Abstract
Delayed leukoencephalopathy is an uncommon complication of hypoxic-ischemic events of different etiologies, including carbon monoxide intoxication. We present a case of a 40-year-old male patient who was admitted with rapidly progressive neurocognitive and behavioral deficits. There was a history of accidental carbon monoxide intoxication one month before, presenting with loss of consciousness and short hospitalization, followed by a complete clinical recovery. The imaging studies in the delayed phase depicted confluent, symmetric supra-tentorial white matter lesions in keeping with diffuse demyelinization. Restricted diffusion and metabolite abnormalities in magnetic resonance proton spectroscopy were also seen. The diagnosis of CO-mediated delayed post-hypoxic leukoencephalopathy was assumed after exclusion of other mimickers. Hyperbaric oxygen therapy was tentatively performed and the patient had a favorable clinical and radiological evolution.
Keywords: Carbon monoxide poisoning, delayed post-ischemic encephalopathy, neurotoxicity syndromes, heroin inhalation syndrome, diffusion magnetic resonance imaging, magnetic resonance spectroscopy
CASE REPORT
A 40-year-old Nepali male living in Portugal for 5 months was admitted with a 2-weeks history of progressive neurobehavioral disturbance with disorientation, incoherent speech and behaviour disturbances. One month earlier he had been hospitalized elsewhere with coma due to acute carbon monoxide (CO) poisoning (carboxyhemoglobin 24% measured in the arterial blood; normal level for non-smokers is <2% and for smokers 5–13%). After a rapid clinical improvement, he was discharged 8 days later with no apparent deficits and returned to normal life. At admission in our hospital, he was alert, mute and showed distractibility, poor understanding, reduced attention and bizarre behaviour. Grasping, choreiform movements in the lower limbs and myoclonic movements of the trunk, without gait disturbance were present. The hematologic, biochemical, serological and vasculitis studies, as well as toxicology screening and carboxyhemoglobin levels were unremarkable. Cerebrospinal fluid analysis was also normal. Electroencephalogram showed diffuse slow brain activity.
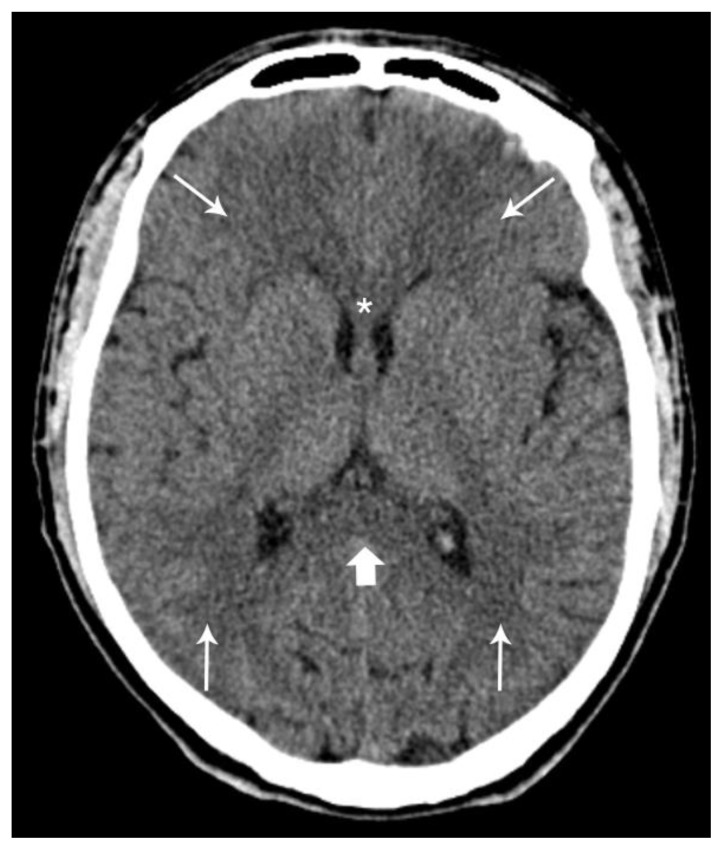
Brain Computed Tomography (CT) depicted diffuse mainly peri-ventricular white matter hypodensity (Figure 1). Brain Magnetic Resonance Imaging (MRI) performed on day 39 post-intoxication showed confluent bilateral relatively symmetric white matter hyperintensity on Proton Density, T2 and Fluid Attenuated Inversion Recovery (FLAIR) sequences with associated restricted diffusion. Relative preservation of gray matter was seen (Figure 2). On single voxel short time to echo (TE) Proton Magnetic Resonance Spectroscopy (1H-MRS) there was a decrease of N-acetyl-aspartate (NAA) and an elevation of Choline (Cho) as well as a lactate peak (Figure 3). Based on the clinical history and laboratory/imaging findings, the diagnosis of delayed post-hypoxic leukoencephalopathy was assumed.
