Abstract

A novel four-component bicyclization strategy has been established, allowing a flexible and practical approach to 37 examples of multicyclic pyrazolo[3,4-b]pyridines from low-cost and readily accessible arylglyoxals, pyrazol-5-amines, aromatic amines, 4-hydroxy-6-methyl-2H-pyran-2-one, and cyclohexane-1,3-diones. The polysubstituted cyclopenta[d]pyrazolo[3,4-b]pyridines were stereoselectively synthesized through a microwave-assisted special [3+2+1]/[3+2] bicyclization with good control of the spatial configuration of exocyclic double bonds. The novel [3+2+1]/[2+2+1] bicyclization resulted in 17 examples of unreported pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones. Reasonable mechanisms for forming two new types of multicyclic pyrazolo[3,4-b]pyridines are also proposed.
Introduction
The development of highly efficient atom- and step-economic synthesis of multiheterocyclic scaffolds, particularly pyridine ring-containing ones, is of chemical and biomedical importance and has been actively pursued in organic and medicinal research for several decades.1,2 The structurally diverse and intriguing cyclopenta[c]pyridine family has been found to exist in monoterpene alkaloids that are represented by oxerine,3 actinidine,4 and aucubinine A and B5 (Figure 1) that showed significant biological activities with respect to abortive and animal stimulating effects.3,4 In addition, a variety of synthetic functionalized pyrazolo[3,4-b]pyridine system represents a core skeleton of pharmaceutical heterocycles with many other biological activities.6,7 A survey of the literature shows that many approaches to cyclopenta[c]pyridines8 and pyrazolo[3,4-b]pyridines9 have been developed, and there are a few reports of the combination of both above bioactive motifs into one compound.10 Hence, an exploration of a direct access to cyclopenta[d]pyrazolo[3,4-b]pyridines would be highly valuable for the discovery of new bioactive compounds.
Figure 1.
Some bioactive cyclopenta[c]pyridine alkaloids.
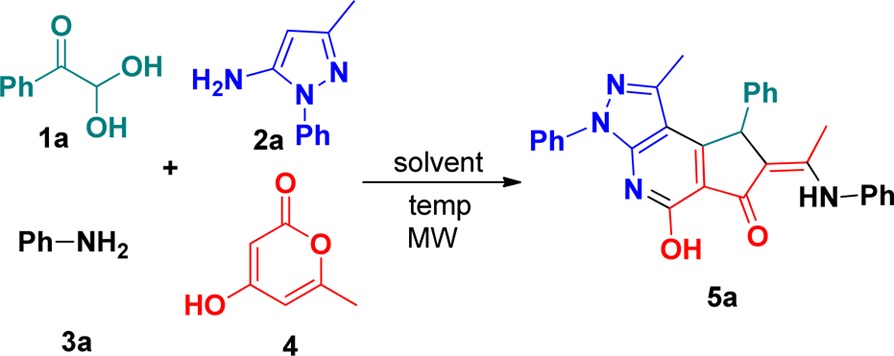
Multicomponent reactions (MCRs) have emerged as effective methods for the assembly of complex cyclic structures by the combination of two or more distinct reactions into a one-pot transformation.11,12 Among them, a branching multicomponent bicyclization not only enhances annulation efficiency but also features bond forming efficiency and high levels of structural complexity and minimizes the generation of waste.13 In recent years, enormous efforts have been made by conducting multicomponent bicyclizations toward the formation of various heterocycles.14,15 Recently, we have also established a new multicomponent reaction of arylglyoxals with electron-rich pyrazol-5-amines and aromatic amines, providing highly substituted pyrazolo[3,4-b]pyridines (Scheme 1).16 When 4-hydroxy-6-methyl-2H-pyran-2-one was trapped in this reaction system, tricyclic cyclopenta[d]pyrazolo[3,4-b]pyridines were unexpectedly obtained through a four-component bicyclization (Scheme 1). Further investigations revealed that replacing 4-hydroxy-6-methyl-2H-pyran-2-one with cyclohexane-1,3-diones delivered the unprecedented pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones. In this paper, we report our interesting observations. This work represents special examples for the regioselective construction of tri- and tetracyclic heterocycles containing a pyrazolo[3,4-b]pyridine unit with the concomitant formation of two new rings and five σ-bonds. In addition, the spatial configuration of exocyclic double bonds has been controlled well in the former reaction, because of the intramolecular hydrogen bond.
Scheme 1. Synthesis of Skeletally Diverse Pyrazolo[3,4-b]pyridine Derivatives.

Results and Discussion
To develop a new four-component bicyclization, we began our investigation with condition optimization by reacting 2,2-dihydroxy-1-phenylethanone 1a with 3-methyl-1-phenyl-1H-pyrazol-5-amine 2a, aniline 3a, and 4-hydroxy-6-methyl-2H-pyran-2-one 4 in DMF solvent under microwave (MW) heating. In our previous report, the multicomponent reaction of arylglyoxals with pyrazol-5-amines and aromatic amines promoted by p-TsOH gave highly substituted pyrazolo[3,4-b]pyridines.16 To continue our efforts on this project, we assume that when 4-hydroxy-6-methyl-2H-pyran-2-one was placed into the system described above, the reaction would proceed in another direction to form cyclopenta[d]pyrazolo[3,4-b]pyridines using appropriate promoters, based on the fact that ring opening of 4-hydroxy-6-methyl-2H-pyran-2-one with a proper nucleophile has been well established.17 With this notion in mind, four reactants with an equivalent molar ratio were treated with common promoters that have been widely used in heterocyclic synthesis [e.g., K2CO3, p-TsOH, and CF3COOH (entries 1–3, respectively, in Table 1)]. Unfortunately, the desired product 5a was not observed at all in the presence of the promoters mentioned above using DMF as a reaction solvent. Further screening of promoters revealed that the use of HOAc led to tricyclic product 5a, albeit with a low yield of 15% (entry 4). A higher yield of 57% was achieved when the reaction was conducted in HOAc at an enhanced temperature of 80 °C. The reaction works more efficiently in HOAc at 110 °C, affording a 74% yield of 5a (entry 7). Further increasing the temperature above 110 °C did not show improvement and even gave diminished chemical yields.
Table 1. Optimization for the Synthesis of 5a under MW Heating.
| entry | solvent | promoter (equiv) | temp (°C) | time (min) | yielda (%) |
|---|---|---|---|---|---|
| 1 | DMF | K2CO3 (1.0) | 80 | 25 | trace |
| 2 | DMF | p-TsOH (1.0) | 80 | 25 | trace |
| 3 | DMF | CF3CO2H (1.0) | 80 | 25 | trace |
| 4 | DMF | HOAc (1.0) | 80 | 25 | 15 |
| 5 | DMF | HOAc (4.0) | 80 | 25 | 39 |
| 6 | HOAc | – | 80 | 25 | 57 |
| 7 | HOAc | – | 110 | 25 | 74 |
Isolated yield.
Once the feasibility of the proposed pathway had been validated, we examined its scope generality by using various readily available arylglyoxals, pyrazol-5-amines, and aromatic amines through a new four-component bicyclization reaction. The experimental results are presented in Scheme 2 and showed that a broad spectrum of substituted arylglyoxals bearing both electron-donating and electron-withdrawing groups were successfully transformed into the corresponding tricyclic products 5a–n in good to excellent yields. Notably, halogen-containing arylglyoxals could be utilized and tolerated well under the optimal reaction conditions, furnishing the desired products in good yields, which offer possibilities for further functionalizations by modern coupling. The variation of nitrogen-tethered substituents on the pyrazole ring, including methyl or phenyl groups, worked well. After successful utilization of different substituents of arylglyoxals and pyrazol-5-amines, we next extended our study to a variety of arylamines with different functional groups such as chloro, methyl, and nitro on the phenyl ring. These functional groups are compatible in the system presented here, providing the desired products in 55–84% yields. Even for a challenging case in which a strong electron-withdrawing effect exists on the ortho position on the aromatic ring (5e), a good yield of 55% was obtained. Alternatively, instead of arylamines, this bicyclization reaction of 1 with 2 and 4 in a 1:2:1 molar ratio could also proceed, leading to the corresponding tricyclic cyclopenta-fused pyrazolo[3,4-b]pyridines 5o–t in 61–82% yields. Interestingly, this protocol provides an unusual pathway for the construction of multifunctionalized tricyclic cyclopenta-fused pyrazolo[3,4-b]pyridines that are challenging to obtain through other methods (Schemes 2 and 3).
Scheme 2. Four-Component Bicyclization for Cyclopenta[d]pyrazolo[3,4-b]pyridines.
Reaction conditions: 1 (1.0 mmol), 2 (1.0 mmol), 3 (1.0 mmol), 4 (1.0 mmol), acetic acid (1.5 mL), 110 °C, MW. Isolated yields based on substrate 2.
