Abstract

Conditions have been developed for the palladium-catalyzed cross-coupling of 3-bromo-2,1-borazaronaphthalenes with potassium alkenyltrifluoroborates. Twenty-seven alkenyl-substituted azaborines have been synthesized through this method, providing access to a family of 2,1-borazaronaphthalenes with alkenyl substitution at the C3 position.
Introduction
2,1-Borazaronaphthalenes serve as versatile isosteres of all-carbon naphthalene substructures, which allows facile emplacement of a variety of substituents about the ring in an efficient and selective manner. Because of electronic desymmetrization brought about by the incorporation of a B—N bond in these aromatic systems, borazines have proven to be far more easily elaborated than naphthalenes themselves. Previous studies have focused on decorating these ring systems by utilizing arylation reactions. Alkenylation reactions are herein examined as a means to elaborate brominated 2,1-borazaronaphthalenes.
2-Alkenylnaphthalenes can be synthesized through a variety of transition-metal-catalyzed transformations, including Heck,1 Mizoroki–Heck,2 Hiyama,3 and Suzuki–Miyaura4 reactions. However, installation of an alkenyl substituent on a functionalized naphthalene is more challenging and often requires four or five steps.5 One method to afford alkenyl-substituted naphthalenes is to perform free-radical bromination of a methyl-substituted derivative followed by a Wittig olefination.6 Aside from the generation of a stoichiometric amount of difficult-to-remove phosphine oxide waste, additional substitution on the naphthalene can affect regiochemical control of free-radical bromination. Similarly, titanium-mediated carbonylation with dichloromethyl methyl ether followed by Wittig olefination afforded 2-alkyl-3-alkenylnaphthalene in moderate yield over two steps.7 In addition to the drawbacks described above, the use of a strong Lewis acid limits functional group tolerability in this approach. Further, there is only one example demonstrating the installation of an alkenyl substituent on a 2-arylnapthalene through the cross-coupling of naphthyl iodide and alkenylboronic acid, which provides 2-aryl-3-alkenylnaphthalene in good yield.8 The naphthyl iodide in this case was synthesized through gold-catalyzed cyclization/iodination, which required preparation of the requisite starting material (1-arylalka-2,3-dienyl acetate). Additionally, to the best of our knowledge, installation of an alkenyl substituent at the C3 position of a 1,2-disubstituted naphthalene has not been reported.
Accessing 2,1-borazaronaphthalenes, a class of azaborines9 that are BN-isosteres of naphthalene, with alkenyl substituents at the C3 position under mild reaction conditions would therefore serve as an example of how functionalized azaborines could be prepared more efficiently than their corresponding all-carbon analogues. Recently, we reported the bromination of 2,1-borazaronaphthalenes in high yield under mild reaction conditions with complete regiochemical control.10 Because of the inherent reactivity of azaborine, bromination occurs selectively at the C3 position of the azaborine in the presence of alkyl/aryl substituents on boron and nitrogen. We therefore envisioned these brominated azaborines as prefunctionalized electrophilic components in Suzuki–Miyaura cross-coupling reactions11 with potassium alkenyltrifluoroborates12 as a route to C3-alkenyl-substituted 2,1-borazaronaphthalenes, a compound class that has not been previously reported.
Results and Discussion
Optimization of the reaction of 3-bromo-2-methyl-2,1-borazaronaphthalene 1 with functionalized alkenyltrifluoroborate 2 was carried out by screening palladium sources, solvents, and bases. Side products represent the sum of homocoupling of the azaborine, protodebromination of the azaborine, and unidentifiable side products. Conditions for the cross-coupling of potassium aryltrifluoroborates with brominated 2,1-borazaronaphthalenes did not afford the desired product in appreciable yield (Table 1, entry 1). Therefore, several other ligands were screened in this reaction (entries 2–4 and 7). Of the ligands tested, triphenylphosphine (PPh3) and 1,1′-bis(diphenylphosphino)ferrocene (dppf) provided the highest conversions to products. Several solvents/ratios were tested for these ligands, and the best result was obtained using toluene as the cosolvent and Pd(dppf)Cl2 as the palladium source (entry 7). Attempts to lower palladium loading resulted in incomplete conversion of the starting material.
Table 1. Optimization of Cross-Coupling with Potassium Alkenyltrifluoroborates.

| entry | palladium source (loading) | base | solvent | P:SPa |
|---|---|---|---|---|
| 1 | XPhos-Pd-G2 (4 mol %) | Cs2CO3 | 1:1 CPME/H2O | trace product |
| 2 | SPhos-Pd-G2 (4 mol %) | K2CO3 | 1:1 CPME/H2O | trace product |
| 3 | Pd2dba3/cataCXium A (4 mol %) | Cs2CO3 | 1:1 CPME/H2O | 0.50:1.0 |
| 4 | (Ph3P)2PdCl2 (6 mol %) | Cs2CO3 | 1:1 CPME/H2O | 2.57:1.0 |
| 5 | (Ph3P)2PdCl2 (6 mol %) | Cs2CO3 | 1:1 toluene/H2O | 2.00:1.0 |
| 6 | (Ph3P)2PdCl2 (6 mol %) | Cs2CO3 | 9:1 toluene/H2O | 2.50:1.0 |
| 7 | Pd(dppf)Cl2(6 mol %) | Cs2CO3 | 1:1 toluene/H2O | 3.10:1.0 |
| 8 | Pd(dppf)Cl2 (6 mol %) | Cs2CO3 | 1:1 CPME/H2O | 1.85:1.0 |
Product:side products.
A representative set of potassium alkenyltrifluoroborates were subjected to the developed reaction conditions with 3-bromo-2-methyl-2,1-borazaronaphthalene as the electrophilic partner (Table 2). Cyclic alkenyltrifluoroborates were successful nucleophiles in the reaction by providing the desired product in yields up to 90% (entries 5, 6, 8, and 10). Alkenyltrifluoroborates with alkyl substituents afforded the desired product in high yields (entries 2, 3, and 9). Vinyltrifluoroborate was a suitable nucleophile for the reaction because the azaborine was obtained in 70% yield (entry 7). Most importantly, cis-1-propenyltrifluoroborate was successfully employed in the reaction, affording the product in high yield without isomerization of the alkene (entry 3). The scalable nature of the cross-coupling reaction was demonstrated by performing the coupling on a 4.5 mmol scale (1 g of azaborine) with one-third palladium loading (2 mol %), which provided the desired product in similar yield (entry 8).