Figure 1.
Forty-year-old male with diffuse post-hypoxic demyelination (1 month after the initial event and before treatment). FINDINGS: Axial non-contrast CT scan shows diffuse hypodensity in the periventricular white matter (small arrows), the splenium (large arrow) and the genu (asterisks) of the corpus callosum. TECHNIQUE: Phillips Brilliance 64; FOV 247; 2 mm slice thickness; 120 Kv; 238 mA; 399 mAs.
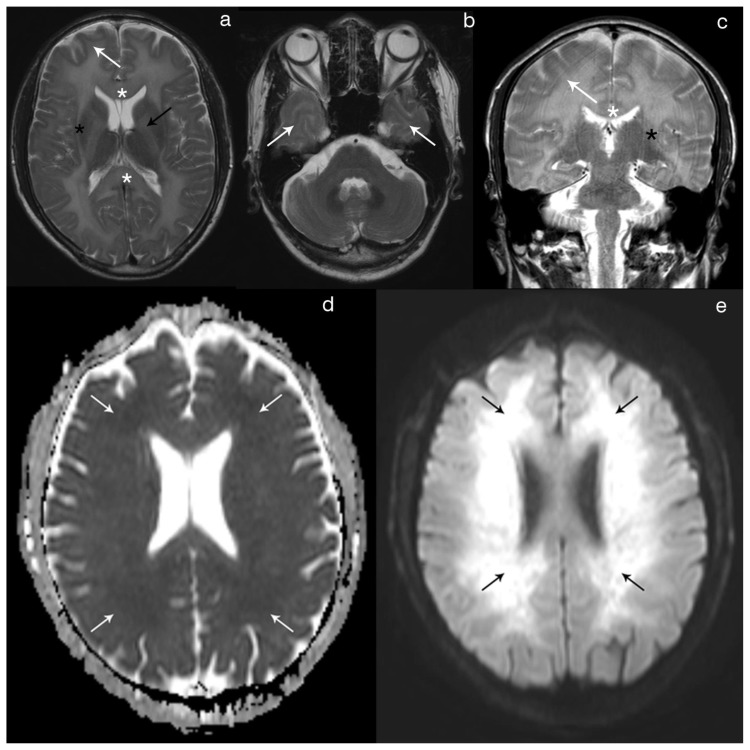
Figure 2.
Forty-year-old male with diffuse post-hypoxic demyelination (1 month after the initial event and before treatment).
FINDINGS: Axial T2 (2a) and coronal T2 (2c) show a diffuse, confluent hyperintensity involving predominantly the periventricular and deep white-matter, as well as the corpus callosum (white asterisks), the internal (black arrow) and the external (black asterisks) capsules. There is also some extension of the signal abnormality to the sub-cortical white-matter (white arrow). The cortex and the deep gray-matter are spared. Axial T2 (2b) at one inferior level depicts the involvement of the anterior temporal sub-cortical white-matter (arrows), with normal findings in the pons and cerebellum. Axial DWI (2e) shows diffuse hyperintensity in the peri-ventricular and deep white-matter with corresponding hypointensity on the axial ADC map (2d) reflecting restricted diffusion.
