Abstract
Aneurysm of the Membranous Septum (AMS) is a rare cardiac disease, mostly associated with other cardiac anomalies, very rare in the absence of other congenital heart defects. A prompt diagnosis is important, due to severe potential complications, but remain challenging. Most of the cases were earlier diagnosed using ventriculography, but, with the availability of echocardiography and cardiovascular magnetic resonance (CMR), this disease can be accurately assessed non-invasively. We report a case of a 62 years old female patient, without other cardiac congenital disease, who was incidentally diagnosed, by means of CMR with a true and isolated AMS. Our report underlines CMR usefulness in AMS diagnosis, thanks to accurate evaluation (both morphologic and functional) provided by this diagnostic tool, which is able to demonstrate clearly the presence of AMS (aneurysm of the membranous septum) and depict its features.
Keywords: aneurysm of the membranous septum, interventricular septum, cardiovascular magnetic resonance
CASE REPORT
We describe a case in which a female patient, without other cardiac congenital defects, was incidentally diagnosed with a true and isolated Aneurysm of the Membranous Septum (AMS) by means of CMR (Cardiovascular Magnetic Resonance).
A 62 years old female patient underwent a CMR in our department after a clinic suspicion of an acute myocarditis. Acute myocarditis was confirmed by CMR and resolved in some weeks. Her medical history was moreover unremarkable.
CMR was performed using a 1,5T General Electric Medical System. Steady State Free Precession (SSFP) images were acquired in short axis and four chambers. Myocardial LE (Late Enhancement) images were acquired 10/15 min following the intravenous administration of contrast medium (BOPTA, MultiHance) in short axis and four chambers.
Imaging Findings
Images showed a saccular formation (20 × 15 mm), with regular contours and a wide base, arising from membranous septum inferior to the aortic valve and protruding into the right ventricular outflow tract. SSPF sequences clearly demonstrated flow from the Left Ventricular Outflow Tract into the formation through the superior portion of the membranous septum, without any shunt flow between the ventricles. SSFP sequences showed also an outpouching or windsock appearance due to aneurysmal distention during ventricular systole. There was neither evidence of any thrombus nor evidence of hyperintensity in LE sequences.
On the basis of the above information, this structure was confidently referable to a true AMS.
Evaluation of short axis sequence through a post - processing software (Segment®) showed regular parameters: left EDV (end diastolic volume) 100 ml, left ESV (end systolic volume) 35 ml, left EF (ejection fraction) 67%; right EDV 90 ml, right ESV 40 ml, right EF 55%.
Management and follow-up
According to the guidelines of the European Society of Cardiology (ESC), our patient received no treatment but was planned a strictly CMR follow-up.
DISCUSSION
Etiology & demographics
Aneurysm of the membranous septum (AMS) is a rare disease, mostly associated with other cardiac anomalies [1], that occurs in 0.3% of patients with congenital heart disease, in up to 19–22,4% with ventricular septal defect [2,3] and in 20% with perimembranous ventricular septal defects [3]. AMS is a very rare condition in the absence of ventricular septal defect [2], such in our case. Despite recent developments in the diagnosis of congenital heart disease, it is still difficult to determine the prevalence of these anomalies [3].
AMS involves the membranous portion of interventricular septum [4] and, although described as an aneurysm, doesn’t appear to be a true aneurysm [5]. AMS, as in our case, is a well - developed, thickened fibrous - walled sac, arising from the right ventricular aspect of the membranous septum, beneath the septal leaflet of the tricuspid valve, sometimes perforated by one or many holes, bulging forward into the right ventricle [6,7]. Absence of myocardium leads to the aneurysm - like behaviour of this structure [8]; the characteristic outpouching or windsock appearance results from aneurysmal distention during ventricular systole [9]. AMS are generally classified as true, false and pseudoaneurysms [3].
