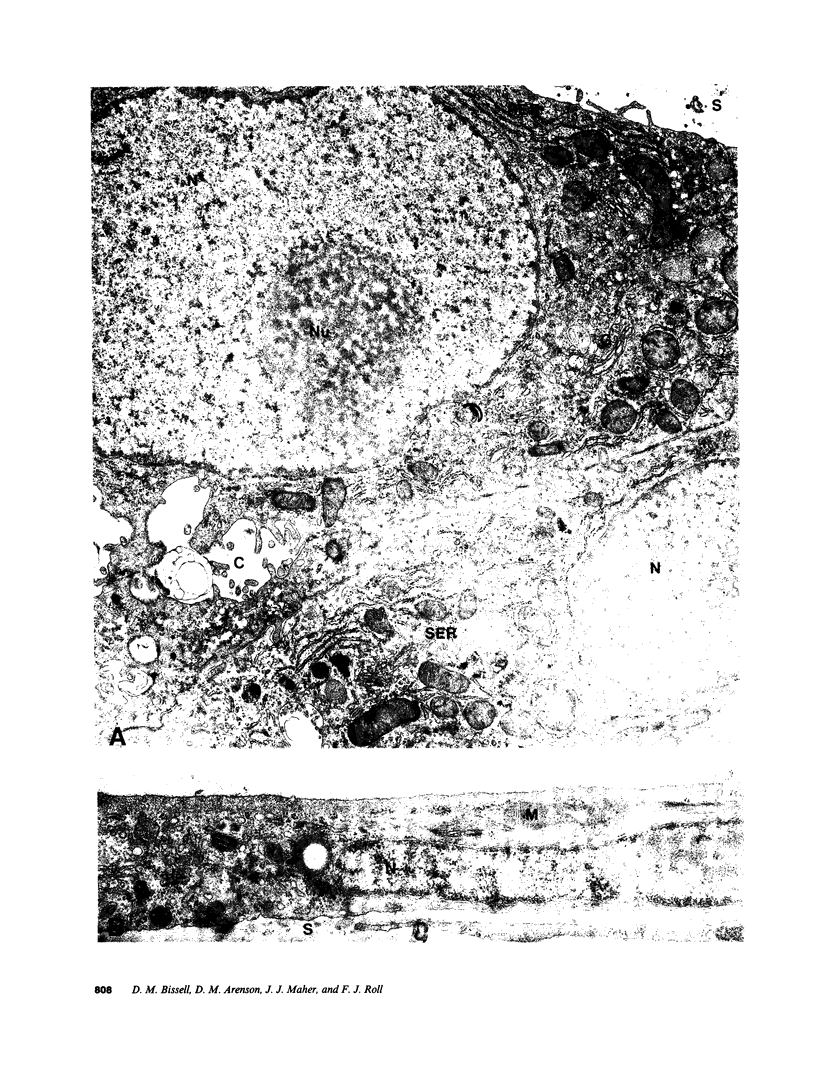
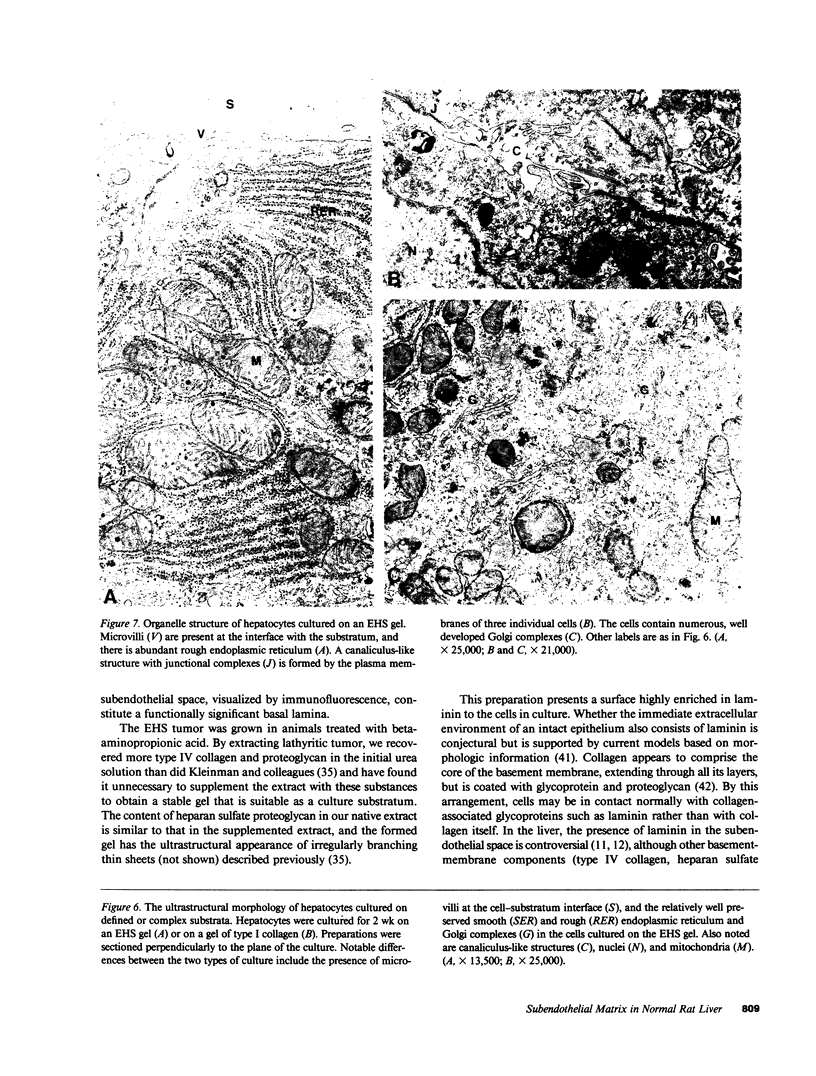
Abstract
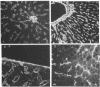
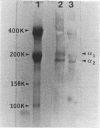
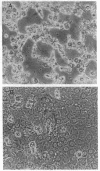
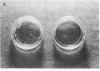
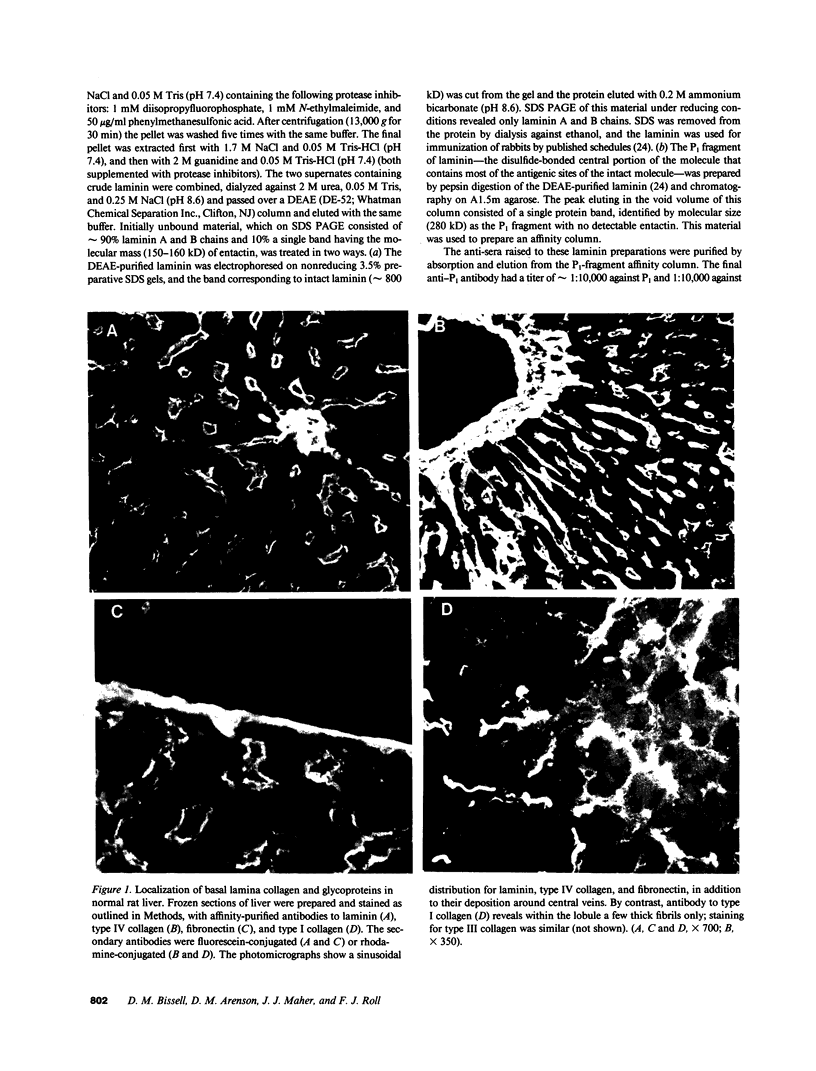
The subendothelial space of normal rat liver contains the constituent proteins of a basal lamina, as judged by immunohistochemical study of tissue sections. However, it is unknown whether these proteins constitute a complex with effects on hepatocellular function. We have examined this question, using normal rat hepatocytes cultured on substrata of matrix proteins as a model of the interaction between cells and basal lamina in vivo. In cultures on a type I collagen substratum, albumin secretion decreased progressively after 2 d. By contrast, when cells were cultured on a laminin-rich gel matrix, albumin secretion was stable for at least 3 wk; other functions and ultrastructural morphology were similarly maintained. None of the individual matrix proteins effectively substituted for the gel matrix, suggesting that full support of hepatocellular function requires a complex of matrix proteins. We speculate that a cause of hepatocellular dysfunction in acute inflammation is disruption of this matrix and alteration of its interaction with the hepatocyte plasma membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Aplin J. D., Hughes R. C. Complex carbohydrates of the extracellular matrix structures, interactions and biological roles. Biochim Biophys Acta. 1982 Dec;694(4):375–418. doi: 10.1016/0304-4157(82)90003-x. [DOI] [PubMed] [Google Scholar]