TECHNIQUE: Philips Intera 3T; Axial T2 (TR 2000/TE 80; Flip angle 90; FOV 230; slice thickness 5mm; NEX 1); Coronal T2 (TR 3000/TE 80; Flip angle 90; FOV 220; slice tickness 4mm; NEX 1); Axial DWI and ADC (TR 3869.16/TE 58.714; Flip angle 18; FOV 240; b value 1000; NEX 2).
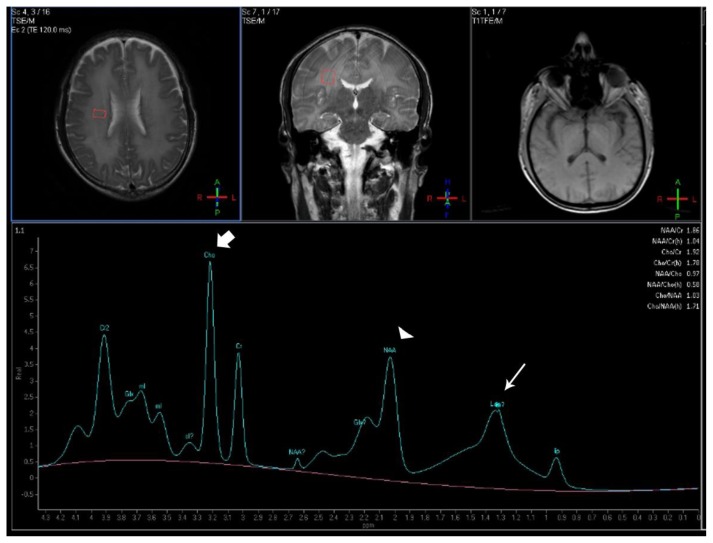
Figure 3.
Forty-year-old male with diffuse post-hypoxic demyelination (one month after the initial event and pre-treatment). FINDINGS: The MRS spectrum obtained in the right deep white matter shows an increased Cho peak (large arrow), a decreased NAA peak (arrowhead) and a peak centered at 1.33ppm compatible with lactate (small arrow). TECHNIQUE: Philips Intera 3T, Single Voxel 1H-MRS, TE=32, TR=2000, NEX=128, voxel size= 2×1,5×2mm; PRESS technique).
Hyperbaric oxygen therapy was empirically started (90 minutes daily sessions, with 100 percent oxygen at 2.5 atmospheres absolute performing a total of 40 sessions) two months after carbon monoxide intoxication. There was an improvement in the imaging findings (Figure 4), as well as in rigidity, language production/comprehension, attention and gait. In the last days of treatment, he was oriented in time and space, had appropriate behaviour and was able to perform almost all activities of daily living. He was discharged firstly to a rehabilitation center and then to join the family in Nepal.
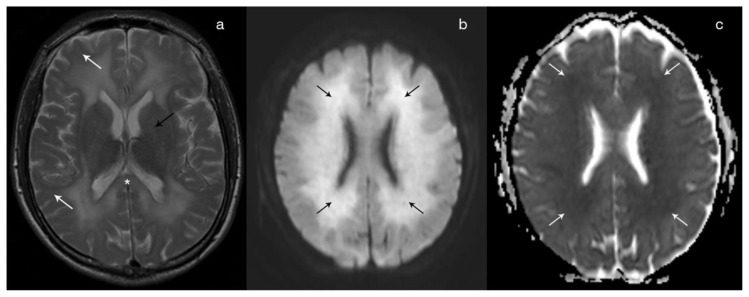
Figure 4.