AMS with regular contours and a wide base are termed true AMS, as in our case, while those with a narrow base and irregular shape are named false AMS [3]. A pseudo-AMS usually occurs as a complication of myocardial infarction, in which the necrotic muscular wall protrudes into the right ventricle, or as a complication of transaortic septal myotomy [3].
The etiology of this anomaly is poorly understood [9]. AMS may develop as a consequence of spontaneous closure, partial or complete, of a ventricular septal defect [2]; other theories suggest either abnormal embryologic development or weakness of the affected tissues [9].
Clinical & imaging findings
AMS may further enlarge [6] and patients may incur potential cardiac complications, such as aortic valve prolapse, right ventricular outflow obstruction, tricuspid valve insufficiency, arrhythmia, rupture, thromboembolism and bacterial endocarditis [2].
True AMS is difficult to identify angiographically, ultrasonographically and pathologically [3]. The first postmortem observation of AMS was reported by Laennec in 1826 [10], Steinberg made the first antemortem diagnosis using ventriculography in 1957 [11]. Conventional left ventriculography is a widely used method, that can well demonstrate aneurysm of the membranous interventricular septum but it bears limitation because of its invasive nature; in addition, detecting the thrombus within the aneurysm is not possible [12]. Nowadays AMS is often detected incidentally during non - invasive imaging (echocardiogram) [13]; nevertheless AMS could not be optimally visualized [13] and detailed morphological assessment is sometimes obscure [12], especially in patients in whom precordial windows are too poor to clearly visualize the cardiac anatomy [5]. According to Canale et al. the echocardiogram sensitivity is 57% in the subcostal four chamber view, 62% in the short axis view, 71% in the apical four chamber view, 87.5% in the long axis view [13]. CMR is a useful diagnostic tool both for morphological and functional assessment because it is capable, as in our case, of three-dimensional anatomical assessment and provides functional data about the blood flow into the aneurysm and integrity of the ventricular membranous septum [13]. CMR is able, as in our case, to demonstrating clearly AMS presence and depict its features (size, absence of holes and/or thrombus) without limitation due to poor acoustic window, that may impair a clear echocardiographic approach.
Phase-contrast MRI is able to quantify the severity of abnormal communication between the cardiac chambers by calculating the ratio between pulmonary and systemic flow (Qp/Qs) [14,15]. PC MRI was found to have 93% specificity and 100% sensitivity for description of shunts with a Qp:Qs greater than 1.5 in comparison with invasive oxymetry [15]. Transthoracic and transesophageal echocardiography usually allow exact localization and sizing of the defect but determination of shunt volumes by Doppler echocardiography has limitations [16]. In general, Doppler echocardiography can provide excellent assessment in smaller, younger children while MRI plays a more important role in the appraisal of the older, larger adolescent or adult with congenital heart disease, especially those that have undergone previous cardiothoracic surgery [16].
Multislice CT is another diagnostic tool, which has similar advantages in morphological and functional assessment compared to CMR, but CMR is preferable because able to provide functional data, without exposure of radiation [13].
Differential Diagnoses
The differential diagnosis of AMS includes anatomically related aneurysms and aneurysmal-like structures arising in and around the left ventricular outflow tract, such as sinus of Valsalva aneurysm [17]. AMS arises from the right ventricular aspect of the membranous septum, beneath the septal leaflet of the tricuspid valve, and bulging forward into the right ventricle [6,7]. It is possible to make a definitive diagnosis only by demonstrate flow from the Left Ventricular Outflow Tract into the AMS through the superior portion of the membranous septum [17]. On the contrary, the criteria for diagnosing a Valsalva sinus aneurysm include an origin above the aortic annulus, a saccular shape and normal dimensions of the adjacent aortic root and ascending aorta [18]. Although angiography is considered the reference standard for confirming the presence of a Valsalva sinus aneurysm, most are initially seen at color Doppler echocardiography [18]. Multiplanar MR imaging with sequences also allows accurate assessment of the origin and size of Valsalva sinus aneurysms and the status of the surrounding cardiac and mediastinal anatomy [18]. The advantages of performing MR imaging in the setting of a known or suspected Valsalva sinus aneurysm include the ability to evaluate the left ventricular hemodynamic pattern, identify aortic regurgitation, and quantify any aortocardiac shunt or turbulent or fistulous blood flow [18]. CT provides detailed anatomic depiction of Valsalva sinus aneurysms and surrounding cardiac structures but MR imaging can be performed without exposing the patient to ionizing radiation or iodinated contrast material, which is a further fundamental advantage [18].
