Abstract
Nodular fasciitis is a benign proliferation of myofibroblasts which presents clinically as a rapidly growing mass with nonspecific features on imaging and high cellular activity on histopathology. Nodular fasciitis can be mistaken for malignant fibrous lesions such as soft tissue sarcoma or breast carcinoma when located within breast tissue. This presents a problem for appropriate treatment planning as the natural history of nodular fasciitis is spontaneous regression. We present the mammographic, sonographic, computed tomography, and histopathologic characteristics of nodular fasciitis in a 68 year female initially presenting with a rapidly enlarging right axillary mass.
Keywords: Nodular fasciitis, Mammography, Axilla, Computed Tomography, Benign mesenchymal tumors of the breast
CASE REPORT
A 68 year old female was referred for diagnostic mammogram for a rapidly growing, painless, palpable lump in the axillary tail of the right breast. Her past medical history included rheumatoid arthritis treated with long standing methotrexate and low dose prednisone therapy. She had a remote history of aggressive Burkitt’s lymphoma treated with chemotherapy and intrathecal methotrexate in 1992. She had undergone a left knee replacement approximately nine months prior, with postoperative course complicated by Clostridium difficile-induced diarrhea and a forty pound weight loss. She had no personal history of breast cancer.
On physical examination, there was a soft, nontender, soft tissue mass in the axillary tail of the right breast. There were no skin changes. There were no associated parasthesias in the right arm. Distal pulses were normal.
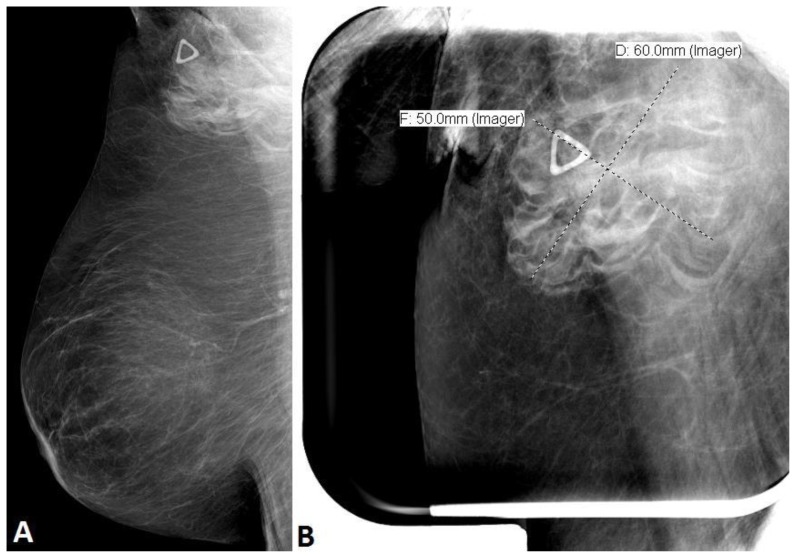
The diagnostic mammogram demonstrated a 6 × 5 cm round equal density mass with ill-defined margins in the right axillary tail seen only on the right mediolateral oblique (MLO) view. There were no associated calcifications or architectural distortion (Figure 1A,B). The patient’s most recent previous screening mammogram one year prior was normal. Given the suspicious findings of a new mass, a BIRADS Category 4 was given and recommendation for a targeted breast ultrasound was made.
Figure 1.
68 year old female with nodular fasciitis in the axillary tail of the right breast. FINDINGS: A. Right mediolateral oblique (MLO) digital mammogram, compression force of 9 daN, 29 kVp, 50 mAs, demonstrated a round equal density mass with ill-defined margins in the right axillary tail. Radiopaque triangular marker indicates this abnormality was palpable to the patient. B. Spot compression MLO digital mammogram, with compression force of 7 daN, 29 kV, 58 mAs, characterizes the 6 × 5 cm mass in the axillary tail of the right breast.