Table 2. Scope of the Cross-Coupling with Potassium Alkenyltrifluoroboratesc.


Alkenyltrifluoroborate ratio of 94:6 cis/trans employed in this reaction.
Reaction completed on a 4.5 mmol scale with 2.0 mol % Pd(dppf)Cl2.
Reaction conditions (unless otherwise noted): 1.0 equiv of 3-bromo-2-methyl-2,1-borazaronaphthalene, 1.1 equiv of potassium alkenyltrifluoroborate, 6.0 mol % Pd(dppf)Cl2, 3.0 equiv of base, 1:1 toluene/H2O, and 60 °C for 18 h.
The developed reaction conditions were extended to cross-coupling of 3-bromo-2-phenyl-2,1-borazaronaphthalene with an array of potassium alkenyltrifluoroborates to demonstrate that azaborine can also be substituted with an aryl group on the boron. Six different alkenyltrifluoroborates were employed in the coupling and provided the desired product in yields up to 90%. The mild reaction conditions of the coupling were demonstrated in the successful coupling of an alkenyltrifluoroborate with an alkyl chloride substituent, affording the desired product in 90% yield (Table 3, entry 2). Cyclic alkenyltrifluoroborates were successfully engaged in the reaction as the desired products were obtained in high yields (entries 3, 4, and 6).
Table 3. Scope of the Cross-Coupling of Brominated B-Phenyl 2,1-Borazaronaphthalenesa.


Reaction conditions (unless otherwise noted): 1.0 equiv of 3-bromo-2-phenyl-2,1-borazaronaphthalene, 1.1 equiv of potassium alkenyltrifluoroborate, 6.0 mol % Pd(dppf)Cl2, 3.0 equiv of base, 1:1 toluene/H2O, and 60 °C for 18 h.
To demonstrate the versatility of this method, an array of brominated 2,1-borazaronaphthalenes were synthesized and subjected to the developed reaction conditions with 1-decenyltrifluoroborate as the nucleophile in the reaction. The cross-coupled products were obtained with secondary cyclic and acyclic groups on boron in yields up to 90% (Table 4, entries 1 and 2). Azaborines with various substitution patterns on the arene of boron were suitable electrophiles for coupling as the cross-coupled products were isolated in high yield (entries 3–5). Substitution on the nitrogen of the 2,1-borazaronaphthalenes did not affect the coupling as reactions with both N-allyl and N-benzyl substituents provided the desired product in high yields (entries 6 and 7).
Table 4. Scope of the Cross-Coupling with Various Brominated 2,1-Borazaronaphthalenesa.
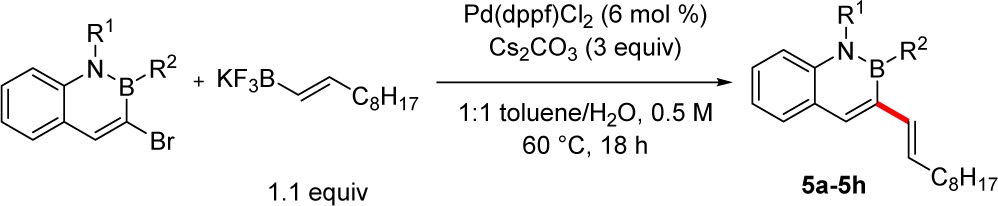

Reaction conditions (unless otherwise noted): 1.0 equiv of 3-bromo-2,1-borazaronaphthalene, 1.1 equiv of potassium alkenyltrifluoroborate, 6.0 mol % Pd(dppf)Cl2, 3.0 equiv of base, 1:1 toluene/H2O, and 60 °C for 18 h.
To demonstrate that the developed reaction conditions can be directly applied to the functionalization of other azaborines, 6-bromo-2-methyl-3-phenyl-2,1-borazaronaphthalene was subjected to the reaction (eq 1). The desired product was obtained in 83% yield to afford C6-alkenyl-substituted 2,1-borazaronaphthalenes, examples of which are absent in the literature.
 |
1 |
The reaction conditions were then extended to cross-coupling of 3,6-dibrominated 2,1-borazaronaphthalene. The addition of 2.2 equiv of 1-propenyltrifluoroborate provides a doubly cross-coupled product in 76% yield (eq 2).
 |
2 |
In conclusion, a general method for Suzuki–Miyaura cross-coupling of brominated 2,1-borazaronaphthalenes with potassium alkenyltrifluoroborates has been developed. Azaborines with alkyl and aryl groups on boron and with or without substitution on nitrogen are suitable reagents for the coupling. Through this route, functionalized 2,1-borazaronaphthalenes with alkenyl substituents can be easily accessed for the first time.
Experimental Section
General Considerations
Toluene was dried over activated alumina. Standard benchtop techniques were employed for handling air-sensitive reagents. Melting points (°C) are uncorrected. NMR spectra were recorded on a 400 or 500 MHz spectrometer. 11B NMR spectra were obtained on a spectrometer equipped with the appropriate decoupling accessories. All 11B NMR chemical shifts were referenced to external BF3·OEt2 (0.0 ppm) with a negative sign indicating an upfield shift. Data are presented as follows: chemical shift (ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quadruplet, m = multiplet, br = broad), coupling constant J (Hz), and integration. Analytical thin-layer chromatography (TLC) was performed on TLC silica gel plates (0.25 mm) precoated with a fluorescent indicator. Visualization of the TLC plates was effected with ultraviolet light. Standard flash chromatography procedures were followed using 100–200 mesh silica gel. HRMS data were obtained by either ESI or CI using a TOF mass spectrometer.
Spectral Information of 2,1-Borazaronaphthalenes
2,1-Borazaronaphthalenes were synthesized13 and brominated10 according to methods previously described in the literature.
3-Bromo-2-(4-trifluoromethylphenyl)-2,1-borazaronaphthalene
The title compound was obtained as a white solid in 90% yield (1.0 mmol scale, 316.7 mg): mp 98–103 °C; 1H NMR (500 MHz, CDCl3) δ 8.49 (s, 1H), 8.07 (s, 1H), 7.98 (d, J = 7.8 Hz, 2H), 7.72 (d, J = 7.8 Hz, 2H), 7.64 (d, J = 7.9 Hz, 1H), 7.51–7.49 (m, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.30–7.27 (m, 1H); 13C NMR (125.8 MHz, CDCl3) δ 147.3, 139.0, 133.6, 131.2 (d, J = 32.5 Hz), 129.7, 129.2, 126.5 (q, J = 275.8 Hz), 125.3, 124.6 (q, J = 3.6 Hz), 122.5, 118.4; 11B NMR (128.38 MHz, CDCl3) δ 32.9; IR (neat) 3367, 2923, 1556, 1427, 1120, 1070 cm–1; HRMS (CI) m/z [M + H]+ calcd for C15H11BNF3Br 352.9825, found 352.9802.