Scheme 3. Domino Synthesis of Cyclopenta[d]pyrazolo[3,4-b]pyridines.

Reaction conditions: 1 (1.0 mmol), 2 (2.0 mmol), 4 (1.0 mmol), acetic acid (1.5 mL), 110 °C, MW. Isolated yields based on substrate 2.
After successfully synthesizing cyclopenta-fused pyrazolo[3,4-b]pyridines 5, we attempted to further probe the reaction scope using dimedone (5,5-dimethylcyclohexane-1,3-dione) (6a) to replace 4-hydroxy-6-methyl-2H-pyran-2-one 4 (Scheme 4). The reaction of 1–3 and 6 proceeded in another direction to form the unprecedented pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones. Encouraged by the interesting results, we screened different Brønsted acid promoters to optimize the reaction conditions for this protocol. The reaction of 2,2-dihydroxy-1-(p-tolyl)ethanone (1e), 2a, 3a, and 6a in HOAc at 110 °C gave product 7a in 25% yield. After optimization, we were pleased to find the use of 1.0 equiv of p-TsOH in DMF at 120 °C pushed this reaction forward, affording a 56% chemical yield of product 7a. An additional 1.0 equiv of CF3SO3H and CF3CO2H in DMF showed poor catalytic activity and almost did not promote this reaction. Using 1.0 equiv of p-TsOH as a promoter and DMF as a solvent, a set of diverse substituted arylglyoxals, with groups such as methyl, methoxy, chloro, and bromo groups, were well incorporated into these current bicyclization reactions. Arylamines containing either electron-withdrawing or -donating substituents did not hamper the reaction process, allowing a bicyclization strategy to structurally complex pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones in moderate yields. Similarly, cyclohexane-1,3-dione can also take part in this domino bicyclization (7p and 7q). Moreover, it allows one-pot access to important tetracyclic products and gives a new bicyclization tool in an atom-efficient fashion, providing a valuable strategy for drug discovery.
Scheme 4. Expanding the Scope of the Bicyclization Reaction.
Reaction conditions: 1 (1.0 mmol), 2 (1.0 mmol), 3 (1.0 mmol), 6 (1.0 mmol), p-TsOH (1.0 mmol), DMF (1.5 mL), 120 °C, MW. Isolated yields based on substrate 2.
In all cases, the complexity of the products illustrates the remarkable chemo- and stereoselectivity of the reaction sequence starting from simple and common reactants. The structural elucidation and attribution of relative stereochemistry were unambiguously determined by X-ray diffraction analysis of single crystals of 5a, 5o, and 7d (see the Supporting Information) and other analysis. As shown in Schemes 2–4, the present four-component reaction can occur at fast speeds and can reach completion within 40 min. Water is nearly a sole byproduct, which makes workup convenient. As mentioned previously, these heterocyclic motifs are widely prevalent in bioactive molecules and pharmaceutical targets.
To understand the mechanistic hypothesis, a preformed 6-methyl-1-phenylpyridine-2,4(1H,3H)-dione 8,18 derived from 4-hydroxy-6-methyl-2H-pyran-2-one and aniline, was reacted with 1c and 2a under the standard conditions. The corresponding tricyclic product 5f was obtained in 75% yield (Scheme 5, eq 1). Treatment with preformed pyrazolo[3,4-b]quinolones 9(19) and aniline 3a generated a trace amount of pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones 7d (Scheme 5, eq 2). When the reaction of 5,5-dimethyl-3-(phenylamino)cyclohex-2-enone 10 with 1c and 2a was conducted, a 53% yield of 7d was isolated (Scheme 5, eq 3). These observations suggested that 4-hydroxy-6-methyl-2H-pyran-2-one and cyclohexane-1,3-diones may be, prior to reacting with arylamines, producing intermediates 8 and 10, which were further converted into the corresponding products 5 and 7, respectively.
Scheme 5. Control Experiments.
On the basis of the experimental results, we proposed mechanisms to explain the structural formation of multifunctionalized pyrazolo[3,4-b]pyridines 5 and 7 (Schemes 6 and 7). In the former, 4-hydroxy-6-methyl-2H-pyran-2-one 4 initially reacted with aryl amines 3 to give intermediate 8, which undergoes Knoevengel condensation with arylglyoxals 1 to give adducts A, followed by Michael addition and tautomerization to form intermediate B. Next, intramolecular cyclization occurs to afford fused pyrazolo[3,4-b]pyridines C. Subsequent ring opening of the pyridine skeleton and cycloisomerization yield final tricyclic cyclopenta-fused pyrazolo[3,4-b]pyridines 5. Similar to the former, the latter is subjected to a sequential enamine formation/Knoevengel condensation/Michael addition/double cyclization sequence, resulting in tetracyclic pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolones 7 (Scheme 7).
Scheme 6. Proposed Mechanism for the Synthesis of 5.

Scheme 7. Proposed Mechanism for the Synthesis of 7.

In conclusion, we have successfully established a new, flexible, and practical four-component bicyclization reaction for the construction of multicyclic pyrazolo[3,4-b]pyridines with good to excellent yields using low-cost and readily accessible arylglyoxals, pyrazol-5-amines, aromatic amines, and 4-hydroxy-6-methyl-2H-pyran-2-one (and cyclohexane-1,3-diones). Two straightforward and operationally simple methods involve complex cascades of sequences consisting of a Knoevengel condensation, Michael addition, and double cyclization and allow selective access to skeletally diverse multicyclic pyrazolo[3,4-b]pyridines by varying reaction substrates. The characteristics of reliable scalability, flexibility of structural modification, and wide substrate scope make this bicyclization strategy a powerful tool for the creation of multiheterocyclic scaffolds of general chemical and biomedical interest. We believe this methodology may be of value to others seeking original synthetic fragments with unique activities for medicinal and pharmaceutical studies.
Experimental Section
General
Microwave irradiation was conducted with Initiator 2.5 Microwave Synthesizers from Biotage (Uppsala, Sweden). The reaction temperatures were measured with an infrared detector during MW heating.
General Procedure for the Synthesis of 5
Example of the Synthesis of 5a
3-Methyl-1-phenyl-1H-pyrazol-5-amine (2a, 1.0 mmol, 173 mg) was introduced into a 10 mL Initiator reaction vial, and 2,2-dihydroxy-1-phenylethanone (1a, 1.0 mmol, 152 mg), aniline (3a, 93 mg), and 4-hydroxy-6-methyl-2H-pyran-2-one (4, 1.0 mmol, 126 mg) as well as acetic acid (1.5 mL) were then successively added. Subsequently, the reaction vial was capped, and then the contents were prestirred for 20 s. The mixture was irradiated (time, 28 min; temperature, 110 °C; absorption level, high; fixed hold time) until TLC [4/1 (v/v) petroleum ether/acetone] revealed that conversion of the starting material 2a was complete. The system was diluted with cold water (20 mL). The solid product was collected by Büchner filtration and purified by flash column chromatography (silica gel, petroleum ether/ethyl acetate mixtures) to afford the pure product 5a.
(Z)-5-Hydroxy-1-methyl-3,8-diphenyl-7-[1-(phenylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5a)
Yellow solid (349 mg, 74% yield): mp 264–266 °C; 1H NMR (400 MHz, CDCl3) δ 11.73 (s, 1H), 10.54 (s, 1H), 8.14 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 7.6 Hz, 2H), 7.37–7.27 (m, 6H), 7.26–7.20 (m, 3H), 7.09 (d, J = 7.2 Hz, 2H), 5.15 (s, 1H), 2.16 (s, 3H), 1.98 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.8, 161.3, 157.6, 156.9, 151.6, 147.0, 143.5, 142.9, 140.0, 139.0, 137.8, 129.3, 129.1, 129.0, 128.8, 127.5, 126.1, 126.0, 124.9, 121.6, 111.5, 49.9, 17.2, 15.4; IR (KBr, ν) 3443, 1624, 1591, 1511, 1414, 1318, 1229, 1026, 766 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C30H24N4O2 471.1829, found 471.1836.
(Z)-7-{1-[(3-Chlorophenyl)amino]ethylidene}-5-hydroxy-1-methyl-3,8-diphenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5b)
Yellow solid (385 mg, 76% yield): mp 276–278 °C; 1H NMR (400 MHz, CDCl3) δ 11.71 (s, 1H), 8.13 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.34–7.27 (m, 5H), 7.19–7.17 (m, 3H), 7.09 (s, 1H), 6.97 (d, J = 8.0 Hz, 1H), 5.13 (s, 1H), 2.15 (s, 3H), 1.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 190.3, 165.3, 161.2, 158.0, 156.6, 151.5, 143.6, 139.7, 139.2, 138.9, 134.9, 130.3, 129.0, 128.9, 127.6, 126.1, 125.9, 124.6, 122.7, 121.6, 115.9, 112.3, 109.4, 49.8, 17.2, 15.4; IR (KBr, ν) 3413, 1699, 1632, 1618, 1509, 1437, 1388, 1306, 1251, 813, 735 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C30H22ClN4O2 505.1440, found 505.1453.