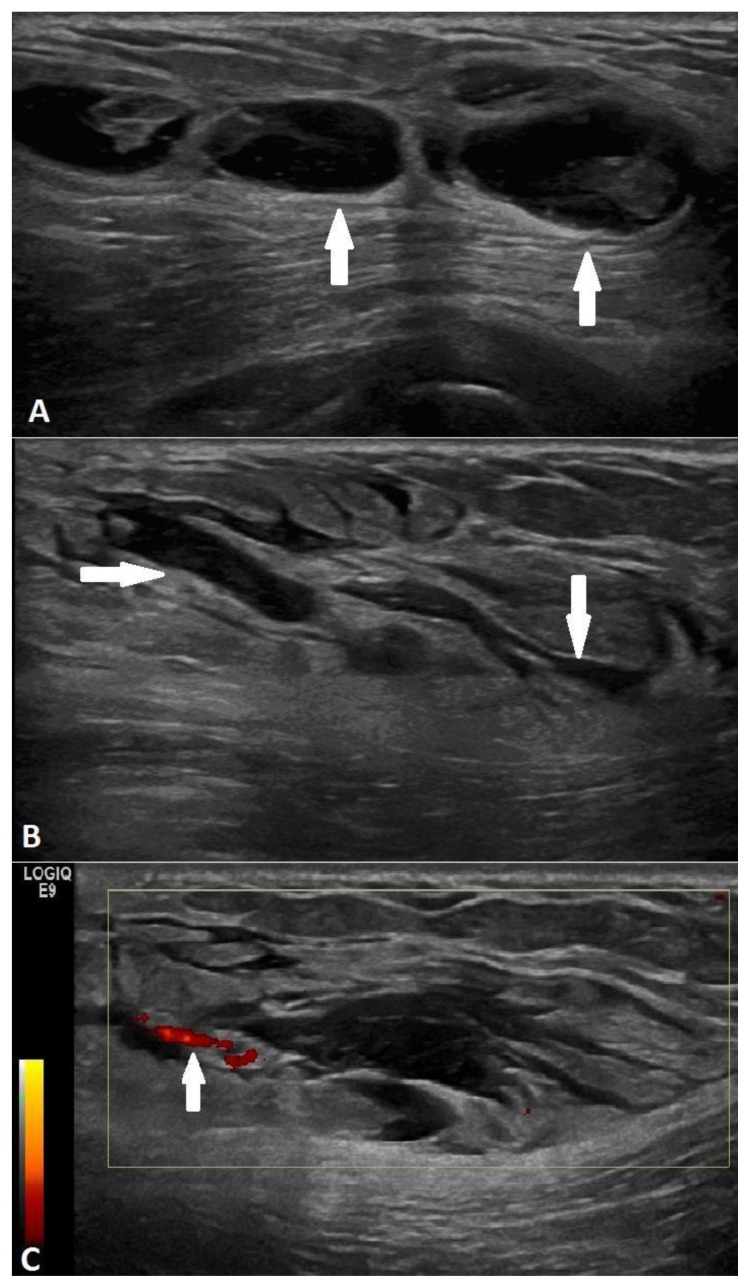
Subsequent targeted ultrasound over the palpable area revealed a multiloculated complex cystic mass in the subcutaneous soft tissue extending deep to and abutting the pectoralis muscle measuring approximately 5.6 × 5.2 cm with extensive surrounding tissue edema (Figure 2A–C). There did not appear to be muscle invasion. Minimal vascularity was seen on Power Doppler (Figure 2C). The final recommendation was a BIRADS Category 4.
Figure 2.
68 year old female with nodular fasciitis in the axillary tail of the right breast. FINDINGS: A. Targeted ultrasound over the area of palpation in the axillary tail revealed a multiloculated complex cystic mass (arrows) with heterogeneous internal echotexture measuring approximately 5.6 × 5.2 cm in size. B. Extensive soft tissue edema surrounded the mass (arrows). C. Minimal vascularity was present within the lesion (arrow) on Power Doppler. TECHNIQUE: Linear 6–15 MHz transducer (frequency 15 MHz) performed on a GE Logiq E9 Ultrasound machine.
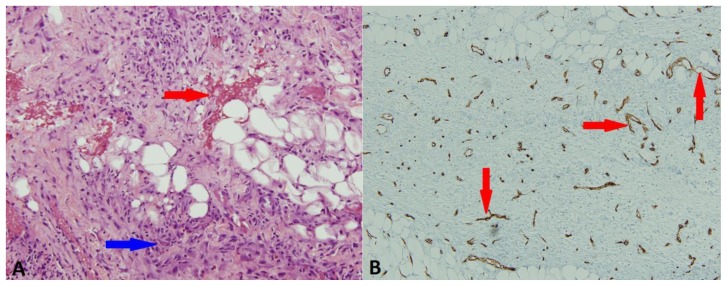
Ultrasound guided aspiration of the cystic component yielded serosanginous fluid. Culture of the fluid revealed no growth after five days. Flow cytometry showed no immunophenotypic evidence of lymphoma or breast carcinoma. Histology from subsequent core needle biopsies obtained with a 14g Achieve biopsy needle revealed spindle cell proliferation composed of fascicles of spindled and stellate cells infiltrating adipose tissue. The spindle cell proliferation was set within a background of loose collagenous/myxoid stroma, increased vascularity highlighted by CD34 immunohistochemical stain, mixed inflammatory cells including lymphocytes, histiocytes, eosinophils, and neutrophils and extravasated red blood cells. Histopathologic features were diagnostic of nodular fasciitis (Figure 3A,B). No evidence of Burkitt lymphoma or other neoplasms was identified.