- Aplin J. D., Hughes R. C. Protein-derivatised glass coverslips for the study of cell-to substratum adhesion. Anal Biochem. 1981 May 1;113(1):144–148. doi: 10.1016/0003-2697(81)90057-9. [DOI] [PubMed] [Google Scholar]
- Barth C. A., Schwarz L. R. Transcellular transport of fluorescein in hepatocyte monolayers: evidence for functional polarity of cells in culture. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4985–4987. doi: 10.1073/pnas.79.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Guzelian P. S. Phenotypic stability of adult rat hepatocytes in primary monolayer culture. Ann N Y Acad Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Stamatoglou S. C., Nermut M. V., Hughes R. C. Interactions of rat hepatocytes with type IV collagen, fibronectin and laminin matrices. Distinct matrix-controlled modes of attachment and spreading. Eur J Cell Biol. 1986 Mar;40(1):72–78. [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carlin B., Jaffe R., Bender B., Chung A. E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981 May 25;256(10):5209–5214. [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary E. C., Yurchenco P. D., Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J Cell Biol. 1985 Jun;100(6):1848–1853. doi: 10.1083/jcb.100.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement B., Guguen-Guillouzo C., Campion J. P., Glaise D., Bourel M., Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984 May-Jun;4(3):373–380. doi: 10.1002/hep.1840040305. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Clément B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983 Jan;143(1):47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Guillouzo A. Modulation of functional activities in cultured rat hepatocytes. Mol Cell Biochem. 