(Z)-5-Hydroxy-8-(4-methoxyphenyl)-1-methyl-3-phenyl-7-[1-(phenylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5c)
Yellow solid (366 mg, 73% yield): mp 276–278 °C; 1H NMR (400 MHz, CDCl3) δ 11.70 (s, 1H), 8.15 (d, J = 7.6 Hz, 2H), 7.46 (t, J = 7.9 Hz, 2H), 7.35 (t, J = 7.6 Hz, 2H), 7.28–7.20 (m, 2H), 7.15–7.03 (m, 4H), 6.82 (d, J = 8.8 Hz, 2H), 5.08 (s, 1H), 3.78 (s, 3H), 2.18 (s, 3H), 1.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.7, 161.2, 158.8, 158.1, 157.6, 151.5, 143.6, 139.1, 137.9, 131.8, 130.0, 129.3, 129.0, 126.0, 125.9, 124.8, 121.5, 115.9, 114.1, 111.6, 109.4, 55.3, 49.1, 17.1, 15.4; IR (KBr, ν) 3420, 1617, 1595, 1574, 1511, 1304, 814, 756 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C31H25N4O3 501.1935, found 501.1945.
(Z)-7-{1-[(3-Chlorophenyl)amino]ethylidene}-5-hydroxy-8-(4-methoxyphenyl)-1-methyl-3-phenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5d)
Yellow solid (354 mg, 66% yield): mp 275–277 °C; 1H NMR (400 MHz, CDCl3) δ 11.66 (s, 1H), 10.53 (s, 1H), 8.17–8.10 (m, 2H), 7.47 (t, J = 6.8 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 7.27 (s, 1H), 7.10 (d, J = 8.0 Hz, 2H), 7.02 (d, J = 8.4 Hz, 2H), 6.83 (d, J = 7.6 Hz, 2H), 5.11 (s, 1H), 3.79 (s, 3H), 2.19 (s, 3H), 1.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.2, 163.1, 160.2, 157.8, 155.5, 142.6, 138.5, 137.9, 130.5, 129.5, 129.4, 129.0, 128.0, 125.0, 120.5, 119.1, 119.0, 113.1, 111.6, 111.4, 111.3, 110.7, 110.5, 54.3, 48.0, 16.2, 14.4; IR (KBr, ν) 3413, 1599, 1654, 1618, 1558, 1173, 926, 778, 642 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C31H24ClN4O3 535.1545, found 535.1566.
(Z)-5-Hydroxy-8-(4-methoxyphenyl)-1-methyl-7-{1-[(2-nitrophenyl)amino]ethylidene}-3-phenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5e)
Yellow solid (301 mg, 55% yield): mp 248–249 °C; 1H NMR (400 MHz, CDCl3) δ 11.47 (s, 1H), 8.10 (d, J = 8.0 Hz, 2H), 7.48–7.41 (m, 6H), 7.02 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 5.96 (s, 1H), 5.05 (s, 1H), 3.78 (s, 3H), 2.32 (s, 3H), 1.80 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.0, 161.2, 158.9, 158.7, 155.8, 149.2, 146.3, 143.6, 138.1, 136.8, 131.4, 129.9, 129.4, 129.0, 127.7, 126.2, 123.9, 121.7, 114.2, 113.2, 102.3, 55.3, 48.7, 18.4, 16.7, 15.4, 14.1; IR (KBr, ν) 3416, 1621, 1589, 1567, 1510, 1498, 1254, 1080, 813, 690 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C31H24N5O5 546.1786, found 546.1791.
(Z)-8-(4-Chlorophenyl)-5-hydroxy-1-methyl-3-phenyl-7-[1-(phenylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5f)
Yellow solid (359 mg, 71% yield): mp 256–258 °C; 1H NMR (400 MHz, CDCl3) δ 11.71 (s, 1H), 10.55 (s, 1H), 8.15 (d, J = 7.6 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.36 (t, J = 7.6 Hz, 2H), 7.28 (m, 3H), 7.22 (m, 1H), 7.14 (d, J = 7.6 Hz, 2H), 7.09 (d, J = 7.2 Hz, 2H), 5.11 (s, 1H), 2.19 (s, 3H), 1.95 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.6, 161.2, 157.6, 143.2, 138.9, 138.6, 137.7, 133.3, 130.3, 129.3, 129.1, 129.0, 127.2, 126.2, 126.1, 124.9, 121.6, 117.4, 116.1, 113.2, 111.1, 49.1, 17.2, 15.5; IR (KBr, ν) 3421, 1619, 1588, 1566, 1489, 1438, 1242, 1079, 759, 633 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C30H22ClN4O2 505.1440, found 505.1445.
(Z)-8-(4-Bromophenyl)-5-hydroxy-1-methyl-3-phenyl-7-[1-(p-tolylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5g)
Yellow solid (474 mg, 84% yield): mp 288–290 °C; 1H NMR (400 MHz, CDCl3) δ 11.65 (s, 1H), 10.62 (s, 1H), 8.15 (d, J = 7.6 Hz, 2H), 7.49–7.43 (m, J = 8.0 Hz, 4H), 7.29 (s, 1H), 7.16 (d, J = 7.6 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 7.2 Hz, 2H), 5.11 (s, 1H), 2.35 (s, 3H3), 2.20 (s, 3H), 1.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.4, 161.2, 158.1, 156.9, 143.1, 139.3, 139.0, 136.3, 135.5, 135.0, 132.0, 130.6, 129.9, 129.0, 126.1, 125.0, 121.6, 121.3, 110.7, 58.4, 49.2, 21.0, 18.4, 17.1, 15.5; IR (KBr, ν) 3214, 1625, 1592, 1566, 1487, 1319, 1079, 809, 689 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C31H24BrN4O2 563.1091, found 563.1092.
(Z)-7-{1-[(4-Chlorophenyl)amino]ethylidene}-5-hydroxy-1,3-dimethyl-8-phenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5h)
Yellow solid (280 mg, 63% yield): mp 278–280 °C; 1H NMR (400 MHz, CDCl3) δ 11.63 (s, 1H), 7.34–7.27 (m, 3H), 7.27–7.21 (m, 2H), 7.16 (d, J = 7.2 Hz, 2H), 7.00 (d, J = 7.6 Hz, 2H), 5.07 (s, 1H), 3.95 (s, 3H), 2.07 (s, 3H), 1.94 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.1, 161.2, 155.6, 147.6, 142.0, 138.4, 137.1, 133.4, 133.0, 130.1, 129.2, 129.1, 112.5, 99.9, 48.6, 34.9, 34.2, 30.9, 16.7, 15.2, 13.9; IR (KBr, ν) 3418, 1668, 1606, 1566, 1496, 1237, 800, 702, 601 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C25H20ClN4O2 443.1283, found 443.1315.
(Z)-5-Hydroxy-8-(4-methoxyphenyl)-1,3-dimethyl-7-[1-(p-tolylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5i)
Yellow solid (277 mg, 61% yield): mp 235–237 °C; 1H NMR (400 MHz, CDCl3) δ 11.58 (s, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.0 Hz, 2H), 6.80 (d, J = 8.8 Hz, 2H), 5.04 (s, 1H), 3.95 (s, 3H), 3.77 (s, 3H), 2.34 (s, 3H), 2.10 (s, 3H), 1.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.8, 161.3, 158.7, 157.7, 157.5, 141.8, 135.9, 135.3, 132.0, 130.0, 129.8, 124.9, 124.8, 116.7, 114.0, 111.2, 55.3, 49.0, 30.0, 21.0, 17.0, 15.3; IR (KBr, ν) 3414, 1625, 1589, 1567, 1438, 1324, 1017, 827, 654 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C27H25N4O3 453.1935, found 453.1964.
(Z)-7-{1-[(4-Chlorophenyl)amino]ethylidene}-5-hydroxy-8-(4-methoxyphenyl)-1,3-dimethyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5j)
Yellow solid (313 mg, 66% yield): mp 250–252 °C; 1H NMR (400 MHz, CDCl3) δ 11.61 (s, 1H), 7.30 (d, J = 7.6 Hz, 2H), 7.06 (d, J = 7.2 Hz, 2H), 7.01 (d, J = 7.2 Hz, 2H), 6.81 (d, J = 7.6 Hz, 2H), 5.03 (s, 1H), 3.95 (s, 3H), 3.78 (s, 3H), 2.10 (s, 3H), 1.94 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 189.6, 161.2, 157.6, 141.5, 138.8, 136.1, 135.1, 133.1, 130.2, 129.8, 128.9, 124.9, 110.7, 107.3, 58.4, 49.0, 34.0, 31.0, 21.0, 18.4, 17.1, 15.3; IR (KBr, ν) 3419, 1616, 1593, 1562, 1432, 1302, 1089, 808, 689 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C26H22ClN4O3 473.1389, found 473.1420.