Figure 3.
68 year old female with nodular fasciitis in the axillary tail of the right breast. A. H&E stain, 20×, demonstrated spindle cell proliferation with extravasated red blood cells (red arrow) and mixed inflammatory background (blue arrow). B. CD34 immunohistochemical stain, 10×, highlighting the increased vascularity (red arrows).
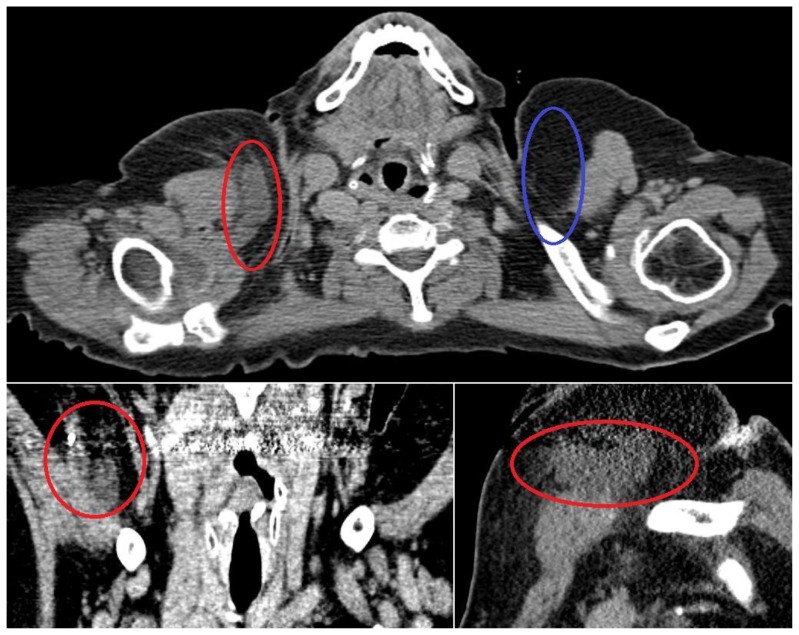
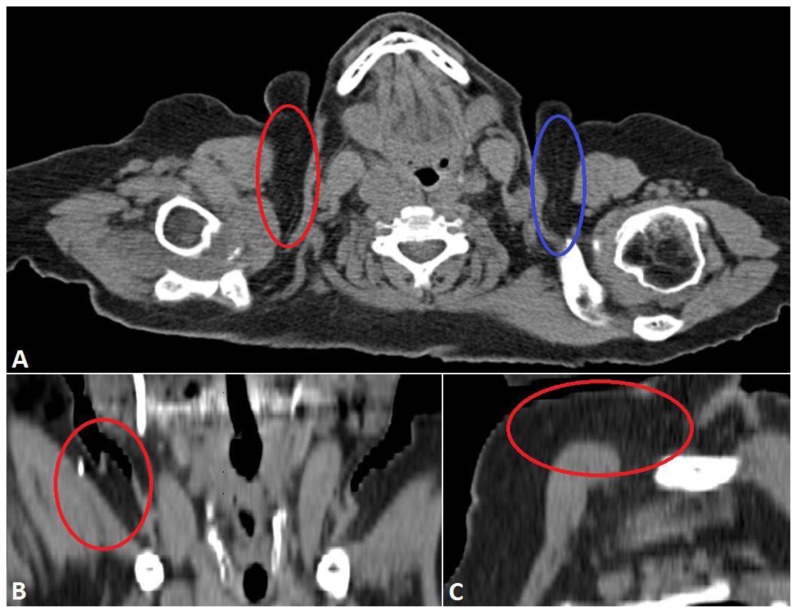
Given its approximation to the chest wall and the clinical history of rapid growth, the patient was referred to cardiothoracic surgery. Subsequent computed tomography (CT) exam of the neck without contrast was performed approximately two weeks after initial presentation at the request of the surgeon. There was a significant decrease in size of the lesion both on clinical exam and on imaging when compared to initial ultrasound (Fig 4). As the lesion had regressed in size in the two weeks since she presented to the breast clinic and there were no concerning imaging findings, conservative clinical and imaging follow up was recommended. A repeat CT examination of the neck 3 months later (Fig 5) demonstrated resolution of the lesion. She is scheduled to return for routine screening mammogram.
Figure 4.