Forty-year-old male with diffuse post-hypoxic demyelination. FINDINGS: Follow-up (67 days after the initial event and post-treatment) axial T2 (4a) shows a partial regression of the T2 hyperintensity in the sub-cortical white matter (white arrow), in the splenium of the corpus callosum (asterisks) and in the internal capsule (black arrow). Axial DWI (4b) shows persistence of high signal in the periventricular white matter (black arrows), although with a reduced intensity in comparison with the previous exam. On the ADC map (4c), the lesions remain hypointense in keeping with restricted diffusion. TECHNIQUE: Philips Intera 3T; Axial T2 (TR 2000/TE 80; Flip angle 90; FOV 230; slice thickness 5mm; NEX 1); Axial DWI and ADC (TR 3869.16/TE 58.714; Flip angle 18; FOV 240; b value 1000; NEX 2).
DISCUSSION
Etiology and demographics
Delayed post-hypoxic leukoencephalopathy (DPHL) represents one uncommon sub-type of acquired leukoencephalopathy and it was first described by Choi et al in a sub-group (2,75%) of a large case series of 2360 patients studied in the context of CO toxicity [1]. Although DPHL is still predominantly associated with CO intoxication (affecting about 10% of the survivors), it can occur after any period of prolonged moderate cerebral hypo-oxygenation [2,3].
Clinical and imaging findings
Affected patients typically present a biphasic pattern: full or near-full recovery after an initial event with subsequent return to usual activities followed by onset of progressive neuropsychiatric and neurological signs and symptoms, usually in a period ranging from 2 to 40 days [4]. Two distinct forms of neurological deterioration have been described, namely akynetic-mutism (generally associated with the involvement of the frontal white- matter tracts) or Parkinsonism (suggesting the affection of the basal ganglia circuits) [5, 6].
The head CT scan may show diffuse supra-tentorial white matter hypodensity although this abnormality is usually very subtle and may be difficult to detect [4]. MRI better depicts the confluent, relatively symmetric involvement of the periventricular white matter and centrum semiovale and less often the U-fibers, sparing the brain stem, the cerebellum as well as the gray matter structures. The lesions show high signal on T2/FLAIR sequences and typically do not exhibit mass effect nor contrast enhancement [4]. Diffusion-weighted imaging (DWI) plays an important role, as it has been shown that a high DWI signal with correspondent low ADC persisting for months is typically associated with this condition [7]. This feature allows the differentiation between DPHL and cytotoxic edema, which usually resolves in a period ranging from 1 to 4 weeks [7]. This prolonged restricted diffusion was also evident in our patient on the initial MRI study and on follow-up and is postulated to correspond to intramyelinic edema [2]. The MRS spectrum acquired in the affected regions shows a reduction of NAA (a neuron-specific marker), reflecting the loss of neuronal tissue and appearance of reactive astrocytosis reported before in pathological examinations performed in DPHL patients [9]. Conversely, the associated elevation of Cho reflects the well-known process of demyelinization associated with this disease, as this metabolite is typically related with membrane turnover and lipid metabolism. A lactate peak has also been described before in association with DPHL [9] and was present in our patient. It probably reflects a shift towards anaerobic metabolism either due to the increment of the energy demand related to remyelinization, or to the reduction of energy supply following the previous ischemic event [9].
There are no widely accepted formal diagnostic criteria for DPHL and the diagnosis depends on the typical radiologic features in the appropriate clinical setting. In case of presumed CO intoxication, blood carboxyhemoglobin level in the acute presentation supports the diagnosis although showing poor association with the clinical evolution or the possible DPHL development [10].
Differential Diagnosis
Some considerations should be given regarding the imaging features of other possible toxic leukoencephalopathies and may allow their differentiation from DPHL.
In the heroin inhalation syndrome (“chasing the dragon”), additionally to the diffuse periventricular cerebral white matter predilection, involvement of the cerebellum and the brainstem is typically seen, helping in the differential with DPHL [12]. Furthermore, restriction diffusion is absent and the Cho peak is generally normal or just slightly decreased [4].
Regarding the metronidazole-induced encephalopathy, key-features are bilateral symmetric hyperintensity on T2/FLAIR of the dentate nuclei, midbrain, dorsal pons, dorsal medulla and corpus callosum [12]. Differently from DPHL, white matter involvement occurs in the sub-cortical region and the callosal commissure lesions show restricted diffusion [12,13].