(Z)-8-(4-Chlorophenyl)-7-{1-[(4-chlorophenyl)amino]ethylidene}-5-hydroxy-1,3-dimethyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5k)
Yellow solid (339 mg, 71% yield): mp 269–270 °C; 1H NMR (400 MHz, CDCl3) δ 11.65 (s, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.4 Hz, 2H), 5.07 (s, 1H), 3.98 (s, 3H), 2.13 (s, 3H), 1.95 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 190.2, 161.2, 156.4, 141.7, 138.6, 136.5, 133.3, 131.6, 130.2, 129.4, 129.0, 126.0, 111.6, 48.9, 34.1, 17.1, 15.3; IR (KBr, ν) 3416, 1663, 1616, 1582, 1499, 1314, 1011, 726 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C25H19Cl2N4O2 477.0893, found 477.0891.
(Z)-7-{1-[(4-Chlorophenyl)amino]ethylidene}-1-cyclopropyl-5-hydroxy-8-(4-methoxyphenyl)-3-methyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5l)
Yellow solid (320 mg, 64% yield): mp 276–278 °C; 1H NMR (400 MHz, CDCl3) δ 11.62 (s, 1H), 10.66 (s, 1H), 7.30 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 5.13 (s, 1H), 3.92 (s, 3H), 3.77 (s, 3H), 1.96 (s, 3H), 1.79–1.46 (m, 1H), 0.90–0.84 (m, 2H), 0.71 (s, 1H), 0.55 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 190.4, 161.2, 158.7, 157.2, 156.2, 147.0, 141.9, 139.4, 136.7, 131.9, 131.2, 129.8, 129.4, 125.9, 125.8, 114.1, 112.2, 55.3, 49.1, 34.1, 17.1, 9.7, 7.3, 7.2; IR (KBr, ν) 3419, 1682, 1609, 1588, 1562, 1496, 1256, 1055, 775 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C28H24ClN4O3 499.1545, found 499.1549.
(Z)-8-(4-Chlorophenyl)-1-cyclopropyl-5-hydroxy-3-methyl-7-[1-(p-tolylamino)ethylidene]-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5m)
Yellow solid (324 mg, 67% yield): mp 252–253 °C; 1H NMR (400 MHz, CDCl3) δ 11.60 (s, 1H), 10.67 (s, 1H), 7.24 (d, J = 6.8 Hz, 2H), 7.13 (m, 4H), 6.97 (d, J = 7.3 Hz, 2H), 5.15 (s, 1H), 3.92 (s, 3H), 2.34 (s, 3H), 1.93 (s, 3H), 1.64–1.54 (m, 1H), 0.89 (s, 2H), 0.68 (s, 1H), 0.54 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 190.8, 161.1, 155.6, 149.3, 149.2, 147.7, 141.9, 140.2, 138.6, 136.9, 133.1, 131.8, 130.8, 130.4, 128.2, 115.9, 112.0, 100.0, 48.2, 34.9, 34.2, 16.8, 15.3, 13.9; IR (KBr, ν) 3293, 1613, 1598, 1564, 1516, 1488, 1264, 1015, 808, 716 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C28H24ClN4O2 483.1596, found 483.1596.
(Z)-8-(4-Chlorophenyl)-7-{1-[(4-chlorophenyl)amino]ethylidene}-1-cyclopropyl-5-hydroxy-3-methyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5n)
Yellow solid (307 mg, 61% yield): mp 234–256 °C; 1H NMR (400 MHz, CDCl3) δ 11.63 (s, 1H), 7.32 (d, J = 7.6 Hz, 2H), 7.25 (d, J = 7.2 Hz, 2H), 7.11 (d, J = 7.2 Hz, 2H), 7.02 (d, J = 7.6 Hz, 2H), 5.17 (s, 1H), 3.93 (s, 3H), 1.95 (s, 3H), 0.90 (s, 2H), 0.62 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 190.3, 161.1, 156.3, 146.8, 138.8, 138.0, 136.5, 133.1, 131.5, 130.1, 129.4, 128.9, 126.0, 118.9, 113.2, 111.8, 49.1, 34.1, 30.9, 17.1, 9.7, 7.5, 7.0; IR (KBr, ν) 3137, 1682, 1608, 1590, 1562, 1497, 1213, 1054, 758, 714 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C27H21Cl2N4O2 503.1050, found 503.1061.
Example of the Synthesis of 5o
3-Methyl-1-phenyl-1H-pyrazol-5-amine (2a, 2.0 mmol, 346 mg) was introduced into a 10 mL Initiator reaction vial, and 2,2-dihydroxy-1-phenylethanone (1a, 1.0 mmol, 152 mg) and 4-hydroxy-6-methyl-2H-pyran-2-one (4, 1.0 mmol, 126 mg) as well as acetic acid (1.5 mL) were then successively added. Subsequently, the reaction vial was capped, and then the contents were prestirred for 20 s. The mixture was irradiated (time, 25 min; temperature, 110 °C; absorption level, high; fixed hold time) until TLC [4/1 (v/v) petroleum ether/acetone] revealed that conversion of starting material 2a was complete. The system was diluted with cold water (20 mL). The solid product was collected by Büchner filtration and purified by flash column chromatography (silica gel, petroleum ether/ethyl acetate mixtures) to afford pure product 5o.
(Z)-5-Hydroxy-1-methyl-7-{1-[3-methyl(1-phenyl-1H-pyrazol-5-yl)amino]ethylidene}-3,8-diphenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5o)
Yellow solid (420 mg, 76% yield): mp 268–269 °C; 1H NMR (400 MHz, CDCl3) δ 11.47 (s, 1H), 10.23 (s, 1H), 8.11 (d, J = 8.0 Hz, 2H), 7.50–7.39 (m, 6H), 7.34–7.31 (m, 2H), 7.26–7.22 (m, 3H), 7.10 (d, J = 6.4 Hz, 2H), 5.96 (s, 1H), 5.06 (s, 1H), 2.31 (s, 3H), 2.13 (s, 3H), 1.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.1, 161.1, 158.3, 156.0, 149.2, 143.7, 139.6, 138.9, 138.0, 136.8, 129.4, 129.0, 128.9, 128.8, 127.7, 127.6, 126.2, 123.9, 121.6, 115.7, 113.1, 109.4, 102.4, 49.4, 16.8, 15.34, 14.1; IR (KBr, ν) 3425, 1620, 1593, 1556, 1510, 1377, 1318, 1130, 725, 669 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C34H27N6O2 551.2204, found 551.2232.
(Z)-8-(4-Bromophenyl)-5-hydroxy-1-methyl-7-{1-[(3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]ethylidene}-3-phenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5p)
Yellow solid (517 mg, 82% yield): mp 258–260 °C; 1H NMR (400 MHz, CDCl3) δ 11.45 (s, 1H), 10.27 (s, 1H), 8.10 (d, J = 8.0 Hz, 2H), 7.49–7.38 (m, 8H), 7.34 (t, J = 6.8 Hz, 1H), 7.29 (d, J = 7.6 Hz, 1H), 6.97 (d, J = 8.3 Hz, 2H), 5.98 (s, 1H), 5.03 (s, 1H), 2.32 (s, 3H), 2.17 (s, 3H), 1.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 190.9, 161.1, 155.8, 149.3, 143.6, 143.4(0), 143.3(8), 138.7, 137.8, 136.7, 132.1, 132.0, 130.4, 129.5, 129.1, 127.9(4), 127.9(2), 126.3(4), 126.3(3), 124.0, 121.7, 121.4, 112.8, 102.4, 48.7, 17.0, 15.5, 14.0; IR (KBr, ν) 3447, 1782, 1626, 1594, 1502, 1409, 1412, 1236, 1129, 1101, 758 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C34H26BrN6O2 629.1309, found 629.1324.
(Z)-8-(4-Fluorophenyl)-5-hydroxy-1-methyl-7-{1-[(3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]ethylidene}-3-phenyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5q)
Yellow solid (416 mg, 73% yield): mp 276–279 °C; 1H NMR (400 MHz, CDCl3) δ 11.48 (s, 1H), 10.31 (s, 1H), 8.09 (d, J = 8.0 Hz, 2H), 7.50–7.40 (m, 6H), 7.34 (t, J = 6.8 Hz, 1H), 7.31–7.27 (m, 1H), 7.11–7.03 (m, 2H), 6.96 (t, J = 8.4 Hz, 2H), 5.98 (s, 1H), 5.05 (s, 1H), 2.32 (s, 3H), 2.14 (s, 3H), 1.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 190.9, 163.1, 159.3 (1JCF = 271.3), 157.9, 156.0, 149.3, 143.4, 138.8, 138.1, 136.6, 135.4 (2JCF = 3.3), 130.4, 130.3 (3JCF = 8), 129.4, 129.0, 127.7, 126.2, 123.9, 121.5, 115.9, 115.8 (4JCF = 21.5), 112.8, 109.2, 102.5, 48.6, 16.8, 15.4, 14.1; IR (KBr, ν) 3464, 1621, 1591, 1576, 1379, 1329, 1225, 1156, 815, 756 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C34H26FN6O2 569.2110, found 569.2136.
