Abstract
Over recent years there has been increasing usage of digital systems within cardiothoracic surgery to quantify air leaks and aid in clinical decision-making regarding the removal of chest drains postoperatively. The literature suggests improved agreement on timing of removal of chest drains and a reduced length of stay of patients. It could be that such devices could be useful tools for the clinician managing cases of pneumothorax.
Methods
This pilot study recruited adults admitted under the medical team with a pneumothorax requiring a chest drain. Participants had the underwater seal device changed for a digital device (Thopaz) which allowed continuous monitoring of the air leak. Drains were removed when either there was no ongoing air leak and the lung had expanded, or surgery was deemed necessary.
Results
Thirteen patients with pneumothorax (four primary, nine secondary) used the device during their admission including one patient treated in the community (the device has internal suction). Data were used to aid the clinician in management of the pneumothorax including the timing of surgery/ removal of drain and commencement of suction.
Discussion
Digital devices appear to be safe and effective and may prove to be a useful tool in the management of pneumothorax.
Keywords: pneumothorax, digital devices, respiratory measurement
Key messages.
Digital drainage devices appear to be safe and easy to integrate into clinical care.
The quantitative measurement of air leak may be useful for clinical decision making.
Introduction
The reported UK hospital admission rates for primary and secondary pneumothorax is 16.7/100 000 for men and 5.8/100 000 for women.1 Although spontaneous pneumothorax is a relatively common medical problem it is recognised that its management can be complex2 with considerable variability in practice3–7 and areas of uncertainty.4 8 There remains debate about the when exactly a drain should be removed once the lung is expanded radiographically,4 6 the use of suction and the benefits of provocative chest drain clamping.4–7 The timing of referral to thoracic surgeons is also controversial.8–10
Over recent years there has been increasing usage of digital drainage systems within cardiothoracic surgery to quantify air leaks and aid in clinical decision-making regarding the removal of chest drains postoperatively. The reported literature suggests improved agreement between surgeons on when to remove chest drains postoperatively11 and reduced length of stay of patients.12
As yet there have been no reported trials of the use of digital devices in the medical patient with pneumothorax, although at least one product, Thopaz, is licensed for use in this situation. We set out to evaluate the feasibility of using such a device for patients admitted with pneumothorax at our institution in a small pilot study.
Materials and methods
Study subjects
The study was approved by both the research and development department of the institution (St George's Hospital NHS Trust) and the local ethics committee (London-Dulwich NRES Committee Health Research Authority). The study period was from 1 September 2012 to 1 April 2013, during which time, consecutive patients admitted under the medical team with pneumothorax were identified by the daily departmental handover lists. Patients between the ages of 18 and 80 with a chest drain in situ for the purpose of treating pneumothorax and who were able to consent were eligible for the study.
Study design
The objectives of this pilot study were (1) to gain and document experience of the use of using digital drainage system in the management of pneumothorax in patients with primary/secondary/iatrogenic pneumothorax; (2) to subjectively evaluate patient, nurse and physician satisfaction of the use of this system in pneumothorax and (3) to gather observational data about the recorded air leak and analyse whether this data appeared to relate to the patient outcome (ie, when the drain was removed and whether the patient required surgery). There was no intention to perform statistical analysis as it was anticipated that the sample size would be small (between 10 and 15 participants were expected based on the admission rates with pneumothorax from the previous year). Qualitative data about the use of the device in management of this condition and the experience of patients and staff was sought.
Methods
Patients suitable for the study were approached by the principal investigator who was independent from the clinical team who had responsibility for the patients care. The study was discussed with each patient and written information provided about the study and written consent was gained. Once a patient had consented to enter the study the principal investigator changed the underwater seal chest drain bottle for the digital device (Thopaz) which took under 2 min. Participants were cared for on respiratory wards where nursing staff and junior doctors were trained on the use of the device.
The device provides continuous recording of the air leak in mL/min and the graphical display screen allows the prior 24 h’ data to be viewed. The physician in charge of the case was able to access this information to guide clinical decision-making. It was suggested (from experience used in surgical cohorts) that it would be reasonable to remove the chest drain when the data showed that the air leak had been <10 mL/min for greater than 6 h. Over the course of the study this advice was changed (see results section for explanation) to a recording of 0 mL/min for more than 12 h. However, it was ultimately the decision of the physician who had responsibility for the case to decide when to remove the drain, if and when to apply suction and if and when to refer for surgery. Physicians could choose to reattach an underwater seal either temporarily or permanently at any time.