Toluene chronic abuse, on the other hand, is mainly characterized by a peculiar pattern of bilateral symmetric hypointensity on T2/FLAIR involving the thalamus, the basal ganglia and sometimes the cortex; white matter involvement starts in the peri-ventricular region, extends to the sub-cortical areas and is associated with diffuse atrophy [14]. Restricted diffusion occurs only in the acute phase; reduced NAA and increased myo-inositol was described [15], differently from DPHL.
Posterior reversible encephalopathy syndrome (PRES) is also a possible toxic complication described in association with multiple pharmacologic treatments (more frequently with cyclosporine therapy), involving mainly the sub-cortical cerebral white matter with a posterior predominance. Infratentorial structures and gray matter can also be affected [4]. Restricted diffusion and hemorrhage are seen in approximately 30% and 17% of cases, respectively [16]. The lactate peak is usually absent, while NAA and creatine peaks are decreased and Cho peak is raised [17].
Finally, both acute or delayed injury from radiation and chemotherapy (most commonly associated with methotrexate; isolated or in association) may also present with multiple patterns of peri-ventricular/deep white matter involvement ranging from single focal lesions to diffuse abnormalities which may also show restricted diffusion and abnormal spectrum with lactate peak. Nevertheless, the clinical history and the possible presence of enhancement, mass effect, hemorrhage and calcification help in the differentiation from DPHL [18].
The metabolic dysmyelinating disorders were discarded due to the age of the patient and the relatively rapid clinical presentation (early sub-acute).
Treatment and prognosis
In the setting of acute CO poisoning, the standard of care is high-flow oxygen, preferentially hyperbaric therapy [19]. However, there are no trials and therefore limited evidence of efficacy in the subacute phase of CO intoxication and/or DPHL. In our case, this therapy was administered despite limited evidence of efficacy. The prognosis of DPHL reported in the literature is relatively favourable (with the majority of patients showing both clinical and neuroradiologic recovery in a period ranging from 3 to 12 months), although some neurologic deficits may persist [2, 20, 21]. Our patient showed a progressive and sustained clinical improvement in pyramidal and extra-pyramidal symptoms (speech, attention, behavioural, rigity and gait), accompanied by a subtle reduction on the extent of the imaging abnormalities in the follow-up MRI study.
TEACHING POINT
Diffuse post-hypoxic leukoencephalopathy (DPHL) represents an uncommon syndrome that typically presents with a neurologic biphasic pattern after any moderate hypoxic-ischemic event, although more commonly seen in association with CO intoxication. Imaging studies depict diffuse confluent bilateral peri-ventricular and deep white matter changes with associated prolonged restricted diffusion, decreased NAA/Cho and presence of a lactate peak in 1H-MRS that gradually improve over time; these clinical and radiologic findings, together with documented abnormal carboxyhemoglobin levels in the acute phase, allow a definitive diagnosis.
Table 1.
Differential diagnosis for toxic encephalopathies.