General Procedure for the Palladium-Catalyzed Cross-Coupling Reaction of Potassium Alkenyltrifluoroborates with Brominated 2,1-Borazaronaphthalenes
To a Biotage 10 mL microwave vial equipped with a magnetic stir bar were added 3-bromo-2,1-alkenyltrifluoroborate (1.1 mmol, 1.1 equiv), Cs2CO3 (3.0 mmol, 977 mg), Pd(dppf)Cl2 (6.0 mol %, 42 mg), and potassium alkenyltrifluoroborate (1.0 mmol, 1.0 equiv). The vial was sealed with a cap, lined with a Teflon septum, and evacuated and purged with argon gas three times. Anhydrous degassed toluene (1.0 mL) and deionized H2O (1.0 mL) were added under argon gas. The reaction mixture was heated at 60 °C for 18 h. After cooling to rt, the reaction mixture was extracted with EtOAc (3 × 10 mL) and dried (MgSO4). After being concentrated in vacuo, the product was isolated by flash column chromatography and eluted with a gradient of EtOAc in hexanes (0 to 10% EtOAc).
N-(3-(2-Methyl-2,1-borazaronaphth-2-yl)allyl)morpholine (3a)
The title compound was obtained as a yellow solid in 88% yield (235.8 mg): mp 89–93 °C; 1H NMR (500 MHz, CDCl3) δ 7.79 (s, 1H), 7.72 (s, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.34–7.37 (m, 1H), 7.18 (d, J = 8.1 Hz, 1H), 7.15–7.12 (m, 1H), 5.24–5.23 (m, 1H), 5.12 (d, J = 2.5 Hz, 1H), 3.67 (t, J = 4.6 Hz, 4H), 3.31 (s, 2H), 2.48 (br, 4H), 0.79 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 149.0, 140.1, 129.6, 128.1, 125.0, 121.0, 117.4, 114.2, 67.3, 64.8, 53.9; 11B NMR (128.38 MHz, CDCl3) δ 36.7; IR (neat) 3319, 2928, 1566, 1434, 1109, 891 cm–1; HRMS (ESI+) m/z [M + H]+ calcd for C16H22BN2O 269.1825, found 269.1820.
2-Methyl-3-((E)-1-propen-1-yl)-2,1-borazaronaphthalene (3b)
The title compound was obtained as a yellow solid in 98% yield (179.3 mg): mp 69–75 °C; 1H NMR (500 MHz, CDCl3) δ 7.76 (s, 1H), 7.65 (s, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.36–7.29 (m, 1H), 7.16 (d, J = 8.0 Hz, 1H), 7.13–7.10 (m, 1H), 6.59 (d, J = 15.6 Hz, 1H), 6.19–6.12 (m, 1H), 1.91 (dd, J = 6.6, 1.5 Hz, 3H), 0.85 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.5, 138.7, 134.8, 129.3, 127.6, 126.5, 125.6, 121.0, 117.4, 19.4; 11B NMR (128.38 MHz, CDCl3) δ 37.2; IR (neat) 3364, 2924, 1614, 1457, 1343 cm–1; HRMS (CI) m/z [M]+ calcd for C12H14BN 183.1219, found 183.1216.
2-Methyl-3-((Z)-1-propen-1-yl)-2,1-borazaronaphthalene (3c)
The title compound was obtained as a brown solid in 87% yield (159.2 mg): mp 98–103 °C; 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H), 7.68 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.37–7.34 (m, 1H), 7.19 (d, J = 8.0 Hz, 1H), 7.18–7.13 (m, 1H), 6.57–6.54 (m, 1H), 5.72–5.79 (m, 1H), 1.85 (dd, J = 7.0, 1.8 Hz, 3H), 0.74 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 140.8, 139.6, 132.1, 129.4, 127.8, 125.3, 125.0, 121.0, 117.6, 14.8; 11B NMR (128.38 MHz, CDCl3) δ 37.3; IR (neat) 3364, 2925, 1611, 1560, 1454 cm–1; HRMS (CI) m/z [M]+ calcd for C12H14BN 183.1219, found 183.1213.
3-(Isopropen-1-yl)-2-methyl-2,1-borazaronaphthalene (3d)
The title compound was obtained as a yellow oil in 69% yield (126.2 mg). 1H NMR (500 MHz, CDCl3) δ 7.80 (s, 1H), 7.68 (s, 1H), 7.62 (d, J = 7.7 Hz, 1H), 7.39–7.36 (m, 1H), 7.19–7.15 (m, 2H), 5.21–5.11 (m, 1H), 5.07 (d, J = 1.9 Hz, 1H), 2.16 (s, 3H), 0.87 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 148.4, 139.6, 139.2, 129.6, 128.0, 125.1, 121.0, 117.4, 113.0, 23.9; 11B NMR (128.38 MHz, CDCl3) δ 36.9; IR (neat) 3368, 2933, 1567, 1462, 1453 cm–1; HRMS (CI) m/z [M]+ calcd for C12H14BN 183.1219, found 183.1220.
4-(2-Methyl-2,1-borazaronaphthyl)-3,6-dihydro-2H-pyran (3e)
The title compound was obtained as a yellow oil in 82% yield (184.5 mg). 1H NMR (500 MHz, CDCl3) δ 7.75 (s, 1H), 7.73 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.34–7.38 (m, 1H), 7.18 (d, J = 8.1 Hz, 1H), 7.16–7.13 (m, 1H), 5.83–5.82 (m, 1H), 4.36–4.35 (m, 2H), 3.97 (t, J = 5.4 Hz, 2H), 2.52–2.49 (m, 2H), 0.84 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 138.6, 137.6, 137.5, 128.5, 127.0, 124.1, 121.9, 120.1, 116.4, 65.2, 63.9, 28.1; 11B NMR (128.38 MHz, CDCl3) δ 36.7; IR (neat) 3325, 2923, 1572, 1458, 1032 cm–1; HRMS (CI) m/z [M]+ calcd for C14H16BNO 225.1325, found 225.1336.