68 year old female with nodular fasciitis in the axillary tail of the right breast imaged two weeks after initial clinical presentation. FINDINGS: A. Axial noncontrast CT scan through the neck demonstrates a nodular soft tissue density in the axillary tail of the right breast (red circle). The contralateral side (blue circle) demonstrates normal subcutaneous fat for comparison. The largest lesion measured over 5 × 1 cm, significantly decreased in size since her biopsy. B. Coronal reformatted CT image demonstrates a soft tissue lesion (red circle) in the right axillary tail abutting the right pectoralis major. Note the biopsy clip in the superolateral aspect of the lesion and the beam hardening artifact related to tooth fillings. C. Sagittal reformatted CT image better shows the size of the lesion, partially obscured by beam hardening artifact related to tooth fillings. TECHNIQUE: Axial CT with soft tissue reconstruction algorithm, 234 mAs, 120 kV, 3 mm slice thickness, noncontrast. Coronal and sagittal reformats obtained from 0.75 mm axial source images at 2 mm slice thickness using TeraRecon software.
Figure 5.
68 year old female with nodular fasciitis in the axillary tail of the right breast imaged 3 ½ months after initial presentation. FINDINGS: A. Axial noncontrast CT scan through the chest demonstrates resolution of previously seen subcutaneous nodular soft tissue densities in the right axillary tail (red circle). Appearance is now similar to the contralateral subcutaneous fat (blue circle). B. Coronal reformatted CT image demonstrating resolution of previously seen soft tissue lesion. Note the small biopsy clip. C. Sagittal reformatted CT image demonstrates resolution of previously seen subcutaneous mass in the right axillary tail. TECHNIQUE: Axial CT with soft tissue reconstruction algorithm, 273 mAs, 120 kV, 3 mm slice thickness, noncontrast. Coronal and sagittal reformats obtained from 3 mm axial source images at 2 mm slice thickness using TeraRecon software.
DISCUSSION
Nodular fasciitis is a benign proliferation of myofibroblasts first described as pseudosarcomatous fibromatosis in 1955 [1]. Because of its rapid growth, radiological, and histopathological appearance it is often mistaken for more malignant entities such as soft tissue sarcoma [2]. Although rare, when present in the breast it can mimic primary breast malignancy. Its benign nature has been well described in the literature, and it has been recently categorized as one of the benign mesenchymal tumors of the breast by the World Health Organization [3].
Etiology & demographics
Nodular fasciitis presents clinically as a rapidly growing soft tissue mass that is often painful and tender regardless of location and presents in patients in the second to fourth decades of life [4]. Incidence is equal between women and men. Approximately 5–15 percent of patients report trauma preceding appearance of the lesion [5]. About half occur in the upper extremities, the remainder occurring in the lower extremities, neck, and thorax [6]. Nodular fasciitis in the breast is rare, however when it does occur it can mimic breast malignancy and thus is an important entity to be aware of. When it occurs in the breast its presentation is similar to other locations in the body with a rapidly growing, palpable, and often tender mass [7].
Clinical & imaging findings
On imaging, nodular fasciitis mimics more aggressive lesions typically prompting biopsy and excision with most cases diagnosed histologically [7]. Because the histological characteristics vary, familiarity with the clinical, radiological, and histological presentation of this entity is essential to arrive at the correct diagnosis.
Nodular fasciitis is a benign soft tissue lesion that can mimic breast cancer both clinically and radiologically [2, 8]. The mammographic appearance of nodular fasciitis varies and ranges from a round mass with indistinct margins and surrounding increase in trabecular density to an irregular, often spiculated mass [9, 10]. These observations are in agreement with the ill-defined equal density mass we observed in our case.
Similarly, nodular fasciitis has a variable and nonspecific sonographic appearance which has not been well established in the literature. Typically circumscribed, its internal echotexture ranges from homogeneous to mixed, sometimes with posterior acoustic enhancement [2, 11]. As in our case, it may not demonstrate any significant vascularity on Doppler ultrasound even in the setting of intense neovascularity demonstrated on angiography [12]. The extensive surrounding soft tissue edema observed in our case has not been previously described and initially was suggestive of an infectious etiology, however no microbacterial growth was observed after five days. The marked soft tissue edema is particularly noteworthy given that our patient had no tenderness associated with the mass, a finding which was worrisome for breast malignancy given its similarly painless presentation. Despite this, the scattered inflammatory cells observed on histology suggest that the soft tissue edema was reactive to the nodular fasciitis.
Nodular fasciitis has been described as a poorly defined soft tissue mass on CT, having attenuation less than that of skeletal muscle [13].
Microscopically, nodular fasciitis is characterized by plump, immature spindle-shaped fibroblasts and myofibroblasts arranged in short irregular bundles accompanied by a dense reticulin meshwork and extravasated erythrocytes [14]. Lymphoid cells, lipid macrophages, and multinucleated giant cells are often described. An important diagnostic criterion is a pattern of intervening ground substance which gives the lesion a “feathery” appearance [12, 14]. Features used to distinguish nodular fasciitis from aggressive lesions such as breast carcinoma or sarcoma include lack of atypia and hyperchromatic nuclei, sparing of the skin, and a limited number of mitoses (not greater than 1 per high power field) [7].