| DHIL | Heroin Inhalation Encephalopathy | Chronic Toluene Abuse | Metronidazole-induced Leukoencephalopathy | Posterior Reversible Encephalopathy Syndrome (PRES) | |
|---|---|---|---|---|---|
| Symptoms/signs | parkinsonism or akinetic-mutism after a complete clinical recovery | 3 progressive stages
|
cerebellar dysfunction, optic atrophy, pyramidal tract signs, cranial nerve abnormalities, personality changes | peripheral neuropathy, ataxic gait, dysarthric speech, seizures, encephalopathy | headache, visual loss, seizures |
| Presentation | early sub-acute (weeks) | early sub-acute (weeks/months) | late sub-acute/chronic (months/years) | early sub-acute (weeks) | acute (hours/days) |
| History | prolonged moderate hypoxic-ischemic event | drug abuse | drug abuse | previous infection and long course antibiotherapy (2g/day for long periods) | hypertension, eclampsia, chemotherapy, immunossupressive therapy, auto-immune disease, renal disease |
| Supra-tentorial gray matter | not involved | cortex is spared; basal ganglia and thalamus may be affected | T2 hypointensity is seen in the thalamus, basal ganglia and cortex | basal ganglia can be rarely involved | involvement of the splenium of the corpus callosum; less commonly sub-cortical white-matter may be affected |
| Supra-tentorial white matter | diffuse periventricular/deep symmetric white matter involvement; corpus callosum and internal capsule may also have abnormal T2/FLAIR hyperintensity | diffuse periventricular/deep symmetric white matter involvement; anterior limb of the internal capsule is spared | Periventricular white matter T2/FLAIR hyperintensity with progression to the sub-cortical area; posterior limb of the internal capsule; diffuse atrophy | cortex is generally involved; basal ganglia and thalamus are less affected | sub-cortical asymmetric white matter involvement with a posterior predominance; hemorrhage may be present |
| Posterior Fossa | not involved | cerebellum and brainstem are typically involved | T2 hypointensity of the red nuclei, substantia nigra and dentate nucleus; the cerebellar white matter, dorsal pons and cerebellar peduncles may be hyperintense; atrophy | not involved | less frequently affected |
| DWI | prolonged restricted diffusion | no restricted diffusion | restricted diffusion only in the initial phase | restricted diffusion is observed in the corpus callosum | usually facilitated diffusion (vasogenic edema); occasionally some lesions show restricted diffusion (cytotoxic edema) |
| H1-MRS | NAA reduced Cho normal or slightly reduced Lactate peak present | NAA reduced Cho normal or slightly reduced Lactate peak present |
NAA reduced in the cerebellum/centrum semiovale; normal in the thalamus Myo-Inositol increased Lactate peak absent |
insufficient data in the literature | NAA mildly decreased Cho reduced Generally, no lactate peak Cr reduced |
| Prognosis | generally favorable, with slowly progressive imaging and clinical recovery | poor (mortality ~50%) | variable | good (lesions are generally reversible in 6–8 weeks | generally favorable (lesions are usually reversible, but not always; death is rarely reported) |
Table 2.
Summary table for delayed hypoxic-ischemic demyelination.
| Etiology | Prolonged moderate hypoxic-ischemic event (++ CO intoxication) |
| Gender/Age | No predilection |
| Incidence | ~10% of CO intoxications |
| Time of Presentation | 2–40 days after the initial event |
| Clinical Presentation | Parkinsonism or Akinetic-Mutism after a complete clinical recovery |
| Diagnosis | Clinical history + suggestive imaging findings; exclusion of other causes |
| CT | Subtle bilateral hypodensity in the peri-ventricular and deep cerebral white matter |
| Conventional MR sequences | Confluent, bilateral and symmetrical peri-ventricular and deep white matter (++ centrum semiovale) hyperintensity on T2/FLAIR; absence of involvement of gray matter or infratentorial structures; also absence of mass effect or enhancement |
| DWI | Prolonged restricted diffusion (lasting longer than usually seen in ischemic cytotoxic edema) |
| MRS | Decreased NAA and increased Cho peak; presence of a lactate peak |
| Prognosis | generally good, with both clinical and radiologic improvement |
| Treatment | ++ symptomatic/supportive; in the context of CO intoxication, hyperbaric oxygen therapy is controversial |