8-[3-(2-Methyl-2,1-borazaronaphthyl)]-1,4-dioxaspiro[4.5]dec-7-ene (3f)
The title compound was obtained as a white solid in 81% yield (227.6 mg): mp 83–88 °C; 1H NMR (500 MHz, CDCl3) δ 7.73 (s, 1H), 7.69 (s, 1H), 7.54–7.56 (m, 1H), 7.32–7.35 (m, 1H), 7.16 (d, J = 8 Hz, 1H), 7.13–7.10 (m, 1H), 5.66–5.65 (m, 1H), 4.05–4.02 (m, 4H), 2.63–2.59 (m, 2H), 2.50–2.49 (m, 2H), 1.93 (t, J = 6.5 Hz, 2H), 0.81 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 140.9, 139.5, 138.7, 129.4, 127.8, 125.2, 121.1, 120.9, 117.3, 108.3, 64.7, 36.5, 31.8, 28.7; 11B NMR (128.38 MHz, CDCl3) δ 37.0; IR (neat) 3308, 2927, 1609, 1460, 1047 cm–1; HRMS (CI) m/z [M]+ calcd for C17H20BNO2 281.1587, found 281.1593.
2-Methyl-3-vinyl-2,1-borazaronaphthalene (3g)
The title compound was obtained as a light yellow solid in 70% yield (118.3 mg): mp 52–56 °C; 1H NMR (500 MHz, CDCl3) δ 7.70 (s, 1H), 7.64 (s, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.33–7.30 (m, 1H), 7.15 (d, J = 8.0 Hz, 1H), 7.10–7.08 (m, 1H), 6.73–6.66 (m, 1H), 5.87–5.85 (m, 1H), 5.74–5.71, (m, 1H), 0.70 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 140.7, 140.3, 139.9, 129.6, 128.2, 125.3, 121.2, 117.5, 114.8; 11B NMR (128.38 MHz, CDCl3) δ 37.2; IR (neat) 3365, 2927, 1610, 1457 cm–1; HRMS (CI) m/z [M]+ calcd for C11H12BN 169.1063, found 169.1063.
3-(4,4-Difluoro-1-cyclohexen-1-yl)-2-methyl-2,1-borazaronaphthalene (3h)
The title compound was obtained as a white solid in 90% yield (233.1 mg): mp 72–76 °C; 1H NMR (500 MHz, CDCl3) δ 7.73 (s, 1H), 7.69 (s, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.41–7.37 (m, 1H), 7.20–7.15 (m, 2H), 5.59–5.56 (m, 1H), 2.78–2.72 (m, 2H), 2.67–2.64 (m, 2H), 2.25–2.17 (m, 2H), 0.82 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 140.8, 139.5, 139.1, 129.4, 128.0, 124.9, 123.3 (t, J = 239.6 Hz), 121.0, 118.2 (t, J = 4.9 Hz), 117.3, 35.3 (t, J = 26.4 Hz), 31.0 (t, J = 24.4 Hz), 27.8 (t, J = 5.2 Hz); 11B NMR (128.38 MHz, CDCl3) δ 36.2; IR (neat) 3397, 2936, 1613, 1560, 1425, 1058, cm–1; HRMS (CI) m/z [M]+ calcd for C15H16BNF2 259.1344, found 259.1327.
3-[(E)-1-Decen-1-yl]-2-methyl-2,1-borazaronaphthalene (3i)
The title compound was obtained as a yellow solid in 75% yield (210.7 mg): mp 58–62 °C; 1H NMR (500 MHz, CDCl3) δ 7.79 (s, 1H), 7.65 (s, 1H), 7.57 (d, J = 7.75 Hz, 1H), 7.34–7.31 (m, 1H), 7.16–7.11 (m, 2H), 6.58 (d, J = 15.5 Hz, 1H), 6.18–6.12 (m, 1H), 2.26–2.22 (m, 2H), 1.53–1.47 (m, 2H), 1.40–1.28 (m, 10H), 0.92 (t, J = 6.9 Hz, 3H), 0.86 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.5, 138.8, 133.4, 132.2, 129.3, 127.6, 125.6, 121.0, 117.4, 34.0, 32.2, 30.0, 29.9, 29.7, 29.6, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 37.1; IR (neat) 3366, 2921, 1556, 1463, 977 cm–1; HRMS (CI) m/z [M]+ calcd for C19H28BN 281.2315, found 281.2328.
tert-Butyl-4-[3-(2-methyl-2,1-borazaronaphthyl)]-3,6-dihydropyridine-1-(2H)-carboxylate (3j)
The title compound was obtained as a white solid in 65% yield (210.6 mg): mp 83–87 °C; 1H NMR (500 MHz, CDCl3) δ 7.75 (s, 1H), 7.69 (s, 1H), 7.57 (d, J = 7.7 Hz, 1H), 7.36–7.33 (m, 1H), 7.17 (d, J = 8.0 Hz, 1H), 7.14–7.11 (m, 1H), 5.71 (br, 1H), 4.07 (br, 2H), 3.64 (t, J = 5.5 Hz, 2H), 4.47 (br, 2H), 1.52 (s, 9H), 0.80 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 155.3, 139.9, 139.6, 138.7, 129.5, 128.0, 125.1, 121.1, 120.7, 117.4, 79.7, 44.3, 40.1, 34.9, 29.4, 28.8; 11B NMR (128.38 MHz, CDCl3) δ 32.0; IR (neat) 3327, 2975, 1677, 1563, 1423, 1163 cm–1; HRMS (CI) m/z [M + H]+ calcd for C19H26BN2O2 325.2087, found 325.2080.
2-Methyl-3-[(Z)-4-phenyl-1-buten-1-yl]-2,1-borazaronaphthalene (3k)
The title compound was obtained as a yellow solid in 83% yield (226.5 mg): mp 74–77 °C; 1H NMR (500 MHz, CDCl3) δ 7.66 (s, 1H), 7.59 (s, 1H), 7.55 (d, J = 7.7 Hz, 1H), 7.38–7.36 (m, 1H), 7.35–7.31 (m, 2H), 7.22–7.13 (m, 5H), 6.58 (dd, J = 11.5, 1.4 Hz, 1H), 5.69 (dt, J = 11.5, 7.2 Hz, 1H), 2.79 (t, J = 7.7 Hz, 2H), 2.70–2.45 (m, 2H), 0.73 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 142.3, 140.6, 139.7, 132.1, 129.9, 129.5, 128.9, 128.6, 127.9, 126.1, 125.3, 121.0, 117.5, 36.7, 30.6; 11B NMR (128.38 MHz, CDCl3) δ 37.4; IR (neat) 3373, 2925, 1556, 1452, 746, 703 cm–1; HRMS (CI) m/z [M]+ calcd for C19H20BN 273.1689, found 273.1683.