(Z)-8-(4-Chlorophenyl)-7-{1-[(1,3-dimethyl-1H-pyrazol-5-yl)amino]ethylidene}-5-hydroxy-1,3-dimethyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5r)
Yellow solid (282 mg, 61% yield): mp 158–160 °C; 1H NMR (400 MHz, CDCl3) δ 11.54 (s, 1H), 7.27 (d, J = 6.4 Hz, 2H), 7.11 (d, J = 7.6 Hz, 2H), 5.74 (s, 1H), 5.09 (s, 1H), 3.97 (s, 3H), 3.71 (s, 3H), 2.21 (s, 3H), 2.12 (s, 3H), 1.90 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.1, 161.1, 155.6, 149.3, 147.6, 142.0, 138.4, 137.1, 133.5, 130.0, 129.1, 117.9, 112.5, 99.8, 48.6, 34.9, 34.2, 30.9, 16.7, 15.2, 13.9; IR (KBr, ν) 3454, 1698, 1659, 1636, 1560, 1207, 1131, 643, 515 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C24H22ClN6O2 461.1501, found 461.1532.
(Z)-8-(3,4-Dichlorophenyl)-7-{1-[(1,3-dimethyl-1H-pyrazol-5-yl)amino]ethylidene}-5-hydroxy-1,3-dimethyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5s)
Yellow solid (322 mg, 65% yield): mp 171–173 °C; 1H NMR (400 MHz, CDCl3) δ 11.53 (s, 1H), 10.63 (s, 1H),7.39 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 2.0 Hz, 1H), 7.08–7.05 (m, 1H), 5.76 (s, 1H), 5.07 (s, 1H), 3.98 (s, 3H), 3.73 (s, 3H), 2.22 (s, 3H), 2.17 (s, 3H), 1.92 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.3, 161.2, 155.6, 147.5, 146.3, 146.1, 145.8, 145.1, 142.2, 139.7, 137.3, 128.9, 128.9, 127.6, 112.9, 99.8, 58.5, 49.4, 34.9, 34.1, 18.4, 16.6 15.1, 13.9; IR (KBr, ν) 3444, 1690, 1620, 1561, 1423, 1339, 1176, 1032, 814 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C24H21Cl2N6O2 495.1111, found 495.1139.
(Z)-7-{1-[(1,3-Dimethyl-1H-pyrazol-5-yl)amino]ethylidene}-5-hydroxy-8-(4-methoxyphenyl)-1,3-dimethyl-7,8-dihydrocyclopenta[d]pyrazolo[3,4-b]pyridin-6(3H)-one (5t)
Yellow solid (307 mg, 67% yield): mp 150–152 °C; 1H NMR (400 MHz, CDCl3) δ 11.54 (s, 1H), 10.70 (s, 1H), 7.07 (d, J = 8.0 Hz, 2H), 6.81 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 5.06 (s, 1H), 3.97 (s, 3H), 3.78 (s, 3H), 3.72 (s, 3H), 2.21 (s, 3H), 2.11 (s, 3H), 1.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 191.4, 161.2, 158.9, 155.4, 147.5, 142.3, 137.4, 131.5, 129.9, 127.7, 126.6, 114.2, 113.0, 100.0, 99.8, 55.3, 48.6, 34.9, 34.1, 18.4, 16.6, 15.2, 13.9; IR (KBr, ν) 3444, 1696, 1620, 1561, 1310, 1254, 1032, 814, 649 cm–1; HRMS (ESI-TOF) m/z calcd for [M – H]− C25H25N6O3 457.1996, found 457.2026.
Example of the Synthesis of 7a
5,5-Dimethylcyclohexane-1,3-dione (6a, 1.0 mmol, 140 mg) and aniline (3a, 1.0 mmol, 93 mg) were introduced into a 10 mL Initiator reaction vial, and 2,2-dihydroxy-1-(p-tolyl)ethanone (1e, 1.0 mmol, 166 mg), 3-methyl-1-phenyl-1H-pyrazol-5-amine (2a, 1.0 mmol, 173 mg), and p-TsOH (1.0 mmol, 172 mg) as well as N,N-dimethylformamide (DMF, 1.5 mL) were then successively added. Subsequently, the reaction vial was capped, and then the contents were prestirred for 20 s. The mixture was irradiated (time, 28 min; temperature, 120 °C; absorption level, high; fixed hold time) until TLC [4/1 (v/v) petroleum ether/acetone] revealed that conversion of starting material 2a was complete. The system was neutralized with diluted chlorhydric acid and then diluted with cold water (40 mL). The solid product was collected by Büchner filtration and purified by flash column chromatography (silica gel, petroleum ether/ethyl acetate mixtures) to afford pure product 7a.
4,4,9-Trimethyl-2,7-diphenyl-1-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7a)
Yellow solid (270 mg, 56% yield): mp 269–271 °C; 1H NMR (400 MHz, CDCl3) δ 8.16–8.14 (m, 2H), 7.50–7.46 (m, 2H), 7.34–7.32 (m, 3H), 7.26–7.24 (m, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.13–7.11 (m, 2H), 7.07 (d, J = 7.6 Hz, 2H), 2.92 (s, 2H), 2.69 (s, 2H), 2.33 (s, 3H), 2.02 (s, 3H), 1.15 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 159.3, 142.6, 140.0, 137.7, 137.6, 132.3, 131.7, 129.9, 128.9(2), 128.8(7), 128.8, 128.4, 128.0, 127.7, 125.8, 122.9, 122.7, 115.2, 114.2, 104.9, 45.3, 37.2, 35.5, 29.0, 21.3, 15.6; HRMS (ESI-TOF) m/z calcd for [M + H]+ C33H31N4 483.2543, found 483.2578.
1-(4-Fluorophenyl)-4,4,9-trimethyl-7-phenyl-2-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7b)
White solid (295 mg, 59% yield): mp 296–297 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.0 Hz, 2H), 7.53–7.49 (m, 2H), 7.33–7.28 (m, 3H), 7.17 (d, J = 8.0 Hz, 2H), 7.03–7.00 (m, 4H), 2.97 (s, 2H), 2.72 (s, 2H), 2.39 (s, 3H), 2.04 (s, 3H), 1.18 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 162.4 (1JCF = 246.2 Hz), 159.3, 142.4, 139.9, 138.2, 134.7, 134.1 (2JCF = 8.1 Hz), 129.6, 129.4, 129.2 (4JCF = 3.5 Hz), 129.0, 128.9, 127.4, 125.9, 122.9, 121.4, 115.0, 114.8 (4JCF = 21.3 Hz), 114.5, 104.7, 45.3, 37.1, 35.6, 29.0, 21.1, 15.6; HRMS (ESI-TOF) m/z calcd for [M + H]+ C33H30FN4 501.2454, found 501.2483.
2-(4-Bromophenyl)-1-(4-chlorophenyl)-4,4,9-trimethyl-7-phenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7c)
White solid (249 mg, 43% yield): mp 280–281 °C; 1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 7.6 Hz, 2H), 7.51–7.48 (m, 2H), 7.38–7.37 (m, 2H), 7.26–7.24 (m, 5H), 7.13–7.10 (m, 2H), 2.98 (s, 2H), 2.71 (s, 2H), 2.05 (s, 3H), 1.17 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 159.2, 142.4, 139.8, 136.4, 134.1, 133.5, 132.4, 131.2, 129.2, 129.1, 128.9, 128.3, 126.0, 122.9, 122.3, 121.1, 115.4, 114.9, 104.4, 45.2, 37.1, 35.6, 29.0, 16.0; HRMS (ESI-TOF) m/z calcd for [M + H]+ C32H27BrClN4 581.1107, found 581.1120.
1-(4-Chlorophenyl)-4,4,9-trimethyl-2,7-diphenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7d)
Pale yellow solid (216 mg, 43% yield): mp 271–272 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 7.6 Hz, 2H), 7.49 (t, J = 8.0 Hz, 2H), 7.38–7.36 (m, 3H), 7.30–7.28 (m, 1H), 7.26–7.22 (m, 4H), 7.12–7.10 (m, 2H), 2.95 (s, 2H), 2.70 (s, 2H), 2.05 (s, 3H), 1.16 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 159.2, 142.3, 139.9, 137.4, 133.8, 133.6, 131.6, 129.1, 128.9, 128.30, 128.0, 127.7, 125.9, 122.8, 121.3, 115.2, 114.7, 104.6, 45.4, 37.1, 35.6, 29.0, 16.0; HRMS (ESI-TOF) m/z calcd for [M + H]+ C32H28ClN4 503.1996, found 503.2015.
