Abstract
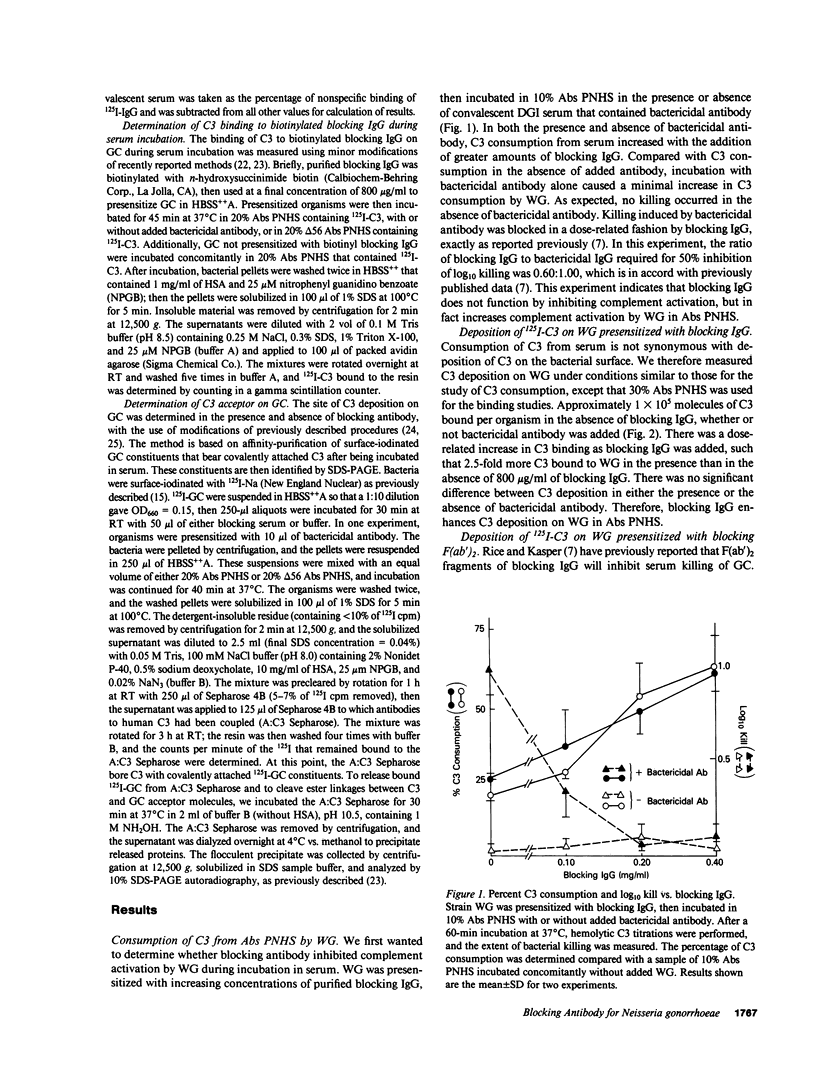
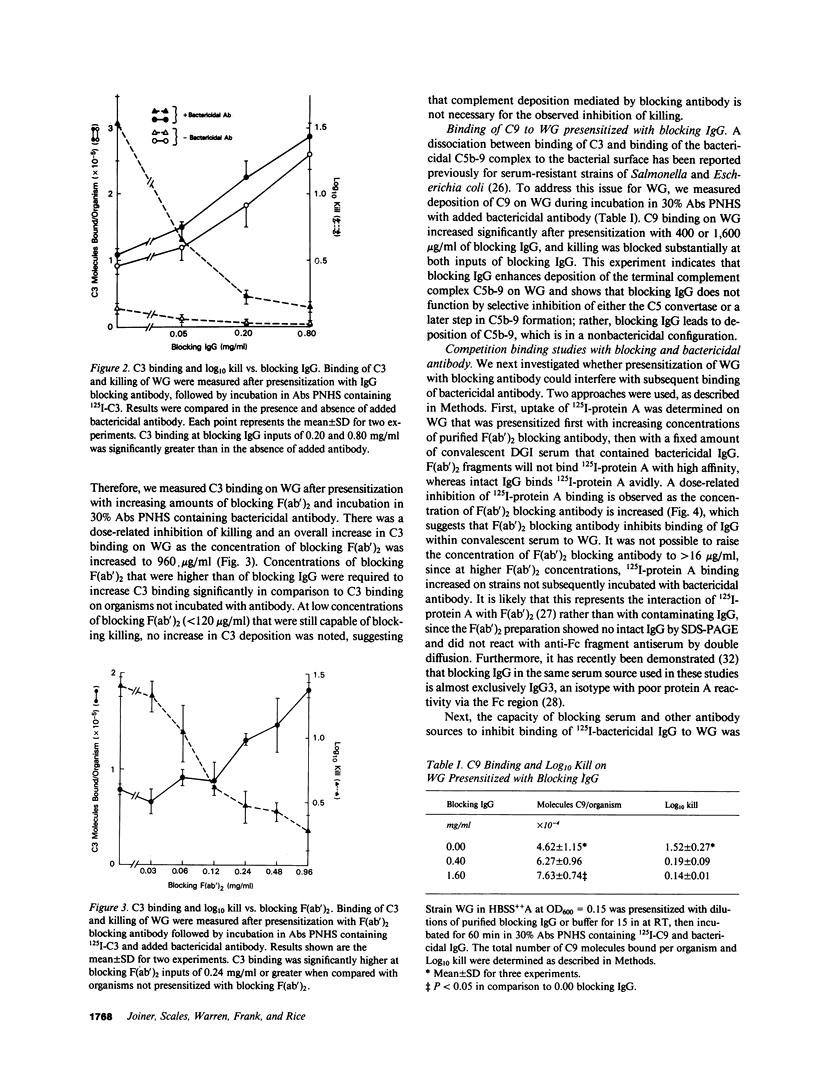
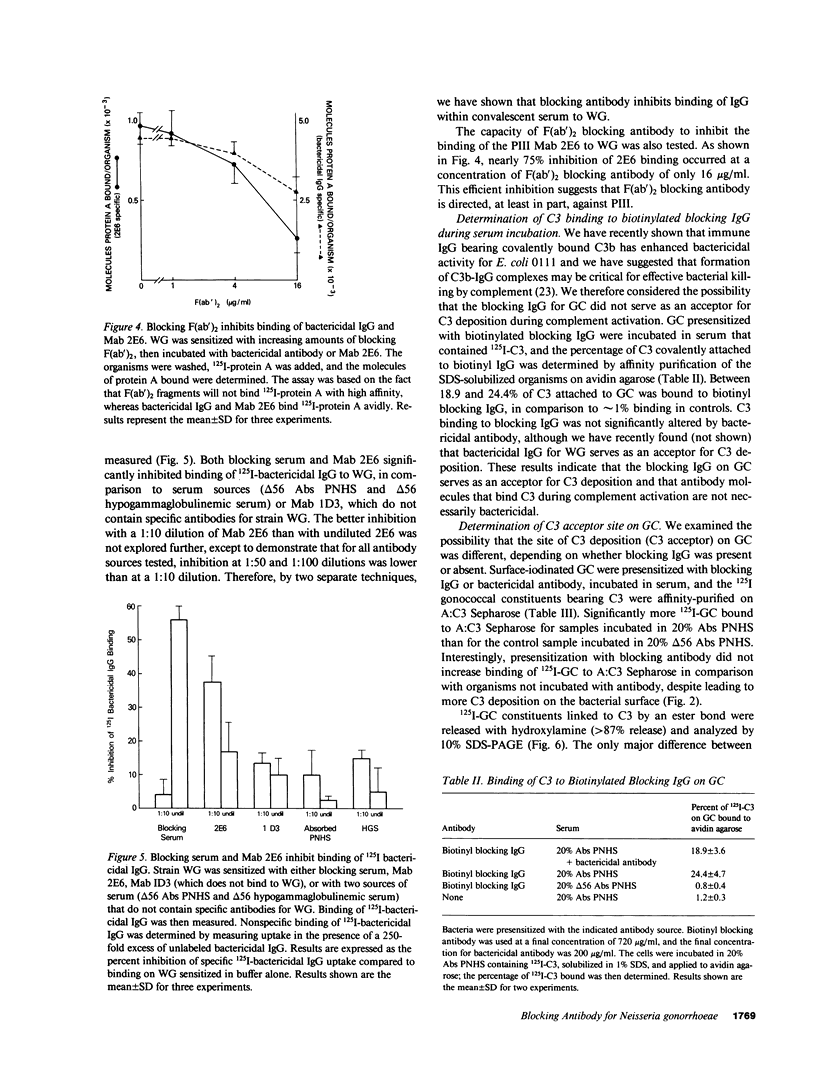
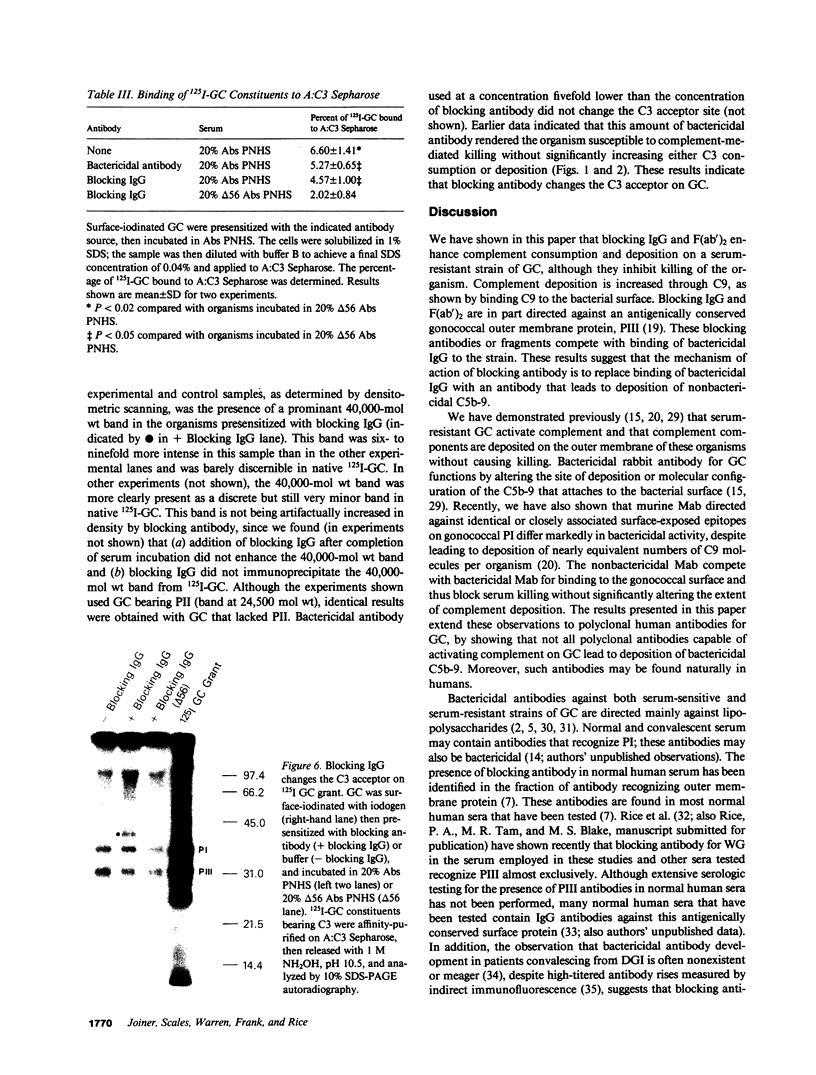
Blocking immunoglobulin G (IgG) inhibits complement-mediated killing of serum-resistant Neisseria gonorrhoeae (GC) in immune human serum. We examined the mechanism of action of blocking IgG. Presensitization of GC with increasing concentrations of blocking IgG or F(ab')2 before incubation with bactericidal antibody and absorbed pooled normal human serum increased consumption and deposition of the third component of human complement (C3) and the ninth component of human complement (C9) but inhibited killing in dose-related fashion. We next showed that blocking IgG or F(ab')2 partially inhibited binding of bactericidal IgG to GC. Also, binding of a monoclonal antibody recognizing GC outer membrane protein PIII was almost completely inhibited by blocking F(ab')2, confirming other work (Rice, P. A., M. R. Tam, and M. S. Blake, manuscript submitted for publication) showing that PIII is a target for blocking antibody. Studies of the C3 deposition site showed that one quarter of the C3 deposited on GC in the presence of blocking IgG bound covalently to the antibody molecule. Finally, 125I-GC constituents with covalently bound C3 were affinity purified on Sepharose bearing antibodies to C3 and identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis. C3 deposition on a 40,000-mol wt surface protein was enhanced six- to ninefold by blocking IgG, which indicates that the site of complement deposition was