3-[(E)-1-Decen-1-yl]-2-phenyl-2,1-borazaronaphthalene (4a)
The title compound was obtained as a white oil in 73% yield (250.3 mg). 1H NMR (500 MHz, CDCl3) δ 8.05 (s, 1H), 7.91 (s, 1H), 7.77–7.75 (m, 2H), 7.69 (d, J = 8.0 Hz, 1H), 7.51–7.47 (m, 3H), 7.42–7.39 (s, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.22 (t, J = 7.5 Hz, 1H), 6.70 (d, J = 15.5 Hz, 1H), 6.07 (dt, J = 15.4, 7.0 Hz, 1H), 2.21 (q, J = 7.0 Hz, 2H), 1.50–1.45 (m, 2H), 1.40–1.35 (m, 10H), 0.96 (t, J = 7.0 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.8, 139.6, 133.2, 132.7, 132.3, 129.5, 128.8, 128.2, 128.0, 125.9, 121.0, 118.0, 33.7, 32.2, 29.9, 29.8, 29.7, 29.6, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 34.4; IR (neat) 3370, 2923, 2852, 1421, 749 cm–1; HRMS (CI) m/z [M + Na]+ calcd for C24H30BNNa 366.2369, found 366.2375.
3-(6-Chloro-1-hexen-1-yl)-2-phenyl-2,1-borazaronaphthalene (4b)
The title compound was obtained as a brown oil in 90% yield (288.9 mg). 1H NMR (500 MHz, CDCl3) δ 8.03 (s, 1H), 7.92 (s, 1H), 7.72 (dd, J = 7.25, 1.5 Hz, 2H), 7.68 (d, J = 7.5 Hz, 1H), 7.50–7.46 (m, 3H), 7.42–7.39 (m, 1H), 7.26 (d, J = 8 Hz, 1H), 7.23–7.20 (m, 1H), 6.68 (d, J = 15.5 Hz, 1H), 6.00 (dt, J = 15.4, 7.0 Hz, 1H), 3.57 (t, J = 6.5 Hz, 2H), 2.23–2.19 (m, 2H), 1.86–1.83 (m, 2H), 1.62–1.58 (m, 2H); 13C NMR (125.8 MHz, CDCl3) δ 140.1, 139.6, 133.4, 133.1, 131.1, 129.5, 128.8, 128.2, 128.1, 125.7, 121.7, 118.0, 45.3, 32.7, 32.3, 27.0; 11B NMR (128.38 MHz, CDCl3) δ 35.0; IR (neat) 3373, 2933, 1558, 1421, 969, 752 cm–1; HRMS (CI) m/z [M]+ calcd for C20H21BNCl 321.1456, found 321.1463.
3-(1-Cyclohexen-1-yl)-2-phenyl-2,1-borazaronaphthalene (4c)
The title compound was obtained as a yellow solid in 79% yield (225.1 mg): mp 88–92 °C; 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 1H), 7.85 (s, 1H), 7.79–7.7.78 (m, 2H), 7.66 (d, J = 7.5 Hz, 1H), 7.45–7.39 (m, 4H), 7.28 (d, J = 8.0 Hz, 1H), 7.22–7.19 (m, 1H), 5.73–5.71 (m, 1H), 2.19–2.13 (m, 4H), 1.66–1.65 (m, 4H); 13C NMR (125.8 MHz, CDCl3) δ 142.3, 140.5, 139.6, 132.8, 129.5, 128.9, 128.1, 128.0, 125.6, 124.2, 121.5, 117.9, 29.9, 26.1, 23.5, 22.6; 11B NMR (128.38 MHz, CDCl3) δ 34.0; IR (neat) 3375, 2920, 1558, 1451, 751 cm–1; HRMS (CI) m/z [M]+ calcd for C20H20BN 285.1689, found 285.1705.
4-(2-Phenyl-2,1-borazaronaphthyl)-3,6-dihydro-2H-pyran (4d)
The title compound was obtained as a white solid in 86% yield (246.8 mg): mp 112–115 °C; 1H NMR (500 MHz, CDCl3) δ 8.02 (s, 1H), 7.91 (s, 1H), 7.76–7.68 (m, 2H), 7.69 (d, J = 7.5 Hz, 1H), 7.47–7.42 (m, 4H), 7.29 (d, J = 8.1 Hz, 1H), 7.24–7.21 (m, 1H), 5.75–5.73 (m, 1H), 4.31–4.29 (m, 2H), 3.84 (t, J = 5.5 Hz, 2H), 2.30 (td, J = 5.3, 2.7 Hz, 2H); 13C NMR (125.8 MHz, CDCl3) δ 140.9, 139.7, 139.4, 132.7, 129.6, 128.9, 128.4, 128.2, 125.3, 122.8, 121.7, 118.0, 66.2, 64.9, 29.5; 11B NMR (128.38 MHz, CDCl3) δ 34.0; IR (neat) 3287, 2930, 1564, 1455, 1229, 758 cm–1; HRMS (CI) m/z [M]+ calcd for C19H18BNO 287.1481, found 287.1487.
2-Phenyl-3-(isopropen-1-yl)-2,1-borazaronaphthalene (4e)
The title compound was obtained as a brown oil in 76% yield (186.2 mg). 1H NMR (500 MHz, CDCl3) δ 7.96 (br, 2H), 7.92–7.81 (m, 2H), 7.70 (d, J = 7.5 Hz, 1H), 7.47–7.43 (m, 4H), 7.29 (d, J = 8.0 Hz, 1H), 7.25–7.22 (m, 1H), 5.06–5.02 (m, 2H), 1.99 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 149.2, 140.9, 139.5, 132.6, 129.5, 128.7, 128.2, 128.0, 125.2, 121.5, 117.8, 113.0, 24.1; 11B NMR (128.38 MHz, CDCl3) δ 33.6; IR (neat) 3367, 2925, 1573, 1463, 757 cm–1; HRMS (CI) m/z [M]+ calcd for C17H16BN 245.1376, found 245.1377.