Nodular fasciitis is categorized into three histologic subtypes: myxoid, cellular, and fibrous [4]. Myxoid lesions, the histologic subtype in our case, typically have higher cellularity and correspondingly shorter clinical presentations compared to the more mature fibrous subtype [15]. While myxoid in nature, mucinous breast carcinoma can be classically distinguished by its clusters of tumor cells floating in pools of extracellular mucin. Different histologic subtypes do often coexist in the same lesion, leading some to suggest that there is an age-related histologic transition from myxoid to cellular and then fibrous as the lesion matures [12]. Nodular fasciitis can be further subdivided based on the basis of its anatomic location: subcutaneous, intramuscular, or intermuscular [12]. Our case was subcutaneous in location which is the most common subtype.
Treatment & prognosis
The recommended treatment for nodular fasciitis is generally conservative, although some advocate for excision[7, 8]. Brown et al recommend conservative management in cases where diagnosis has been established with satisfactory core biopsy, describing a case where clinical regression was observed within 16 days without any therapeutic intervention [7]. Numerous other reports describe spontaneous regression of nodular fasciitis [9, 14, 16]. As the lesion is often diagnosed after excision, the natural history is not completely understood. The CT scan of our patient demonstrated a significant decrease in the size of the lesion within a period of approximately two weeks consistent with relatively rapid spontaneous regression. Follow up CT examination 3 months later performed at the discretion of the attending surgeon demonstrated resolution of the lesion. In cases of suspected nodular fasciitis in the breast based, conservative management may be considered if satisfactory core biopsy demonstrates no findings to suggest malignancy.
Differential Diagnoses
Lymphadenitis
Abscess
Hematoma
Non-breast malignancy
Primary breast malignancy
Nodular Fasciitis
In the setting of a rapid clinical presentation with the described imaging findings, the differential diagnosis would include inflammatory diseases such as lymphadenitis, abscess, hematoma, non-breast malignancy such as lymphoma, and primary breast malignancy (Table 2).
Table 2.
Differential diagnoses table for Nodular Fasciitis of the axillary tail of the breast
| Clinical Presentation | Mammography | Ultrasound | CT | |
|---|---|---|---|---|
| Lymphadenitis |
|
|
|
Enlarged, morphologically abnormal lymph nodes |
| Non-puerperal Abscess |
|
|
|
|
| Hematoma |
|
|
|
|
| Non-breast Malignancy (including Lymphoma) |
|
|
|
|
| Primary Breast Malignancy |
|
|
|
|
| Inflammatory Process (Nodular Fasciitis) |
|
Varies. Spherical mass with indistinct margins and surrounding increase in trabecular density OR an irregular, spiculated mass | Variable, nonspecific; not well established in the literature; typically well circumscribed, homogeneous to mixed internal echotexture, sometimes with posterior acoustic enhancement; may have surrounding soft tissue edema | Poorly defined, having attenuation less than that of skeletal muscle |
Lymphadenitis
Inflammation of lymph nodes by bacterial or granulomatous infections, such as tuberculosis, is known as lymphadenitis. The most common location of intramammary lymph nodes is in the axillary tail of the breast. Inflammation of these nodes resulting in lymphadenitis is most commonly due to bacterial agents that are located in the normal flora of the skin. Focal lymphadenitis is prominent in streptococcal infection, tuberculosis, nontuberculous mycobacterial infection, tularemia, plague, and cat-scratch disease. Multifocal lymphadenitis is common in infectious mononucleosis, cytomegalovirus infection, toxoplasmosis, brucellosis, secondary syphilis, and disseminated histoplasmosis [17, 18].
The axillary nodes receive drainage from the arm, thoracic wall, and breast. Infections, including cat scratch disease, are other common causes of axillary lymphadenopathy. Fever typically accompanies lymphadenopathy for the majority of the infectious etiologies [19]. Other causes of lymphadenitis are more rare disorders including histiocytic necrotizing lymphadenitis, also known as Kikuchi-Fujimoti disease, which typically presents with fever and lymphadenitis, though typically cervical [20]. In the absence of upper extremity lesions with lymphadenopathy, primary or metastatic malignancy is in the differential diagnosis [21].