1983;53-54(1-2):35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- Hadley M. A., Byers S. W., Suárez-Quian C. A., Kleinman H. K., Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985 Oct;101(4):1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Leyshon W. C., Ledbetter S. R., Tyree B., Suzuki S., Kato M., Kimata K., Kleinman H. K. Isolation of two forms of basement membrane proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8098–8105. [PubMed] [Google Scholar]
- Hogan B. L., Cooper A. R., Kurkinen M. Incorporation into Reichert's membrane of laminin-like extracellular proteins synthesized by parietal endoderm cells of the mouse embryo. Dev Biol. 1980 Dec;80(2):289–300. doi: 10.1016/0012-1606(80)90405-4. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Roll F. J., Huang S., Bissell D. M. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Ho J., Mehra R., Bruce-Robertson A. Hepatotrophic effects of insulin on glucose, glycogen, and adenine nucleotides in hepatocytes isolated from fasted adult rats. Gastroenterology. 1980 Mar;78(3):556–570. [PubMed] [Google Scholar]
- Johansson S., Hök M. Substrate adhesion of rat hepatocytes: on the mechanism of attachment to fibronectin. J Cell Biol. 1984 Mar;98(3):810–817. doi: 10.1083/jcb.98.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
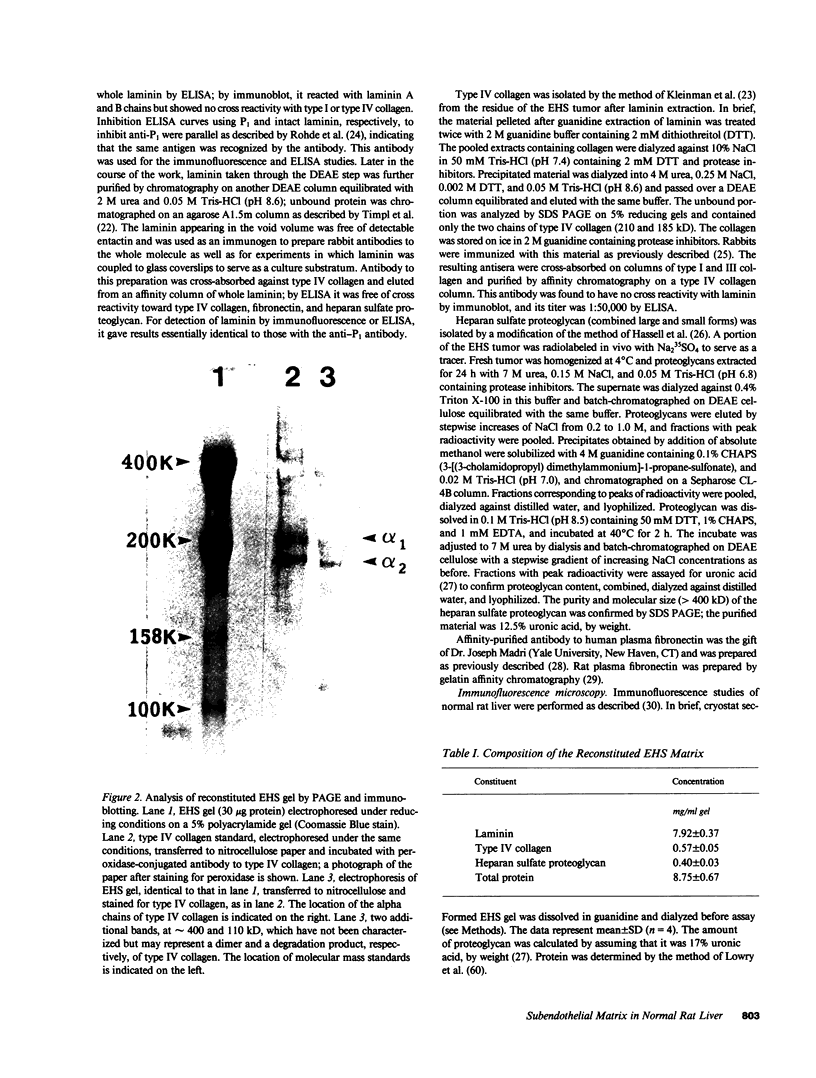
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Ouellet C., Goyette R., Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985 Sep;101(3):914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Martin G. R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982 Oct;95(1):340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Barwick K. W. Use of avidin-biotin complex in an ELISA system: a quantitative comparison with two other immunoperoxidase detection systems using keratin antisera. Lab Invest. 