Analysis
At the end of the patient episode, data from each device was downloaded and correlated with the patient's medical notes. Each patient was asked to complete a brief questionnaire about their satisfaction with the device. After 120 days following discharge the patient's medical records were searched to determine if there had been a recurrence of the pneumothorax and whether the patient was still alive. At the end of the study period nurses who had been involved with caring for patients who had used the device were asked to complete a brief questionnaire on their experience of using the device. Physicians who had clinical responsibility for patients using the device were asked to comment on their experience of using the device.
Results
Baseline characteristics of participants
Fifteen patients were admitted over the study periods that were suitable for the study. Thirteen patients were approached to enter the study, all of whom agreed to participate. Their characteristics and the outcome of their admission are shown in table 1.
Table 1.
Patient characteristics and clinical outcome
| Study no. | Age | Sex | Primary/secondary | Prior PTX | Time drain in situ/until VATS (days) | Time on device (days) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 46 | F | Primary | 0 | 2 | 1 | No recurrence |
| 2 | 50 | M | Secondary | 1 (2010) | 23 | 1 | No recurrence |
| 3 | 63 | M | Secondary | 1 (2005) | 30 | 29 | No recurrence Died of respiratory failure |
| 4 | 65 | M | Secondary | 0 | 14 | 1 | No recurrence. 2 drains fell out |
| 5 | 50 | M | Secondary | 0 | 2 | 1 | No recurrence |
| 6 | 59 | M | Secondary | 2 (2012 ×2) | 2 | 2 | No recurrence |
| 7 | 42 | M | Primary | 0 | 9 | 5 | VATS as inpatient |
| 8 | 48 | M | Secondary | 0 | 6 | 4 | No recurrence |
| 9 | 33 | M | Primary | 0 | 3 | 2 | No recurrence |
| 10 | 27 | M | Primary | 0 | 7 | 5 | No recurrence |
| 11 | 64 | M | Secondary | 1 (2012) | 92 | 7 | No recurrence following valve insertion |
| 12 | 38 | M | Secondary | 3 (2011) | 8 | 5 | Inpatient VATS |
| 13 | 70 | M | Secondary | 0 | 11 | 7 | No recurrence died of laryngeal cancer |
PTX, pneumothorax; VATS, video assisted thoracoscopic surgery
Data gathered from the device and clinical course
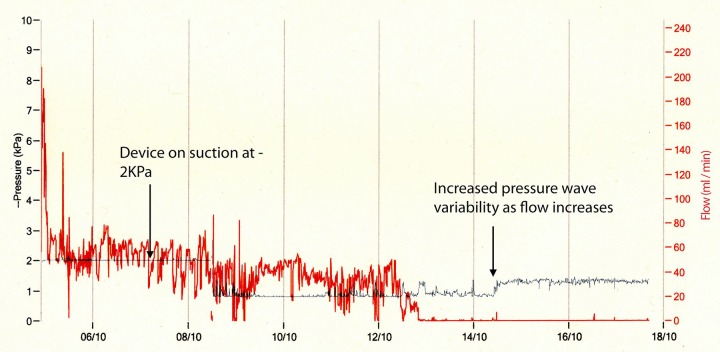
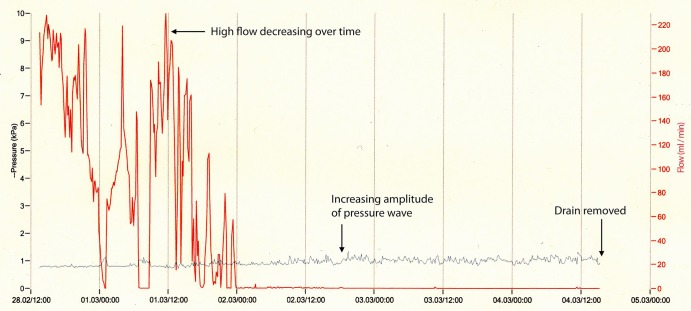
Data from each device were downloaded and viewed (examples are shown in figures 1–3). Figure 1 shows the trace from a patient with primary pneumothorax in a man never smoker. The red line shows that flow is relatively high initially (up to 230 mL/min) and decreases over time so that by the beginning of day 4 the air flow is less than 10 mL/min. The blue line shows the pressure recordings over this time. As the flow decreases, the amplitude of this pressure recording increases. The patient is able to regulate their own pleural pressure which Medela suggests is another indication that the drain could now be removed based on research by Brunellia et al.13 Figure 2 shows the trace recorded from a patient with secondary pneumothorax who had a persistent air leak. The air leak can be seen to be very gradually decreasing over time with a smaller variability in the degree of air leak. The blue line is initially flat as the device is delivering suction at −2 Kpa consistently. Again as the air leak resolves the amplitude of this line increases indicating that the patient is able to maintain their own pleural pressures and the drain is removed.