8-(2-Phenyl-2,1-borazaronaphthyl)-1,4-dioxaspiro[4.5]dec-7-ene (4f)
The title compound was obtained as a white solid in 81% yield (277.8 mg): mp 108–112 °C; 1H NMR (500 MHz, CDCl3) δ 7.98 (s, 1H), 7.90 (s, 1H), 7.80–7.78 (m, 2H), 7.65 (d, J = 7.5 Hz, 1H), 7.45–7.39 (m, 4H), 7.28 (d, J = 8.0 Hz, 1H), 7.21–7.18 (m, 1H), 6.62–5.60 (m, 1H), 4.05–3.98 (m, 4H), 2.47–2.46 (m, 2H), 2.38 (td, J = 6.3, 1.6 Hz, 2H), 1.79 (t, J = 6.5 Hz, 2H); 13C NMR (125.8 MHz, CDCl3) δ 141.9, 141.1, 139.6, 132.7, 129.4, 128.9, 128.1, 128.1, 125.3, 121.5, 121.1, 117.8, 108.2, 64.5, 36.4, 31.6, 29.0; 11B NMR (128.38 MHz, CDCl3) δ 34.4; IR (neat) 3326, 1560, 1421, 1112, 755 cm–1; HRMS (CI) m/z [M]+ calcd for C22H22BNO2 343.1744, found 343.1756.
2-Isopropyl-3-[(E)-1-decen-1-yl]-2,1-borazaronaphthalene (5a)
The title compound was obtained as a colorless oil in 81% yield (247.8 mg). 1H NMR (500 MHz, CDCl3) δ 7.92 (s, 1H), 7.67 (s, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.39–7.35 (m, 1H), 7.24 (d, J = 8.0 Hz, 1H), 7.18–7.15 (m, 1H), 6.69 (dd, J = 15.6, 0.4 Hz, 1H), 6.13–6.18 (m, 1H), 2.31–2.26 (m, 2H), 1.96–1.92 (m, 1H), 1.57–1.54 (m, 2H), 1.43–1.35 (m, 10H), 1.23 (d, J = 7.3 Hz, 6H), 0.97 (t, J = 6.9 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.6, 138.4, 132.2, 131.1, 129.3, 127.6, 125.7, 121.2, 117.7, 33.8, 32.2, 30.0, 29.9, 29.7, 29.6, 23.0, 19.6, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 38.8; IR (neat) 3424, 2924, 1560, 1427, 969 cm–1; HRMS (CI) m/z [M]+ calcd for C21H32BN 309.2628, found 309.2633.
2-Cyclopropyl-3-[(E)-1-decen-1-yl]-2,1-borazaronaphthalene (5b)
The title compound was obtained as a yellow solid in 90% yield (276.3 mg): mp 51–55 °C; 1H NMR (500 MHz, CDCl3) δ 7.92 (s, 1H), 7.56 (d, J = 7.7 Hz, 1H), 7.32–7.29 (m, 1H), 7.18 (s, 1H), 7.11 (t, J = 7.9 Hz, 2H), 6.69 (d, J = 15.6 Hz, 1H), 6.38–6.24 (m, 1H), 2.25 (q, J = 7.2 Hz, 2H), 1.52–1.48 (m, 2H), 1.39–1.28 (m, 10H), 0.92–0.89 (m, 5H), 0.66–0.55 (m, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.5, 138.5, 132.8, 132.4, 129.3, 127.6, 125.5, 121.0, 117.4, 34.0, 32.2, 30.0, 29.9, 29.7, 29.6, 23.0, 14.4, 6.0; 11B NMR (128.38 MHz, CDCl3) δ 37.1; IR (neat) 3374, 3010, 2920, 1557, 1428, 1105 cm–1; HRMS (CI) m/z [M]+ calcd for C21H30BN 307.2471, found 307.2484.
3-[(E)-1-Decen-1-yl]-2-(4-fluorophenyl)-2,1-borazaronaphthalene (5c)
The title compound was obtained as a yellow oil in 88% yield (317.6 mg). 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.85 (s, 1H), 7.71–7.65 (m, 3H), 7.41–7.37 (m, 1H), 7.25 (d, J = 8.5 Hz, 1H), 7.22–7.19 (m, 1H), 7.17–7.14 (m, 2H), 6.62 (dd, J = 15.6, 0.7 Hz, 1H), 6.01 (dt, J = 15.5, 7.0 Hz, 1H), 2.17 (qd, J = 7.3, 1.3 Hz, 2H), 1.48–1.49 (m, 2H), 1.36–1.29 (m, 10H), 0.92 (t, J = 7.0 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 163.6 (d, J = 252 Hz), 139.8, 139.3, 134.9 (d, J = 7.4 Hz), 132.4, 132.3, 129.3, 127.9, 125.7, 121.6, 117.8, 115.1 (d, J = 19.6 Hz), 33.5, 32.0, 29.7, 29.6, 29.5, 29.4, 22.8, 14.2; 11B NMR (128.38 MHz, CDCl3) δ 33.9; IR (neat) 3375, 2923, 1595, 1224, 832 cm–1; HRMS (CI) m/z [M]+ calcd for C24H29BNF 361.2377, found 361.2368.
3-[(E)-1-Decen-1-yl]-2-(3-methoxyphenyl)-2,1-borazaronaphthalene (5d)
The title compound was obtained as a colorless oil in 81% yield (302.1 mg). 1H NMR (500 MHz, CDCl3) δ 8.04 (s, 1H), 7.94 (s, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.43–7.40 (m, 2H), 7.32 (d, J = 7.2 Hz, 1H), 7.29–7.27 (m, 2H), 7.22 (t, J = 7.5 Hz, 1H), 7.01 (dd, J = 8.2, 2.7 Hz, 1H), 6.68 (d, J = 15.6 Hz, 1H), 6.10–6.04 (m, 1H), 3.89 (s, 3H), 2.21–2.16 (m, 2H), 1.47–1.43 (m, 2H), 1.36–1.28 (m, 10H), 0.93 (t, J = 6.9 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 159.4, 139.8, 139.5, 132.5, 132.3, 129.5, 129.4, 128.0, 125.9, 125.5, 121.7, 118.3, 118.0, 114.4, 55.4, 33.7, 32.2, 29.9, 29.8, 29.6, 29.6, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 34.8; IR (neat) 3366, 2924, 1558, 1451, 1236, 971, 761 cm–1; HRMS (CI) m/z [M + H]+ calcd for C25H33BNO 374.2655, found 374.2661.