1-(4-Chlorophenyl)-9-cyclopropyl-4,4-dimethyl-2,7-diphenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7e)
Yellow solid (238 mg, 45% yield): mp 264–265 °C; 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.0 Hz, 2H), 7.49–7.46 (m, 2H), 7.37–7.36 (m, 3H), 7.29–7.21 (m, 5H), 7.12–7.10 (m, 2H), 2.96 (s, 2H), 2.71 (s, 2H), 1.37–1.36 (m, 1H), 1.16 (s, 6H), 0.93–0.92 (m, 2H), 0.51–0.48 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 159.0, 147.2, 137.4, 133.5, 133.4, 133.1, 131.6, 129.1, 128.8, 128.3, 128.1, 127.8, 127.7, 125.9, 123.0, 122.7, 122.6, 115.3, 114.4, 105.0, 45.1, 37.2, 35.6, 28.9, 10.9, 7.9; HRMS (ESI-TOF) m/z calcd for [M + H]+ C34H30ClN4 529.2153, found 529.2159.
4,4,7,9-Tetramethyl-1,2-di-p-tolyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7f)
White solid (169 mg, 39% yield): mp 255–256 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.21–7.18 (m, 4H), 7.13–7.10 (m, 4H), 3.91 (s, 3H), 2.79 (s, 2H), 2.64 (s, 3H), 2.30 (s, 3H), 2.28 (s, 3H), 1.77 (s, 2H), 1.07 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 158.7, 148.9, 138.9, 137.7, 137.3, 132.5, 131.4, 130.3, 129.5, 128.7, 128.6, 128.2, 122.1, 114.5, 114.3, 102.7, 45.1, 36.8, 35.4, 34.3, 29.0, 21.3, 15.6; HRMS (ESI-TOF) m/z calcd for [M + H]+ C29H31N4 435.2543, found 435.2541.
2-(4-Methoxyphenyl)-4,4,7,9-tetramethyl-1-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7g)
White solid (202 mg, 45% yield): mp 240–241 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.20 (s, 1H), 7.18 (d, J = 1.2 Hz, 2H), 7.15 (s, 1H), 7.11 (d, J = 8.0 Hz, 2H), 6.93 (d, J = 8.8 Hz, 2H), 3.91 (s, 3H), 3.76 (s, 3H), 2.78 (s, 2H), 2.62 (s, 2H), 2.28 (s, 3H), 1.77 (s, 3H), 1.07 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 159.1, 158.6, 148.9, 138.9, 137.2, 132.6, 131.5, 130.4, 129.3, 128.7, 122.2, 114.6, 114.3, 114.1, 102.7, 55.8, 45.1, 36.7, 35.4, 34.3, 29.00, 21.3, 15.6; HRMS (ESI-TOF) m/z calcd for [M + H]+ C29H31N4O 451.2492, found 451.2491.
4,4,7,9-Tetramethyl-2-phenyl-1-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7h)
White solid (218 mg, 52% yield): mp 260–261 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.40 (d, J = 7.6 Hz, 3H), 7.25–7.18 (m, 4H), 7.10 (d, J = 7.6 Hz, 2H), 3.91 (s, 3H), 2.80 (s, 2H), 2.66 (s, 2H), 2.28 (s, 3H), 1.78 (s, 3H), 1.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.8, 140.3, 137.7, 137.4, 132.1, 129.9, 128.9, 128.4, 127.9, 127.7, 122.7, 114.9, 114.4, 103.1, 45.2, 37.2, 35.5, 34.5, 29.0, 21.3, 15.5; HRMS (ESI-TOF) m/z calcd for [M + H]+ C28H29N4 421.2386, found 421.2396.
2-(4-Bromophenyl)-4,4,7,9-tetramethyl-1-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7i)
Yellow solid (189 mg, 38% yield): mp 280–281 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.61 (d, J = 2.0 Hz, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.22–7.19 (m, 4H), 7.14 (s, 2H), 3.91 (s, 3H), 2.79 (s, 2H), 2.67 (s, 2H), 2.30 (s, 3H), 1.78 (s, 3H), 1.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.7, 140.3, 137.7, 136.8, 132.1, 131.3, 129.6, 129.3, 129.2, 128.6, 127.7, 122.5, 121.9, 115.0, 114.7, 102.9, 45.2, 37.2, 35.5, 34.5, 28.9, 21.3, 15.4; HRMS (ESI-TOF) m/z calcd for [M + H]+ C28H28BrN4 499.1491, found 499.1497.
1-(4-Bromophenyl)-4,4,7,9-tetramethyl-2-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7j)
White solid (209 mg, 42% yield): mp 251–252 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.51 (d, J = 2.0 Hz, 1H), 7.49 (d, J = 2.0 Hz, 1H), 7.27–7.21 (m, 4H), 7.13 (d, J = 8.4 Hz, 2H), 3.92 (s, 3H), 2.80 (s, 2H), 2.65 (s, 2H), 2.32 (s, 3H), 1.83 (s, 3H), 1.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.7, 140.0, 138.3, 134.7, 133.7, 132.1, 130.9, 129.7, 129.3, 129.1, 127.3, 121.8, 121.1, 114.8, 114.8, 102.8, 45.2, 37.1, 35.5, 34.5, 28.9, 21.1, 15.9; HRMS (ESI-TOF) m/z calcd for [M + H]+ C28H28BrN4 499.1491, found 499.1496.
1-(4-Bromophenyl)-4,4,7,9-tetramethyl-2-phenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7k)
White solid (179 mg, 37% yield): mp 281–282 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.51 (s, 1H), 7.49 (s, 1H), 7.43 (d, J = 7.6 Hz, 3H), 7.27–7.25 (m, 4H), 3.93 (s, 3H), 2.82 (s, 2H), 2.68 (s, 2H), 1.85 (s, 3H), 1.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.8, 140.0, 137.4, 133.7, 132.0, 130.9, 129.2, 129.1, 128.8, 128.3, 127.7, 121.8, 121.1, 115.0, 114.9, 102.7, 45.2, 37.1, 35.5, 34.5, 28.9, 15.9; HRMS (ESI-TOF) m/z calcd for [M + H]+ C27H26BrN4 485.1335, found 485.1370.
4,4,7,9-Tetramethyl-1-phenyl-2-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7l)
White solid (227 mg, 54% yield): mp 280–281 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.30 (s, 5H), 7.19 (d, J = 8.0 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 3.91 (s, 3H), 2.80 (s, 2H), 2.66 (s, 2H), 2.30 (s, 3H), 1.75 (s, 3H), 1.08 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.8, 140.2, 137.9, 135.1, 133.2, 132.3, 131.4, 129.5, 129.0, 127.6, 127.6, 127.4, 122.5, 114.8, 114.5, 102.9, 45.4, 37.2, 35.5, 34.5, 29.0, 21.1, 15.5; HRMS (ESI-TOF) m/z calcd for [M + H]+ C28H29N4 421.2386, found 421.2397.
2-(3-Bromo-4-methylphenyl)-4,4,7,9-tetramethyl-1-phenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7m)
White solid (204 mg, 41% yield): mp 276–278 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.51 (d, J = 2.0 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.33 (s, 5H), 7.20 (dd, J = 8.0, 2.0 Hz, 1H), 3.91 (s, 3H), 2.80 (s, 2H), 2.69 (s, 2H), 1.74 (s, 3H), 1.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.8, 140.3, 138.0, 136.3, 132.7, 132.3, 131.1, 130.7, 128.5, 127.9, 127.8, 127.7, 126.4, 124.5, 122.5, 115.0, 114.7, 102.9, 45.1, 37.1, 35.5, 34.6, 29.0, 22.6, 15.3; HRMS (ESI-TOF) m/z calcd for [M + H]+ C28H28BrN4 499.1491, found 499.1509.
2-(4-Bromophenyl)-4,4,7,9-tetramethyl-1-phenyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinoline (7n)
Yellow solid (208 mg, 43% yield): mp 279–281 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 8.4 Hz, 2H), 7.32 (s, 5H), 7.22 (d, J = 8.4 Hz, 2H), 3.92 (s, 3H), 2.81 (s, 2H), 2.69 (s, 2H), 1.76 (s, 3H), 1.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.8, 140.2, 136.7, 132.7, 132.3, 132.2, 131.4, 131.2, 129.2, 128.0, 127.9, 122.4, 122.0, 115.1, 114.9, 102.8, 45.3, 37.2, 35.5, 34.5, 29.0, 15.4; HRMS (ESI-TOF) m/z calcd for [M + H]+ C27H26BrN4 485.1335, found 485.1358.