Treatment & prognosis
Patients with AMS who are asymptomatic should be followed closely in terms of potential cardiac complications [2]; European Society of Cardiology (ESC) states that follow-up at 3 to 5 year intervals may be reasonable [19]. Surgery is usually considered in AMS cases complicated with either thrombus and related systemic emboli, rupture or accompanying significant VSD [13].
Thus, as in our case, information provided by CMR are useful for planning patient care and follow - up, also thank to high reproducibility of CMR data.
TEACHING POINT
Aneurysm of the Membranous Septum is a thickened fibrous - walled sac arising from the right ventricular aspect of the membranous septum and bulging forward into the right ventricle; absence of myocardium leads to the aneurysm like behaviour of this structure. It is possible to make a definitive diagnosis by assessing its origin and demonstrating flow from the Left Ventricular Outflow Tract into the aneurysm through the superior portion of the membranous septum.
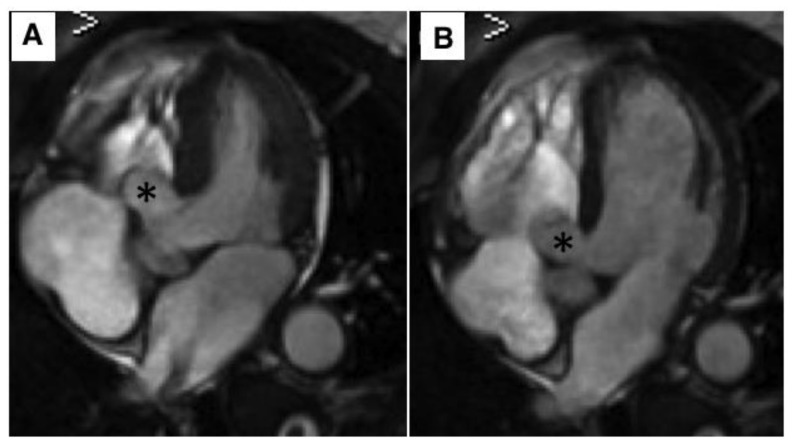
Figure 1.
62 year old female with aneurysm of the membranous septum.
Technique: MRI 1,5T; Steady State Free Precession (SSFP) four chambers view; TR (repetition time) 3,6 msec, TE (echo time) 1,6 msec, Flip angle 65°, Slice thickness 4mm, Image matrix 256 × 256.
Finding: Steady State Free Precession (SSFP) four chambers view in systole (a) and diastole (b) show an AMS (20 × 15 mm) arising from membranous septum beneath the septal leaflet of the tricuspid valve, bulging into right ventricle, without any thrombus or shunt flow between the ventricles (asterisk).
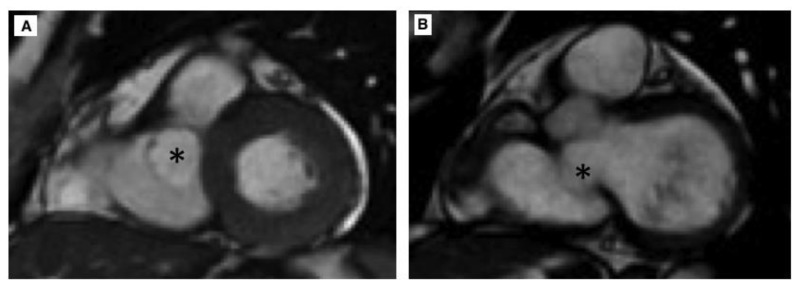
Figure 2.