In one series of 31 patients with isolated axillary masses, 9 had breast cancer (5 in the contralateral breast) and 9 had metastases from other sites [21]. The history in a patient with lymphadenopathy should focus upon the following: localizing signs or symptoms suggesting infection or malignancy; exposures likely to be associated with infection (eg, cat scratches [cat scratch disease], undercooked meat [toxoplasmosis], tick bite [Lyme disease]), travel to areas with high rates of endemic infection, high risk behavior (eg, sexual behavior, injection drug use); constitutional symptoms such as fever, night sweats, or weight loss suggesting tuberculosis, lymphoma, or other malignancy; use of medications that can cause lymphadenopathy; and foreign travel [19]. The time course of the adenopathy also can help narrow the diagnostic possibilities. Radiologic tests can define node size and distribution more precisely than physical examination, but add little to diagnosis. Imaging by computed tomography (CT), ultrasound, Doppler technology, or magnetic resonance imaging (MRI) can help distinguish enlarged lymph nodes from other structures and define the pathologic process, especially cystic changes. Imaging can provide clues to diagnosis but usually cannot replace biopsy [19].
Abscess
Non-puerperal abscess is a relatively uncommon benign breast entity, representing 1–2% of all symptomatic breast processes [22]. Non-puerperal abscesses affect a wide age range (mid-teens through eighth decade), with peak incidence in the mid to late fourth decade [23]. Abscesses are usually low density, radiopaque lesions on mammography although they can also appear as high density, spiculated masses [24, 25]. Ultrasound findings include complex cystic lesions (roughly 50% of cases) and non-specific heterogeneously hypoechoic masses [26–28]. An abscess may have a similar appearance as a hematoma on CT exam, although a clinical history of a fever and an elevated white blood cell count may suggest the diagnosis [25].
Hematoma
Breast hematomas and seromas can be seen after biopsy, trauma, or surgery [25]. Hematomas will become smaller over time and eventually resorb [25]. Hematomas may appear as an irregular spiculated, high density mass on mammography. Similar to an abscess, hematomas may appear as complex cystic masses on ultrasound. On CT, hematomas appear as a fluid collection which may be irregular and peripherally enhancing with punctate foci or air and/or air-fluid level [25]. As a hematoma resolves, the blood filled mass will change to serous fluid, forming a seroma; seromas appear as well-defined high-attenuation fluid collections [25].
Non-breast Malignancy
Metastatic involvement of the breast is a very uncommon condition, accounting for 0.5–6% of all breast malignancies [29, 30]. The most common sources of primary tumors are lymphoma, melanoma, rhabdomyoscarcoma, lung carcinoma, ovarian carcinoma, renal cell carcinoma, leukemia, thyroid and cervical carcinoma [31]. Metastatic lesions may manifest mammographically as single or multiple masses or as diffuse skin thickening and at ultrasound, they tend to have circumscribed margins with low-level internal echoes [32].
Breast lymphomas represent approximately 1% of all non-Hodgkin lymphomas, the frequency of primary and secondary cases typically equal [33–35]. The most frequent morphologic type is diffuse large B-cell lymphoma [33]. Mammographic appearance ranges from discrete masses to diffuse involvement of the parenchyma and skin [36]. Classification of lymphoma increasingly depends on immunophenotypic, genetic, and molecular features of the tumor, in addition to tissue architecture. Core needle biopsy provides tissue for evaluation of architecture and flow cytometry and is a relatively low- morbidity, inexpensive alternative to open biopsy [37]. The CT appearance of breast lymphomas is varied and ranges from unilateral, bilateral, single, multiple, round/oval masses, or diffuse masses [38]. Management of lymphoma of the breast depends on the stage of the disease; radiation therapy is often considered for early stage (I and II) disease [39].
Breast carcinoma
Metaplastic carcinoma of the breast manifests as a rapidly growing, mammographically ill-defined round mass with associated architectural distortion on mammograms [40]. Complex echogenicity with solid and cystic components may be seen sonographically and is related to hemorrhagic or cystic necrosis seen pathologically [40].
TEACHING POINT
While rare in the breast, nodular fasciitis commonly presents as a rapidly growing mass with concerning and nonspecific imaging features and high cellular activity on histopathology. Its location as a painless mass in the axillary tail of the breast in our patient was suspicious for a malignant process. Nonspecific clinical examination, mammographic, and sonographic findings in our case illustrate the need for image guided versus surgical biopsy to exclude malignancy. It is important to be aware of the clinical, radiological, and histological appearance of nodular fasciitis in order to arrive at the correct diagnosis so that appropriate management can be planned for this self-limited lesion.
Table 1.