1,2-Bis(4-methoxyphenyl)-4,4,7,9-tetramethyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7o)
Yellow solid (186 mg, 40% yield): mp 254–256 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.24 (s, 1H), 7.22 (s, 1H), 7.17 (d, J = 8.8 Hz, 2H), 6.93 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 3.91 (s, 3H), 3.76 (s, 3H), 3.74 (s, 3H), 2.78 (s, 2H), 2.62 (s, 2H), 1.78 (s, 3H), 1.07 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 159.1, 159.0, 158.8, 140.1, 133.6, 131.2, 130.5, 128.7, 125.5, 122.3, 120.6, 114.6, 114.3, 114.0, 113.1, 103.0, 55.4, 55.2, 45.3, 37.1, 35.4, 34.5, 29.0, 15.4; HRMS (ESI-TOF) m/z calcd for [M + H]+ C29H31N4O2 467.2441, found 467.2449.
9-Cyclopropyl-2-(4-methoxyphenyl)-7-methyl-1-(p-tolyl)-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7p)
Brown solid (206 mg, 46% yield): mp 272–273 °C; 1H NMR (400 MHz, CDCl3) δ 7.20 (s, 1H), 7.18 (s, 1H), 7.04 (t, J = 8.4 Hz, 4H), 6.83 (d, J = 8.8 Hz, 2H), 4.08 (s, 3H), 3.80 (s, 3H), 3.13 (d, J = 17.6 Hz, 2H), 2.85 (d, J = 6.0 Hz, 2H), 2.30 (s, 5H), 1.36–1.27 (m, 1H), 0.75–0.73 (m, 2H), 0.30.36 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 159.4, 158.9, 145.3, 137.0, 132.1, 130.6, 130.1, 130.0, 128.7, 128.3, 128.2, 122.8, 115.8, 114.1, 114.0, 103.4, 55.4, 34.7, 24.4, 23.0, 21.3, 10.6, 7.4; HRMS (ESI-TOF) m/z calcd for [M + H]+ C29H29N4O 449.2335, found 449.2340.
7,9-Dimethyl-1,2-di-p-tolyl-3,4,5,7-tetrahydro-2H-pyrazolo[3,4-b]pyrrolo[4,3,2-de]quinolone (7q)
Brown solid (142 mg, 35% yield): mp 274–275 °C; 1H NMR (400 MHz, CDCl3-d) δ 7.16 (s, 1H), 7.14 (s, 1H), 7.07–7.04 (m, 4H), 6.83 (d, J = 8.8 Hz, 2H), 4.10 (s, 3H), 3.80 (s, 3H), 3.12–3.10 (m, 2H), 2.86 (t, J = 6.0 Hz, 2H), 2.33 (s, 3H), 2.31–2.28 (m, 2H), 1.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.5, 158.9, 140.2, 137.3, 132.2, 130.6, 130.0, 128.6, 128.4, 122.4, 115.7, 114.4, 114.0, 102.8, 55.4, 34.4, 31.3, 24.5, 23.0, 21.3, 15.5; HRMS (ESI-TOF) m/z calcd for [M + H]+ C27H27N4 407.2230, found 407.2251.
Acknowledgments
We are grateful for financial support from the NSFC (21332005, 21232004, 21272095, 21472071, and 21102124), the PAPD of Jiangsu Higher Education Institutions, the Jiangsu Science and Technology Support Program (BE2011045), the Qing Lan Project (12QLG006), the Robert A. Welch Foundation (D-1361), and the National Institutes of Health (R33DA031860).
Supporting Information Available
1H and 13C NMR spectra for all pure products and X-ray crystal data (CIF) for 5a, 5q, and 7d. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Melhado A. D.; Brenzovitch W. E.; Lackner A. D.; Toste F. D. J. Am. Chem. Soc. 2010, 132, 8885. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Adams G. L.; Carroll P. J.; Smith A. B. III. J. Am. Chem. Soc. 2013, 135, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Snyder S. A.; Breazzano S. P.; Ross A. G.; Lin Y.; Zografos A. L. J. Am. Chem. Soc. 2009, 131, 1753. [DOI] [PubMed] [Google Scholar]
- a Ondachi P. W.; Comins D. L. J. Org. Chem. 2010, 75, 1706. [DOI] [PubMed] [Google Scholar]; b Inaba Y.; Hasuda T.; Hitotsuyanagi Y.; Aoyagi Y.; Fujikawa N.; Onozaki A.; Watanabe A.; Kinoshita T.; Takeya K. J. Nat. Prod. 2013, 76, 1085. [DOI] [PubMed] [Google Scholar]; c Bull J. A.; Mousseau J. J.; Pelletier G.; Charette A. B. Chem. Rev. 2012, 112, 2642. [DOI] [PubMed] [Google Scholar]; d Montenegro H. E.; Ramirez-Lopez P.; de la Torre M. C.; Asenjo M.; Sierra M. A. Chem.—Eur. J. 2010, 16, 3798. [DOI] [PubMed] [Google Scholar]
- For isolation of oxerine, see:; a Benkrief R.; Skaltsounis A.-L.; Tillequin F.; Koch M.; Pusset J. Planta Med. 1991, 57, 79. [DOI] [PubMed] [Google Scholar]; For syntheses of oxerine, see:; b Aoyagi Y.; Inariyama T.; Arai Y.; Tsuchida S.; Matuda Y.; Kobayashi H.; Ohta A.; Kurihara T.; Fujihira S. Tetrahedron 1994, 50, 13575. [Google Scholar]; c Jones K.; Fiumana A. Tetrahedron Lett. 1996, 37, 804. [Google Scholar]; d Jones K.; Fiumana A.; Escudero-Hernandez M. L. Tetrahedron 2000, 56, 3976. [Google Scholar]; e Ohba M.; Izuta R.; Shimizu E. Tetrahedron Lett. 2000, 41, 10251. [Google Scholar]; f Zhao J.; Yang X.; Jia X.; Luo S.; Zhai H. Tetrahedron 2003, 59, 9379. [Google Scholar]
- Sakan T.; Fujino A.; Murai F.; Butsugan Y.; Suzui A. Bull. Chem. Soc. Jpn. 1959, 32, 315. [Google Scholar]
- a Hattori M.; Kawata Y.; Inoue K.; Shu Y.-Z.; Che Q.-M.; Namba T.; Kobashi K. Phytother. Res. 1990, 4, 66. [Google Scholar]; b Baghdikian B.; Ollivier E.; Faure R.; Debrauwer L.; Rathelot P.; Balansard G. J. Nat. Prod. 1999, 62, 211. [DOI] [PubMed] [Google Scholar]; c Baghdikian B.; Guiraud-Dauriac H.; Ollivier E.; N’Guyen A.; Dumenil G.; Balansard G. Planta Med. 1999, 65, 164. [DOI] [PubMed] [Google Scholar]
- a Cappelli A.; Nannicini C.; Gallelli A.; Giuliani G.; Valenti S.; Mohr G. P.; Anzini M.; Mennuni L.; Ferrari F.; Caselli G.; Giordani A.; Pereis W.; Makovec F.; Giorgi G.; Vomero S. J. Med. Chem. 2008, 51, 2137. [DOI] [PubMed] [Google Scholar]; b de Mello H.; Echevarria A.; Bernardino A. M.; Canto-Cavalheiro M.; Leon L. L. J. Med. Chem. 2004, 47, 5427. [DOI] [PubMed] [Google Scholar]; c Tuccinardi T.; Schenone S.; Bondavalli F.; Brullo C.; Bruno O.; Mosti L.; Zizzari A. T.; Tintori C.; Manetti F.; Ciampi O.; Trincavelli M. L.; Martini C.; Martinelli A.; Botta M. ChemMedChem 2008, 3, 898. [DOI] [PubMed] [Google Scholar]; d Manetti F.; Schenone S.; Bondavalli F.; Brullo C.; Bruno O.; Ranise A.; Mosti L.; Menozzi G.; Fossa P.; Trincavelli M. L.; Martini C.; Martinelli A.; Tintori C.; Botta M. J. Med. Chem. 2005, 48, 7172. [DOI] [PubMed] [Google Scholar]
- a Lin R.; Connolly P. J.; Lu Y.; Chiu G.; Li S.; Yu Y.; Huang S.; Li X.; Emanuel S. L.; Middleton S. A.; Gruninger R. H.; Adams M.; Fuentes-Pesquera A. R.; Greenberger L. M. Bioorg. Med. Chem. Lett. 2007, 17, 4297. [DOI] [PubMed] [Google Scholar]; b Witherington J.; Bordas V.; Garland S. L.; Hickey D. M. B.; Ife R. J.; Liddle J.; Saunders M.; Smith D. G.; Ward R. W. Bioorg. Med. Chem. Lett. 2003, 13, 1577. [DOI] [PubMed] [Google Scholar]; c Revesz L.; Blum E.; Di Padova F. E.; Buhl T.; Feifel R.; Gram H.; Hiestand P.; Manning U.; Neumann U.; Rucklin G. Bioorg. Med. Chem. Lett. 2006, 16, 262. [DOI] [PubMed] [Google Scholar]