62 year old female with aneurysm of the membranous septum.
Technique: MRI 1,5T; Steady State Free Precession (SSFP) short axis view; TR (repetition time) 3,6 msec, TE (echo time) 1,6 msec, Flip angle 65°, Slice thickness 4mm, Image matrix 256 × 256.
Finding: Steady State Free Precession (SSFP) short axis view in systole (a) and diastole (b) show AMS (asterisk) adjacent to interventricular septum, inferior to the aortic valve, protruding in right ventricular chamber.
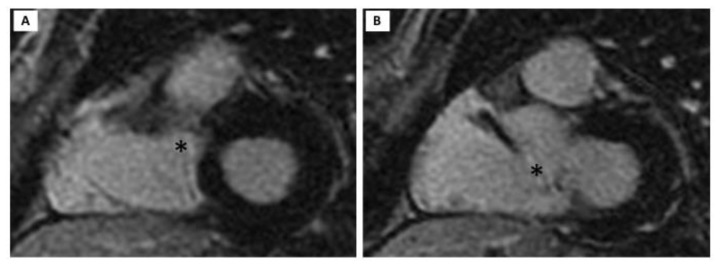
Figure 3.
62 year old female with aneurysm of the membranous septum.
Technique: MRI 1,5T; myocardial LE (Late Enhancement) short axis view acquired 10/15 min following the intravenous administration of contrast medium (BOPTA, MultiHance); TR (repetition time) 3,6 msec, TE (echo time) minimum, TI (Inversion Time) 180 msec, Flip angle 20°, Image Matrix 256 × 256.
Finding: myocardial LE (Late Enhancement) short axis view in systole (a) and diastole (b) show AMS (asterisk) without evidence of hyperintensity.
Table 1.
Summary table for AMS (Aneurysm of the Membranous Septum)
| Incidence | Rare, although it is still difficult to determine its prevalence. AMS occurs in 0.3% of patients with congenital heart disease, in up to 19–22,4% with ventricular septal defect and in 20% with perimembranous ventricular septal defects. |
| Etiology | AMS develops, more often, as a consequence of spontaneous closure of a ventricular septal defect. |
| Findings on imaging |
|
| Prognosis | AMS may further enlarge. Patients may incur potential cardiac complications, such as aortic valve prolapse, right ventricular outflow obstruction, tricuspid valve insufficiency, arrhythmia, rupture, thromboembolism and bacterial endocarditis. |
| Treatment | Patients with AMS who are asymptomatic should be followed closely while surgery is usually considered in cases complicated with concurrent heart diseases, hemodynamic abnormalities and/or symptoms. |
Table 2.
Differential diagnoses for AMS (Aneurysm of the Membranous Septum)
| Aneurysm of Membranous Septum (AMS) | Valsalva Sinus Aneurysm (VSA) | |
|---|---|---|
| US | AMS is often detected incidentally during echocardiogram but morphological assessment may be difficult. Doppler echocardiography may identify shunt flow between the ventricles. | Most VSA are initially seen at color Doppler echocardiography but global assessment may be difficult. |
| CT | CT is able to demonstrating AMS presence and features but bears limitation due to exposure of radiation | CT is able to demonstrating VSA presence and features but bears limitation due to exposure of radiation. |
| MRI SSPF | SSPF MRI sequences demonstrate AMS presence and describe its feature. Also SSFP sequences show flow from the Left Ventricular Outflow Tract into the AMS through the superior portion of the membranous septum. | MRI SSFP sequences allow accurate assessment of the origin and size of VSA. Also, SSFP sequences identify any aorto-cardiac shunt or fistulous blood flow. |
| MRI PC | PC MRI is able to quantify the severity of abnormal communication between the cardiac chambers and AMS. | MRI PC quantifies any aorto-cardiac shunt or fistulous blood flow. |
| MRI LE | In some cases, AMS shows hyperintensity in LE sequences. | In some cases, VSA shows hyperintensity in LE sequences. |
| Ventriculography | Ventriculography can well demonstrate AMS but it is an invasive procedure and is not able to demonstrate a thrombus within the aneurysm. | Ventriculography is considered the reference standard for confirming the presence of a VSA but it is an invasive procedure. |
Table 3.