3-[(E)-1-Decen-1-yl]-2-(4-trifluorophenyl)-2,1-borazaronaphthalene (5e)
The title compound was obtained as a light brown oil in 83% yield (341.3 mg). 1H NMR (500 MHz, CDCl3) δ 8.03 (s, 1H), 7.91 (s, 1H), 7.80 (d, J = 7.7 Hz, 2H), 7.70–7.68 (m, 3H), 7.42–7.39 (m, 1H), 7.27 (d, J = 8.1 Hz, 1H), 7.22–7.21 (m, 1H), 6.56 (dd, J = 15.6, 1.0 Hz, 1H), 5.60–5.94 (m, 1H), 2.14 (m, 2H), 1.42–1.39 (m, 2H), 1.27–1.32 (m, 10H), 0.90 (t, J = 5.1 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 140.1, 139.1, 133.1, 132.8, 131.9, 130.4 (d, J = 32.3 Hz), 129.4, 128.1, 126.3 (q, J = 272.2 Hz), 125.8, 124.6 (q, J = 3.7 Hz), 121.9, 117.9, 33.5, 32.0, 29.6, 29.5, 29.3, 22.8, 14.2; 11B NMR (128.38 MHz, CDCl3) δ 34.5; IR (neat) 3395, 2924, 1559, 1323, 969, 836 cm–1; HRMS (CI) m/z [M]+ calcd for C25H29BNF3 411.2345, found 411.2353.
1-Allyl-3-[(E)-1-decen-1-yl]-2-methyl-2,1-borazaronaphthalene (5f)
The title compound was obtained as a light yellow oil in 62% yield (199.0 mg). 1H NMR (500 MHz, CDCl3) δ 7.84 (s, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.39 (dd, J = 4.7, 1.2 Hz, 2H), 7.17–7.13 (m, 1H), 6.66 (dd, J = 15.5, 1.0 Hz, 1H), 6.10–6.01 (m, 2H), 5.15 (dd, J = 10.5, 1.4 Hz, 1H), 4.99 (dd, J = 17.2, 1.3 Hz, 1H), 4.72 (dd, J = 4.1, 2.0 Hz, 2H), 2.27–2.22 (m, 2H), 1.54–1.49 (m, 2H), 1.41–1.27 (m, 10H), 0.93–0.89 (m, 6H); 13C NMR (125.8 MHz, CDCl3) δ 141.2, 137.7, 134.7, 132.7, 131.3, 130.2, 127.8, 126.7, 120.8, 115.9, 115.5, 49.8, 33.8, 32.2, 30.1, 29.9, 29.7, 29.6, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 38.7; IR (neat) 2923, 1556, 1405, 1212, 968 cm–1; HRMS (CI) m/z [M]+ calcd for C22H32BN 321.2628, found 321.2633.
1-Benzyl-3-[(E)-1-decen-1-yl]-2-methyl-2,1-borazaronaphthalene (5g)
The title compound was obtained as a light brown oil in 90% yield (333.9 mg). 1H NMR (500 MHz, CDCl3) δ 7.97 (s, 1H), 7.68 (d, J = 7.0 Hz, 1H), 7.35–7.27 (m, 5H), 7.19–7.16 (m, 3H), 6.78 (dd, J = 15.6, 0.6 Hz, 1H), 6.19 (dt, J = 15.5, 6.9 Hz, 1H), 5.38 (s, 2H), 2.36–2.37 (m, 2H), 1.63–1.57 (m, 2H), 1.50–1.39 (m, 10H), 1.00–0.98 (m, 6H); 13C NMR (125.8 MHz, CDCl3) δ 141.3, 138.8, 138.02, 132.7, 131.4, 130.2, 129.0, 127.9, 127.1, 126.9, 126.1, 120.9, 115.9, 51.4, 33.8, 32.2, 30.1, 29.9, 29.8, 29.7, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 39.0; IR (neat) 2924, 2853, 1610, 1492, 1402, 1360, 967 cm–1; HRMS (CI) m/z [M]+ calcd for C26H34BN 371.2784, found 371.2791.
3-[(E)-1-Decen-1-yl]-2-(4-methylphenyl)-2,1-borazaronaphthalene (5h)
The title compound was obtained as a light yellow oil in 80% yield (285.6 mg). 1H NMR (500 MHz, CDCl3) δ 8.04 (s, 1H), 7.91 (s, 1H), 7.68 (d, J = 7.5 Hz, 1H), 7.57–7.52 (m, 2H), 7.42–7.37 (m, 2H), 7.29–7.25 (m, 2H), 7.21 (t, J = 7.5 Hz, 1H), 6.68 (d, J = 15.5 Hz, 1H), 6.07 (dt, J = 15.4, 7.0 Hz, 1H), 2.47 (s, 3H), 2.20 (q, J = 6.9 Hz, 2H), 1.49–1.46 (m, 2H), 1.39–1.33 (m, 10H), 0.94 (t, J = 6.9 Hz, 3H); 13C NMR (125.8 MHz, CDCl3) δ 139.7, 137.5, 133.9, 132.7, 132.2, 130.3, 129.5, 128.1, 128.0, 125.9, 121.6, 117.9, 33.7, 32.2, 30.0, 29.9, 29.7, 29.6, 23.0, 21.9, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 34.7; IR (neat) 3369, 2923, 2852, 1612, 1557, 1450, 969 cm–1; HRMS (CI) m/z [M]+ calcd for C25H32BN 357.2628, found 357.2632.
6-[(E)-1-Decen-1-yl]-2-methyl-3-phenyl-2,1-borazaronaphthalene (6)
The title compound was obtained as a brown oil in 83% yield (296.3 mg). 1H NMR (500 MHz, CDCl3) δ 7.85 (s, 1H), 7.77 (s, 1H), 7.57 (d, J = 1.5 Hz, 1H), 7.47–7.41 (m, 5H), 7.33–7.31 (m, 1H), 7.16 (d, J = 8.5 Hz, 1H), 6.46 (d, J = 16.0 Hz, 1H), 6.23 (dt, J = 15.7, 6.9 Hz, 1H), 2.27–2.22 (m, 2H), 1.52–1.48 (m, 2H), 1.39–1.28 (m, 10H), 0.92 (t, J = 7.0 Hz, 3H), 0.83 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 145.1, 141.8, 139.0, 131.3, 130.0, 129.6, 128.5, 128.4, 127.2, 126.3, 126.0, 125.3, 117.7, 33.4, 32.2, 29.8, 29.8, 29.6, 29.6, 23.0, 14.4; 11B NMR (128.38 MHz, CDCl3) δ 36.4; IR (neat) 3372, 2924, 2853, 1617, 1577, 1456, 962, 762 cm–1; HRMS (CI) m/z [M]+ calcd for C25H32BN 357.2628, found 357.2621.