altered by blocking antibody. These studies show that blocking IgG competes with bactericidal antibody for binding to GC, but enhances rather than blocks complement activation, and leads to complement deposition at new sites that do not result in serum killing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks G. F., Israel K. S., Petersen B. H. Bactericidal and opsonic activity against Neisseria gonorrhoeae in sera from patients with disseminated gonococcal infection. J Infect Dis. 1976 Nov;134(5):450–462. doi: 10.1093/infdis/134.5.450. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Berger M., Joiner K. A., Frank M. M. Classical complement pathway activation by antipneumococcal antibodies leads to covalent binding of C3b to antibody molecules. Infect Immun. 1983 Nov;42(2):594–598. doi: 10.1128/iai.42.2.594-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., MacKeen L. A., Clark R. A. Dissemination of gonococcal infection is associated with delayed stimulation of complement-dependent neutrophil chemotaxis in vitro. Infect Immun. 1982 Nov;38(2):563–572. doi: 10.1128/iai.38.2.563-572.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Guttman R. M., Waisbren B. A. Bacterial blocking activity of specific IgG in chronic Pseudomonas aeruginosa infection. Clin Exp Immunol. 1975 Jan;19(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- HESS E. V., HUNTER D. K., ZIFF M. GONOCOCCAL ANTIBODIES IN ACUTE ARTHRITIS. JAMA. 1965 Feb 15;191:531–534. doi: 10.1001/jama.1965.03080070015004. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H. Isolation of IgG3 from normal human sera and from a patient with multiple myeloma by using protein A-sepharose 4B. Scand J Immunol. 1975;4(7):633–640. doi: 10.1111/j.1365-3083.1975.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Olsen D. A., Buchanan T. M. Analysis of the antigen specificity of the human serum immunoglobulin G immune response to complicated gonococcal infection. Infect Immun. 1984 Feb;43(2):706–709. doi: 10.1128/iai.43.2.706-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inganäs M., Johansson S. G., Bennich H. H. Interaction of human polyclonal IgE and IgG from different species with protein A from Staphylococcus aureus: demonstration of protein-A-reactive sites located in the Fab'2 fragment of human IgG. Scand J Immunol. 1980;12(1):23–31. doi: 10.1111/j.1365-3083.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Fries L. F., Schmetz M. A., Frank M. M. IgG bearing covalently bound C3b has enhanced bactericidal activity for Escherichia coli 0111. J Exp Med. 1985 Sep 1;162(3):877–889. doi: 10.1084/jem.162.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R. C., Hammer C. H., Leive L., Frank M. M. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab')2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983 Nov;131(5):2563–2569. [PubMed] [Google Scholar]
- Joiner K. A., Goldman R., Schmetz M., Berger M., Hammer C. H., Frank M. M., Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984 Jan;132(1):369–375. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Hammer C., Frank M. M. Bactericidal but not nonbactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae. J Immunol. 