1983 Jan;48(1):98–107. [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Michalopoulos G., Sattler C. A., Sattler G. L., Pitot H. C. Cytochrome P-450 induction by phenobarbital and 3-methylcholanthrene in primary cultures of hepatocytes. Science. 1976 Sep 3;193(4256):907–909. doi: 10.1126/science.948753. [DOI] [PubMed] [Google Scholar]
- Oh E., Pierschbacher M., Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981 May;78(5):3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977 Jan 1;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego H., Medline A., Blendis L. M., Rankin J. G., Kreaden D. A. Collagenisation of the Disse space in alcoholic liver disease. Gut. 1979 Aug;20(8):673–679. doi: 10.1136/gut.20.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970 Nov;49:707–721. doi: 10.1016/s0002-9343(70)80135-8. [DOI] [PubMed] [Google Scholar]
- Rohde H., Wick G., Timpl R. Immunochemical characterization of the basement membrane glycoprotein laminin. Eur J Biochem. 1979 Dec;102(1):195–201. doi: 10.1111/j.1432-1033.1979.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Ponce-Noyola P. The extracellular matrix of the liver. Coll Relat Res. 1982 Mar;2(2):151–175. doi: 10.1016/s0174-173x(82)80031-9. [DOI] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Furthmayr H. A new method of iodinating collagens for use in radioimmunoassay. Anal Biochem. 1979 Jul 15;96(2):489–499. doi: 10.1016/0003-2697(79)90611-0. [DOI] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Rubin K., Johansson S., Pettersson I., Ocklind C., Obrink B., Hök M. Attachment of rat hepatocytes to collagen and fibronectin; a study using antibodies directed against cell surface components. Biochem Biophys Res Commun. 