Summary of Nodular Fasciitis of the axillary tail of the breast
| Etiology | Unknown |
| Incidence | Rare |
| Gender Ratio | Incidence equal between men and women |
| Age predilection | 2nd to 4th decades of life |
| Risk factors | No known; 5–15% patients report history of trauma preceding appearance of the lesion |
| Treatment | Conservative, although some advocate for local excision |
| Prognosis | Often spontaneously regresses |
| Findings on Imaging | Variable appearance on mammography, US, and CT. Ill-defined nodular lesion, related to a fascia, with infiltration of fat and/or muscle tissue |
ABBREVIATIONS
- CT
computed tomography
- H&E
Hematoxylin and eosin stain
- MLO
mediolateral oblique
- MRI
magnetic resonance imaging
REFERENCES
- 1.Konwaler BE, Keasbey L, Kaplan L. Subcutaneous pseudosarcomatous fibromatosis (fasciitis) American journal of clinical pathology. 1955;25:241–252. doi: 10.1093/ajcp/25.3.241. [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrom J, Buckingham J, Bell S, Jain S. Nodular fasciitis of the breast simulating breast cancer on imaging. Australasian radiology. 2001;45:67–70. doi: 10.1046/j.1440-1673.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. Journal of clinical pathology. 2013;66:465–470. doi: 10.1136/jclinpath-2012-201078. [DOI] [PubMed] [Google Scholar]
- 4.Kempson R, Fletcher C, Evans H, et al. Fibrous and myofibroblastic tumors. In: Rosai J, editor. Tumors of the Soft Tissues. 3rd ed. Bethesda, MD: Armed Forces Institute of Pathology; 2001. pp. 23–112. [Google Scholar]
- 5.Gelfand JM, Mirza N, Kantor J, et al. Nodular fasciitis. Archives of dermatology. 2001;137:719. [PubMed] [Google Scholar]
- 6.Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007;27:173–187. doi: 10.1148/rg.271065065. [DOI] [PubMed] [Google Scholar]
- 7.Brown V, Carty NJ. A case of nodular fascitis of the breast and review of the literature. Breast. 2005;14:384–387. doi: 10.1016/j.breast.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Tomita S, Thompson K, Carver T, Vazquez WD. Nodular fasciitis: a sarcomatous impersonator. Journal of pediatric surgery. 2009;44:e17–19. doi: 10.1016/j.jpedsurg.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Ozben V, Aydogan F, Karaca FC, Ilvan S, Uras C. Nodular Fasciitis of the Breast Previously Misdiagnosed as Breast Carcinoma. Breast Care (Basel) 2009;4:401–402. doi: 10.1159/000261502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black A, Kissin M, Jackson P, Birdsall S, Shipley J, Gusterson B. Nodular fasciitis-Part I. Simulating breast carcinoma in an 84-year-old woman. The Breast. 1994;3:48–49. [Google Scholar]
- 11.Nikolaidis P, Gabriel HA, Lamba AR, Chan NG. Sonographic appearance of nodular fasciitis. Journal of ultrasound in medicine. 2006;25:281–285. doi: 10.7863/jum.2006.25.2.281. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, De Schepper A, Vanhoenacker F, et al. Nodular fasciitis: correlation of MRI findings and histopathology. Skeletal radiology. 2002;31:155–161. doi: 10.1007/s00256-001-0462-z. [DOI] [PubMed] [Google Scholar]
- 13.Meyer CA, Kransdorf MJ, Jelinek JS, Moser RP., Jr MR and CT appearance of nodular fasciitis. Journal of computer assisted tomography. 1991;15:276–279. doi: 10.1097/00004728-199103000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Duncan SF, Athanasian EA, Antonescu CR, Roberts CC. Resolution of nodular fasciitis in the upper arm. Radiology Case Reports. 2006;1:17–20. doi: 10.2484/rcr.v1i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price E, Jr, WMS Nodular fasciitis: a clinicopathologic analysis of 65 cases. American journal of clinical pathology. 1961;35:122–136. doi: 10.1093/ajcp/35.2.122. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Ehara S, Nishida J, Satoh T. Rapid involution of proliferative fasciitis. Skeletal radiology. 2004;33:300–302. doi: 10.1007/s00256-003-0738-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Suen M, Metreweli C. Mammographic, sonographic and histopathological correlation of benign axillary masses. Clinical radiology. 1997;52:130–135. doi: 10.1016/s0009-9260(97)80106-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Giurescu ME, Philpotts LE, Horvath LJ, Tocino I. Clinical importance of unilaterally enlarging lymph nodes on otherwise normal mammograms. Radiology. 1997;203:329–334. doi: 10.1148/radiology.203.2.9114083. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher Robert H., M, MSc . Evaluation of peripheral lymphadenopathy in adults. In: Boxer Laurence A., MDEJST, MD, editor. Section Editor. Jun 19, 2012. [Google Scholar]