1985 Mar;134(3):1920–1925. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Joiner K., Hieny S., Kirchhoff L. V., Sher A. gp72, the 72 kilodalton glycoprotein, is the membrane acceptor site for C3 on Trypanosoma cruzi epimastigotes. J Exp Med. 1985 May 1;161(5):1196–1212. doi: 10.1084/jem.161.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Leith D. K., Morse S. A. Cross-linking analysis of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1980 Jul;143(1):182–187. doi: 10.1128/jb.143.1.182-187.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. P., Goldenberg D. L., Rice P. A. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983 Nov;62(6):395–406. [PubMed] [Google Scholar]
- Ratnoff W. D., Fearon D. T., Austen K. F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6(4):361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Ochs H. D., Buchanan T. M. Immunoglobulin class responsible for gonococcal bactericidal activity of normal human sera. J Immunol. 1979 May;122(5):1771–1779. [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Artenstein M. S. Cross-reactivity of Neisseria gonorrhoeae and Neisseria meningitidis and the nature of antigens involved in the bactericidal reaction. J Infect Dis. 1974 Sep;130(3):240–247. doi: 10.1093/infdis/130.3.240. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Waisbren B. A., Brown I. A factor in the serum of patients with persisting infection that inhibits the bactericidal activity of normal serum against the organism that is causing the infection. J Immunol. 1966 Sep;97(3):431–437. [PubMed] [Google Scholar]