- a Sezer S.; Gumrukcu Y.; Bakirci I.; Yagiz Unver M.; Tanyeli C. Tetrahedron: Asymmetry 2012, 23, 662. [Google Scholar]; b Bonaga L. V. R.; Zhang H.-C.; Maryanoff B. E. Chem. Commun. 2004, 2394. [DOI] [PubMed] [Google Scholar]; c Deprele S.; Kashemirov B. A.; Hogan J. M.; Ebetino F. H.; Barnett B. L.; Evdokimov A.; McKenna C. E. Bioorg. Med. Chem. Lett. 2008, 18, 2878. [DOI] [PubMed] [Google Scholar]; d Dotsenko V. V.; Krivokolysko S. G.; Litvinov V. P. Monatsh. Chem. 2008, 139, 271. [Google Scholar]; e Robert N.; Hoarau C.; Marsais F. Tetrahedron 2007, 63, 3702–3706. [Google Scholar]; f Zhao J.; Yang X.; Jia X.; Luo S.; Zhai H. Tetrahedron 2003, 59, 9379. [Google Scholar]; g Guenter M.; Gais H.-J. J. Org. Chem. 2003, 68, 8037. [DOI] [PubMed] [Google Scholar]; h Ohba M.; Izuta R. Heterocycles 2001, 55, 823. [Google Scholar]; i Ohba M.; Izuta R.; Shimizu E. Tetrahedron Lett. 2000, 41, 10251. [Google Scholar]; j Jones K.; Fiumana A. Tetrahedron Lett. 1996, 37, 8049. [Google Scholar]
- a Shekarrao K.; Kaishap P. P.; Saddanapu V.; Addlagatta A.; Gogoi S.; Boruah R. C. RSC Adv. 2014, 4, 24001. [Google Scholar]; b Dodiya D. K.; Trivedi A. R.; Kataria V. B.; Shah V. H. Curr. Org. Chem. 2012, 16, 400. [Google Scholar]; c El-borai M. A.; Rizk H. F.; Abd-Aal M. F.; El-Deeb I. Y. Eur. J. Med. Chem. 2012, 48, 92. [DOI] [PubMed] [Google Scholar]; d Kendre D. B.; Toche R. B.; Jachak M. N. J. Heterocycl. Chem. 2008, 45, 1281. [Google Scholar]; e Diaz-Ortiz A.; Carrillo J. R.; Cossio F. P.; Gomez-Escalonilla M. J.; De la Hoz A.; Moreno A.; Prieto P. Tetrahedron 2000, 56, 1569. [Google Scholar]
- Winters G.; Schiatti P.; Selva D. Farmaco, Ed. Sci. 1985, 40, 845. [PubMed] [Google Scholar]
- For reviews, see:; a Groenendaal B.; Ruijter E.; Orru R. V. A. Chem. Commun. 2008, 5474. [DOI] [PubMed] [Google Scholar]; b Padwa A. Chem. Soc. Rev. 2009, 38, 3072. [DOI] [PubMed] [Google Scholar]; c Tietze L. F.; Kinzel T.; Brazel C. C. Acc. Chem. Res. 2009, 42, 367. [DOI] [PubMed] [Google Scholar]; d Domling A.; Wang W.; Wang K. Chem. Rev. 2012, 112, 3083. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ruijter E.; Scheffelaar R.; Orru R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234. [DOI] [PubMed] [Google Scholar]; f Jiang B.; Rajale T.; Wever W.; Tu S.-J.; Li G. Chem.—Asian J. 2010, 5, 2318. [DOI] [PubMed] [Google Scholar]
- a Tietze L. F.; Brasche G.; Gerike K.. Domino Reactions in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]; b Zhu J. P.; Bienayme H.. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]; c Toure B. B.; Hall D. G. Chem. Rev. 2009, 109, 4439. [DOI] [PubMed] [Google Scholar]; d Ganem B. Acc. Chem. Res. 2009, 42, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ismabery N.; Lavila R. Chem.—Eur. J. 2008, 14, 8444.18576454 [Google Scholar]; f Tietze L. F. Chem. Rev. 1996, 96, 115. [DOI] [PubMed] [Google Scholar]
- a Li M.; Shao P.; Wang S.-W.; Kong W.; Wen L.-R. J. Org. Chem. 2012, 77, 8956. [DOI] [PubMed] [Google Scholar]; b Terzidis M. A.; Zarganes-Tzitzikas T.; Tsimenidis C.; Stephanidou-Stephanatou J.; Tsoleridis C. A.; Kostakis G. E. J. Org. Chem. 2012, 77, 9018. [DOI] [PubMed] [Google Scholar]; c Ausmees K.; Kriis K.; Pehk T.; Werner F.; Järving I.; Lopp M.; Kanger T. J. Org. Chem. 2012, 77, 10680. [DOI] [PubMed] [Google Scholar]; d Sun J.; Sun Y.; Gong H.; Xie Y.-J.; Yan C.-G. Org. Lett. 2012, 14, 5172. [DOI] [PubMed] [Google Scholar]
- a Fan W.; Ye Q.; Xu H.-W.; Jiang B.; Wang S.-L.; Tu S.-J. Org. Lett. 2013, 15, 2258. [DOI] [PubMed] [Google Scholar]; b Jiang B.; Wang X.; Xu H.-W.; Tu M.-S.; Tu S.-J.; Li G. Org. Lett. 2013, 15, 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang B.; Feng B.-M.; Wang S.-L.; Tu S.-J.; Li G. Chem.—Eur. J. 2012, 18, 9823. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jiang B.; Tu S.-J.; Kaur P.; Wever W.; Li G. J. Am. Chem. Soc. 2009, 131, 11660. [DOI] [PubMed] [Google Scholar]; e Li T.-J.; Yin H.-M.; Yao C.-S.; Wang X.-S.; Jiang B.; Tu S.-J.; Li G. Chem. Commun. 2012, 11966. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Jiang B.; Li C.; Shi F.; Tu S.-J.; Kaur P.; Wever W.; Li G. J. Org. Chem. 2010, 75, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Raikar S. N.; Malinakova H. C. J. Org. Chem. 2013, 78, 3832. [DOI] [PubMed] [Google Scholar]; b Xia L.; Li S.; Chen R.-J.; Liu K.; Chen X.-C. J. Org. Chem. 2013, 78, 3120. [DOI] [PubMed] [Google Scholar]; c Wang X.; Wang S.-Y.; Ji S.-J. Org. Lett. 2013, 15, 1954. [DOI] [PubMed] [Google Scholar]; d Qian W.-Y.; Amegadzie A.; Winternheimer D.; Allen J. Org. Lett. 2013, 15, 2986. [DOI] [PubMed] [Google Scholar]; e Xu Z.-G.; Moline F. D.; Cappelli A. P.; Hulme C. Org. Lett. 2013, 15, 2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B.; Ye Q.; Fan W.; Wang S.-L.; Tu S.-J.; Li G. Chem. Commun. 2014, 50, 6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a De March P.; Moreno-Manas M.; Roca J. L. J. Heterocycl. Chem. 1984, 21, 1371. [Google Scholar]; b Svetlik J.; Pronayova N.; Hanus V. J. Heterocycl. Chem. 2000, 37, 395. [Google Scholar]; c Grigoryeva O. A.; Fedotova O. V.; Shkel A. A. Chem. Heterocycl. Compd. 2011, 46, 1509. [Google Scholar]; d Gelin S.; Chantegrel B.; Nadi A. I. J. Org. Chem. 1983, 48, 4078. [Google Scholar]; e Jiang B.; Feng B.-M.; Wang S.-L.; Tu S.-J.; Li G. Chem.—Eur. J. 2012, 18, 9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the preparation of 6-methyl-1-phenylpyridine-2,4(1H,3H)-dione 8, see:Selness S. R.; Boehm T. L.; Walker J. K.; Devadas B.; Durley R. C.; Kurumbail R.; Shieh H.; Xing L.; Hepperle M.; Rucker P. V.; Jerome K. D.; Benson A. G.; Marrufo L. D.; Madsen H. M.; Hitchcock J.; Owen T. J.; Christie L.; Promo M. A.; Hickory B. S.; Alvira E.; Naing W.; Blevis-Bal R.; Devraj R. V.; Messing D.; Schindler J. F.; Hirsch J.; Saabye M.; Bonar S.; Webb E.; Anderson G.; Monahan J. B. Bioorg. Med. Chem. Lett. 2011, 21, 4059. [DOI] [PubMed] [Google Scholar]
- For the preparation of pyrazolo[3,4-b]quinolin-5(4H)-ones 9, see:Hua G.-P.; Xu J.-N.; Tu S.-J.; Wang Q.; Zhang J.-P.; Zhu X.-T.; Li T.-J.; Zhu S.-L.; Zhang X.-J. Youji Huaxue 2005, 25, 1610. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