2-Methyl-3,6-di[(E)-1-propen-1-yl]-2,1-borazaronaphthalene (7)
The title compound was obtained as a yellow solid in 76% yield (169.4 mg): mp 106–110 °C; 1H NMR (500 MHz, CDCl3) δ 7.75 (s, 1H), 7.60 (s, 1H), 7.48 (d, J = 1.5 Hz, 1H), 7.38 (dd, J = 8.3, 1.9 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.62 (dd, J = 15.7, 0.7 Hz, 1H), 6.48 (dd, J = 15.7, 1.5 Hz, 1H), 6.26–6.14 (m, 2H), 1.95–1.93 (m, 6H), 0.86 (s, 3H); 13C NMR (125.8 MHz, CDCl3) δ 137.4, 137.6, 133.9, 130.1, 130.0, 125.7, 125.4, 124.5, 124.3, 123.1, 116.6, 18.3, 17.8; 11B NMR (128.38 MHz, CDCl3) δ 37.2; IR (neat) 3371, 2957, 2910, 1610, 1557, 1436 cm–1; HRMS (CI) m/z [M]+ calcd for C15H18BN 223.1532, found 223.1532.
Acknowledgments
This research was supported by the NIGMS (R01 GM-081376) and Eli Lilly. Frontier Scientific is acknowledged for their generous donation of potassium organotrifluoroborates. Frontier Scientific and Johnson Matthey are acknowledged for their donation of palladium salts. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining the HRMS data.
Supporting Information Available
Copies of 1H, 13C, and 11B NMR spectra for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ehle A. R.; Zhou Q.; Watson M. P. Org. Lett. 2012, 14, 1202. [DOI] [PubMed] [Google Scholar]
- Martinez R.; Voica F.; Genet J.-P.; Darses S. Org. Lett. 2007, 9, 3213. [DOI] [PubMed] [Google Scholar]
- Grirrane A.; Garcia H.; Corma A. J. Catal. 2013, 302, 49. [Google Scholar]
- Kuroda J.; Inamoto K.; Hiroya K.; Doi T. Eur. J. Org. Chem. 2009, 2251. [Google Scholar]
- a Green I. R.; Hugo V. I.; Oosthuizen G.; Giles R. G. F. Synth. Commun. 1996, 26, 867. [Google Scholar]; b Giles R. G. F.; Green I. R.; Hugo V. I.; Mitchell P. R. K.; Yorke S. C. J. Chem. Soc., Perkin Trans. 1 1984, 2383. [Google Scholar]
- a Sun Y.-P.; Sears D. F. Jr.; Saltiel J.; Mallory F. B.; Mallory C. W.; Buser C. A. J. Am. Chem. Soc. 1988, 110, 6974. [Google Scholar]; b Saltiel J.; Choi J.-O.; Sears D. F. Jr.; Eaker D. W.; Mallory F. B.; Mallory C. W. J. Phys. Chem. 1994, 98, 13162. [Google Scholar]
- Ito T.; Ikemoto T.; Yamano T.; Mizuno Y.; Tomimatsu K. Tetrahedron: Asymmetry 2013, 14, 3525. [Google Scholar]
- Kong W.; Fu C.; Ma S. Eur. J. Org. Chem. 2010, 6545. [Google Scholar]
- For recent reviews of azaborines, see:; a Liu Z.; Marder T. B. Angew. Chem., Int. Ed. 2008, 47, 242. [DOI] [PubMed] [Google Scholar]; b Campbell P. G.; Marwitz A. J. V.; Liu S.-Y. Angew. Chem., Int. Ed. 2012, 51, 6074. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bosdet M. J. D.; Piers W. E. Can. J. Chem. 2009, 87, 8. [Google Scholar]
- Molander G. A.; Wisniewski S. R. J. Org. Chem. 2014, 79, 6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews of the Suzuki–Miyaura reaction, see:; a Miyaura N.; Suzuki A. Chem. Rev. 1995, 95, 2457. [Google Scholar]; b Bellina F.; Carpita A.; Rossi R. Synthesis 2004, 2419. [Google Scholar]; c Kotha S.; Lahiri K.; Kashinath D. Tetrahedron 2002, 58, 9633. [Google Scholar]; d Sandrock D. L.Alkylboron Reagents. In Science of Synthesis, Cross Coupling and Heck-Type Reactions 1; Molander G. A., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2013; Vol. 1. [Google Scholar]; e Miyaura N.Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Halides. In Metal-Catalyzed Cross-Coupling Reactions; de Meijere A., Diederich F., Eds.; Wiley-VCH: Weinheim, Germany, 2003; Vol. 1. [Google Scholar]; f Boronic Acids; Hall D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]; g Molander G. A.; Canturk B. C. Angew. Chem., Int. Ed. 2009, 48, 9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews of potassium organotrifluoroborates, see:; a Molander G. A.; Figueroa R. Aldrichimica Acta 2005, 38, 49. [Google Scholar]; b Molander G. A.; Ellis N. Acc. Chem. Res. 2007, 40, 275. [DOI] [PubMed] [Google Scholar]; c Stefani H. A.; Cella R.; Vieira A. S. Tetrahedron 2007, 63, 3623. [Google Scholar]; d Darses S.; Genet J. P. Chem. Rev. 2008, 108, 288. [DOI] [PubMed] [Google Scholar]; e Molander G. A.; Jean-Gerard L.. Organotrifluoroborates: Organoboron Reagents for the Twenty-First Century. In Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Hall D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2011; Vol. 2, pp 507–550. [Google Scholar]; f Molander G. A.; Jean-Gerard L.. Cross-Coupling Reactions of Organotrifluoroborate Salts. In Organic Reactions; Denmark S. E., Ed.; John Wiley & Sons, Inc.: New York, 2013; Vol. 79, pp 1–316. [Google Scholar]
- Wisniewski S. R.; Guenther C. G.; Argintaru O. A.; Molander G. A. J. Org. Chem. 2014, 79, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