ABBREVIATIONS
- ADC
Apparent coefficient map
- Cho
Choline
- CO
Carbon monoxide
- Cr
creatine
- CT
Computed Tomography
- DPHL
Diffuse post-hypoxic leukoencephalopathy
- DWI
Diffusion-wheighted imaging
- FLAIR
Fluid Attenuated Inversion Recovery
- 1H- MRS
Proton Magnetic Resonance Spectroscopy
- MRI
Magnetic Resonance Imaging
- NAA
N-acetyl-aspartate
- TE
time to echo
REFERENCES
- 1.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433–35. doi: 10.1001/archneur.1983.04050070063016. [DOI] [PubMed] [Google Scholar]
- 2.Shprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Tormoehlen LM. Toxic leukoencephalopathies. Neurol Clin. 2011;29(3):591–605. doi: 10.1016/j.ncl.2011.05.005. Review. [DOI] [PubMed] [Google Scholar]
- 4.Beppu T. The Role of MR Imaging in Assessment of Brain Damage from Carbon Monoxide Poisoning: A Review of the Literature. AJNR Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3489. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MS, Marsden CD. Neurological sequelae following carbon monoxide poisoning clinical course and outcome according to the clinical types and brain computed tomography scan findings. Mov Disord. 1994;9(5):550–8. doi: 10.1002/mds.870090508. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao CL, Kuo HC, Huang CC. Delayed encephalopathy after carbon monoxide intoxication--long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurol Taiwan. 2004;13(2):64–70. [PubMed] [Google Scholar]
- 7.Kim JH, Chang KH, Song IC, Kim KH, Kwon BJ, Kim HC, Kim JH, Han MH. Delayed encephalopathy of acute carbon monoxide intoxication: diffusivity of cerebral white matter lesions. AJNR Am J Neuroradiol. 2003;24(8):1592–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012;32(5):1285–97. doi: 10.1148/rg.325115760. discussion 1297–9. [DOI] [PubMed] [Google Scholar]
- 9.Gottfried JA, Mayer SA, Shungu DC, Chang Y, Duyn JH. Delayed posthypoxic demyelination. Association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology. 1997;49(5):1400–4. doi: 10.1212/wnl.49.5.1400. [DOI] [PubMed] [Google Scholar]
- 10.Yardan T, Cevik Y, Donderici O, Kavalci C, Yilmaz FM, Yilmaz G, et al. Elevated serum S100B protein and neuron-specific enolase levels in carbon monoxide poisoning. Am J Emerg Med. 2009;27(7):838–42. doi: 10.1016/j.ajem.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Keogh CF, Andrews GT, Spacey SD, Forkheim KE, Graeb DA. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon”. AJR Am J Roentgenol. 2003;180(3):847–50. doi: 10.2214/ajr.180.3.1800847. [DOI] [PubMed] [Google Scholar]
- 12.Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652–8. doi: 10.3174/ajnr.A0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cecil KM, Halsted MJ, Schapiro M, Dinopoulos A, Jones BV. Reversible MR imaging and MR spectroscopy abnormalities in association with metronidazole therapy. J Comput Assist Tomogr. 2002 Nov-Dec;26(6):948–51. doi: 10.1097/00004728-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O. Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am J Neuroradiol. 2002;23(7):1173–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Aydin K, Sencer S, Ogel K, Genchellac H, Demir T, Minareci O. Single-voxel proton MR spectroscopy in toluene abuse. Magn Reson Imaging. 2003;21(7):777–85. doi: 10.1016/s0730-725x(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 16.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189(4):904–12. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Kim SH, Lee SH, Baek HJ, Shon HS, Kim SS. Serial MR spectroscopy in relapsing reversible posterior leukoencephalopathy syndrome. Neurologist. 2009;15(6):338–41. doi: 10.1097/NRL.0b013e3181914af6. [DOI] [PubMed] [Google Scholar]
- 18.Barkovich AJ, Raybaud C. Pediatric Neuroimaging. 5th edition. Lippincot Williams&Williams; 2012. pp. 114–119. [Google Scholar]
- 19.Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347(14):1057–67. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Pan X, Wan Y, Zhang Q, Liang W. Factors affecting the prognosis of patients with delayed encephalopathy after acute carbon monoxide poisoning. Am J Emerg Med. 2011;29(3):261–4. doi: 10.1016/j.ajem.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27(8):1763–5. [PMC free article] [PubMed] [Google Scholar]