Classification of AMS (Aneurysm of the Membranous Septum) types
| AMS TYPE | Characteristics |
|---|---|
| True | Is a congenital disease. Has regular contours and a wide base. |
| False | Is a congenital disease. Has irregular shape and a narrow base. |
| Pseudoaneurysm | Is an acquired disease occurring as a complication of myocardial infarction or of transaortic septal myotomy. |
ABBREVIATIONS
- AMS
Aneurysm of the membranous septum
- CMR
Cardiovascular Magnetic Resonance
- EDV
End Diastolic Volume
- EF
Ejection Fraction
- ESV
End Systolic Volume
- FIESTA
Fast Imaging Employing Steady State Acquisition
- IVS
Interventricular Septum
- LE
Late Enhancement
- PC
Phase Contrast
- SSFP
Steady State Free Precession
- TE
Echo Time
- TR
Repetition Time
- VSA
Valsalva Sinus Aneurysm
REFERENCES
- 1.Langer C, Horstkotte D, Piper C. Aneurysm of the membranous septum causes pre syncopes and transient bilateral blindness. Eur Heart J. 2007 Apr;28(7):784. doi: 10.1093/eurheartj/ehl251. [DOI] [PubMed] [Google Scholar]
- 2.Yavuz S, Eris C, Goncu T, Sezen M, Ata Y, Turk T. An Incidental Aneurysm of the Interventricular Membranous Septum. Archives of Iranian Medicine. 2010 Jul;13(4) [PubMed] [Google Scholar]
- 3.Loukas M, Shane Tubbs R, Louis RG, Jr, Curry B. Pseudoaneurysm of the membranous septum, case report and review of the literature. Surg Radiol Anat. 2006;28:564–568. doi: 10.1007/s00276-006-0136-6. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza J, Kalache K, Goncalves LF, Lee W, Chaiworapongsa T, Schoen ML, Devers P, Treadwell M, Mazor M, Romero R. Prenatal diagnosis of membranous ventricular septal aneurysms and their association with absence of atrioventricular valve ‘offsetting’. Ultrasound Obstet Gynecol. 2004;24:787–792. doi: 10.1002/uog.1769. [DOI] [PubMed] [Google Scholar]
- 5.Reddy SC, Chopra PS, Rao PS. Aneurysm of the membranous ventricular septum resulting in pulmonary outflow tract obstruction in congenitally corrected transposition of the great arteries. Am Heart J. 1997 Jan;133(1):112–9. doi: 10.1016/s0002-8703(97)70256-4. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz AT, Ozal E, Arslan M, Tatar H, Ozturk OY. Aneurysm of the membranous septum in adult patients with perimembranous ventricular septal defect. Eur J Cardiothorac Surg. 1997 Feb;11(2):307–11. doi: 10.1016/s1010-7940(96)01058-5. [DOI] [PubMed] [Google Scholar]
- 7.Pedra CA, Yoo SJ, Soederberg B, Freedom RM. Aneurysm of the Membranous Septum in Critical Pulmonary Stenosis: Spontaneous Rupture After Balloon Dilatation. Pediatr Cardiol. 2001;22(4):359–362. doi: 10.1007/s002460010248. [DOI] [PubMed] [Google Scholar]
- 8.Bijulal S, Sivasankaran S, Sanjay G, Tharakan J. Membranous Septal Aneurysm : An Unusual Cause for Right Ventricular Outflow Tract Obstruction in a Malaligned Ventricular Septal Defect with Aortomitral Discontinuity (Double - Outlet Right Ventricle) Associated with Visceral Heterotaxy. Pediatr Cardiol. 2009 Feb;30(2):200–2. doi: 10.1007/s00246-008-9299-8. [DOI] [PubMed] [Google Scholar]