1979 Nov 14;91(1):86–94. doi: 10.1016/0006-291x(79)90586-2. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Kjéllen L., Unger E., Hök M., Farquhar M. G. Heparan sulfate proteoglycans are concentrated on the sinusoidal plasmalemmal domain and in intracellular organelles of hepatocytes. J Cell Biol. 1985 Mar;100(3):975–980. doi: 10.1083/jcb.100.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Catanese V. M., Rubin D. F. Collagen fractionation: separation of native types I, II and III by differential precipitation. Anal Biochem. 1976 Mar;71(1):114–118. doi: 10.1016/0003-2697(76)90016-6. [DOI] [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P., Mosselmans R., Ronveaux M. F. Adult rat hepatocytes in primary monolayer culture. Ultrastructural characteristics of intercellular contacts and cell membrane differentiations. J Cell Biol. 1977 Sep;74(3):858–877. doi: 10.1083/jcb.74.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., Rao C. N., Hassell J. R., Liotta L. A., Martin G. R., Kleinman H. K. Interactions of basement membrane components. Biochim Biophys Acta. 1983 Dec 27;761(3):278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Furthmayr H. Self-assembly of basement membrane collagen. Biochemistry. 1984 Apr 10;23(8):1839–1850. doi: 10.1021/bi00303a040. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985 Jun 25;260(12):7636–7644. [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Models for the self-assembly of basement membrane. J Histochem Cytochem. 1986 Jan;34(1):93–102. doi: 10.1177/34.1.3510247. [DOI] [PubMed] [Google Scholar]