- 20.Ohta K, Endo N, Kaizaki Y. Axillary and intramammary lymphadenopathy caused by Kikuchi-Fujimoto disease mimicking malignant lymphoma. Breast Cancer. 2013;20:97–101. doi: 10.1007/s12282-009-0182-0. [DOI] [PubMed] [Google Scholar]
- 21.De Andrade J, Marana H, Sarmento FJ, Murta E, Velludo M, Bighetti S. Differential diagnosis of axillary masses. Tumori. 1996;82:596. doi: 10.1177/030089169608200617. [DOI] [PubMed] [Google Scholar]
- 22.Dixon JM. Periductal mastitis/duct ectasia. World journal of surgery. 1989;13:715–720. doi: 10.1007/BF01658420. [DOI] [PubMed] [Google Scholar]
- 23.Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. The American journal of surgery. 2004;188:407–410. doi: 10.1016/j.amjsurg.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Tabár L, Dean P. Teaching atlas of mammography. Thieme; 2012. [PubMed] [Google Scholar]
- 25.Harish MG, Konda SD, MacMahon H, Newstead GM. Breast Lesions Incidentally Detected with CT: What the General Radiologist Needs to Know1. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007;27:S37–S51. doi: 10.1148/rg.27si075510. [DOI] [PubMed] [Google Scholar]
- 26.Lequin M, Van Spengler J, Van Pel R, van Eijck C, van Overhagen H. Mammographic and sonographic spectrum of non-puerperal mastitis. European journal of radiology. 1995;21:138–142. doi: 10.1016/0720-048x(95)00699-q. [DOI] [PubMed] [Google Scholar]
- 27.Tan H, Li R, Peng W, Liu H, Gu Y, Shen X. Radiological and clinical features of adult non-puerperal mastitis. British Journal of Radiology. 2013;86 doi: 10.1259/bjr.20120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu P, Kurihara Y, Kanemaki Y, et al. High-resolution MRI in detecting subareolar breast abscess. American Journal of Roentgenology. 2007;188:1568–1572. doi: 10.2214/AJR.06.0099. [DOI] [PubMed] [Google Scholar]
- 29.Amichetti M, Perani B, Boi S. Metastases to the breast from extramammary malignancies. Oncology. 1990;47:257–260. doi: 10.1159/000226826. [DOI] [PubMed] [Google Scholar]
- 30.Bohman L, Bassett L, Gold R, Voet R. Breast metastases from extramammary malignancies. Radiology. 1982;144:309–312. doi: 10.1148/radiology.144.2.7089284. [DOI] [PubMed] [Google Scholar]
- 31.Vizcaino I, Torregrosa A, Higueras V, et al. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. European radiology. 2001;11:1659–1665. doi: 10.1007/s003300000807. [DOI] [PubMed] [Google Scholar]
- 32.Feder JM, de Paredes ES, Hogge JP, Wilken JJ. Unusual Breast Lesions: Radiologic-Pathologic Correlation1. Radiographics : a review publication of the Radiological Society of North America, Inc. 1999;19:S11–S26. doi: 10.1148/radiographics.19.suppl_1.g99oc07s11. [DOI] [PubMed] [Google Scholar]
- 33.Topalovski M, Crisan D, Mattson JC. Lymphoma of the breast: a clinicopathologic study of primary and secondary cases. Archives of pathology & laboratory medicine. 1999;123:1208–1218. doi: 10.5858/1999-123-1208-LOTB. [DOI] [PubMed] [Google Scholar]
- 34.Ha CS, Dubey P, Goyal LK, Hess M, Cabanillas F, Cox JD. Localized primary non-Hodgkin lymphoma of the breast. American journal of clinical oncology. 1998;21:376–380. doi: 10.1097/00000421-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Giardini R, Piccolo C, Rilke F. Primary non? Hodgkin’s lymphomas of the female breast. Cancer. 1992;69:725–735. doi: 10.1002/1097-0142(19920201)69:3<725::aid-cncr2820690320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 36.Meyer J, Kopans D, Long J. Mammographic appearance of malignant lymphoma of the breast. Radiology. 1980;135:623–626. doi: 10.1148/radiology.135.3.7384445. [DOI] [PubMed] [Google Scholar]
- 37.Amador-Ortiz C, Chen L, Hassan A, et al. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. American journal of clinical pathology. 2011;135:516–524. doi: 10.1309/AJCP3WZ8ZDRJQDOU. [DOI] [PubMed] [Google Scholar]
- 38.Ginat DT, Puri S. FDG PET/CT manifestations of hematopoietic malignancies of the breast. Academic radiology. 2010;17:1026–1030. doi: 10.1016/j.acra.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin’s Lymphomas. Journal of the National Comprehensive Cancer Network. 2010;8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 40.Park JM, Han BK, Moon WK, Choe YH, Ahn SH, Gong G. Metaplastic carcinoma of the breast: mammographic and sonographic findings. Journal of clinical ultrasound. 2000;28:179–186. doi: 10.1002/(sici)1097-0096(200005)28:4<179::aid-jcu5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]