- 9.Shah AM, Rivenes SM, Fraser CD, Miller Hance WC. Aneurysm of the Atrioventricular Membranous Septum Appearing as a Right Atrial Cystic Mass. Anesthesia and Analgesia. 2007 Dec;105(6):1569–1571. doi: 10.1213/01.ane.0000290303.88801.5b. [DOI] [PubMed] [Google Scholar]
- 10.Salazar J, Gutierrez A, Cay E, Ballester C, Salazar JJ, Placer L. Cerebral embolism and thrombus in a membranous interventricular septal aneurysm. The Annals of Thoracic Surgery. 2003 Jul;76(1):286–287. doi: 10.1016/s0003-4975(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 11.Assey ME, Stalheim RM, Usher BW. Ventricular Septal Defect with Aneurysm of the Membranous Septum Presenting as a Systolic and Early Diastolic Murmur. Chest. 1979 Apr;75(4):504–6. doi: 10.1378/chest.75.4.504. [DOI] [PubMed] [Google Scholar]
- 12.Komatsua S, Sato Y, Omori Y, Hirayama A, Okuyama Y, Kasiwase K, Fujisawa Y, Koshimune Y, Kiyomoto M, Shimizu T, Kodama K. Aneurysm of the membranous interventricular septum demonstrated by multislice computed tomography. International Journal of Cardiology. 2007;114(1):123–124. doi: 10.1016/j.ijcard.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Catakoglu AB, Aytekin V, Aytekin S, Duran C, Polat B, Demiroglu C. Aneurysm of the membranous septum detected during left ventriculography and demonstrated by cardiac magnetic resonance imaging. International Journal of Cardiology. 2009;136(3):e60–e62. doi: 10.1016/j.ijcard.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Caroff J, Bière L, Trebuchet G, Nedelcu C, Sibileau E, Beregi JP, Aubé C, Furber A, Willoteaux S. Applications of phase-contrast velocimetry sequences in cardiovascular imaging. Diagnostic and Interventional Imaging. 2012;93:159–170. doi: 10.1016/j.diii.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg A, Jha S. Phase-contrast MRI and applications in congenital heart disease. Clinical Radiology. 2012;67:e399–e410. doi: 10.1016/j.crad.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Marx GR, Geva T. MRI and Eehoeardiography in Children: How Do They Compare? Seminars in Roentgenology. 1998;XXXIII(3 (July)):281–292. doi: 10.1016/s0037-198x(98)80009-6. [DOI] [PubMed] [Google Scholar]
- 17.Sugioka K, Watanabe H, Gersony DR, Hozumi T, Homma S, Suehiro S, Takeuchi K, Yoshikawa J. Giant Aneurysm of the Membranous Ventricular Septum Extending Outside the Heart: Diagnosis by Transthoracic Color Flow Doppler Echocardiography. Journal of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002 Feb;15(2):188–91. doi: 10.1067/mje.2002.116322. [DOI] [PubMed] [Google Scholar]
- 18.Bricker AO, Avutu B, Mohammed TL, Williamson EE, Syed IS, Julsrud PR, Schoenhagen P, Kirsch J. Valsalva sinus aneurysms: findings at CT and MR imaging. Radiographics. 2010 Jan;30(1):99–110. doi: 10.1148/rg.301095719. [DOI] [PubMed] [Google Scholar]
- 19.Cozijnsen MA, Cozijnsen L, Maas ACP, Bakker-de Boo M, Bouma BJ. A ventricular septal defect with a giant appendiform aneurysm of the membranous septum. Neth Heart J. 2013;21:152–154. doi: 10.1007/s12471-012-0343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]