Figure 1.
Primary pneumothorax in a never smoker.
Figure 2.
Secondary pneumothorax with slow resolution of air leak.
Figure 3.
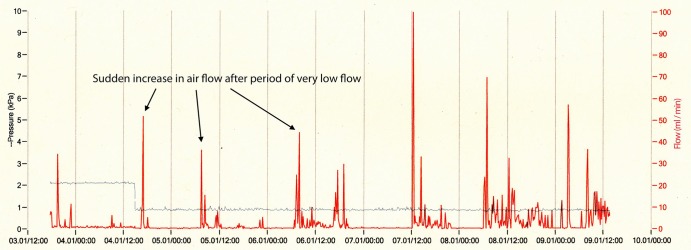
Primary pneuothorax with ongoing air leak.
Figure 3 shows the trace from a patient with primary pneumothorax who had an on-going air leak. Although the leak is relatively small (maximum 100 mL/min) and reads as little as 1 mL for several hours at a time it is persistent. There is a spike in the waveform at intervals of 15–20 h which heralds a period of several hours of increased flow. The pleural pressure wave is of low amplitude. This patient required surgery for on-going air leak.
Clinical outcome
Patient records were checked at 120 days after admission. There were no episodes of recurrence in patients whose pneumothorax had resolved. One patient had a persistent air leak (92 days) which resolved following insertion of endobronchial valve. Two patients had died, one at day 58th day (after the original admission) of respiratory failure and the other at day 24th day due to metastatic laryngeal cancer. Two patients underwent VATS during their inpatient admission and two patients have been referred to surgeons as outpatients. The median hospital stay was 3.5 days (range 1–92 days) and the median time device was in situ was 4 days (range 1–29 days).
Patient experience of using the device
Patient satisfaction with the device was high with most patients reporting no problems with the system during their admission. One patient became very anxious about the device and the data being generated. They kept an independent log of the recordings from the device and tried to alter the settings themselves. Another patient commented that “I prefer the Thopaz system over the bottle due to its precise measurements, flexibility and ease of transport”. It was possible for one patient to be treated within the community, with close follow-up by the principal investigator. Another patient asked for the device to be changed back to an underwater seal as he felt that it was the connection of his drain to the device which had caused it to fall out; it was our opinion that the reason for the drain falling out was not related to the Thopaz device.
Nursing experience of using the device
Nurse satisfaction with the device was high and all who returned the questionnaire rating their overall impression of the device as very good or excellent. All of those surveyed (nine) said that they preferred the Thopaz to the conventional underwater seal. One of the nursing sisters commented that “the Thopaz drains have been very easy to integrate onto the ward. The main benefits I can see are that the patient can mobilise while on suction and the drain does not have to be kept on the floor”.
Physician experience of using the device
The experience of the five senior thoracic physicians with clinical responsibility for the study patients was mixed. There was generally good agreement on the management of each case according to the clinical course and data that the device provided. Four of the five physicians said that they would be happy to change to using this device routinely. Issues raised included concerns about when it was reasonable to remove the drain. Surgical units suggested removal of drains was safe when the flow rate was less than 20 mL for 6 h. This was used as a guide for the first few patients. However, patient 7 had flow which was intermittent, and flow was 0 for up to 12 h before restarting (see figure 3). This patient required video assisted thoracoscopic surgery (VATS). There were no operative findings to specifically explain the intermittent nature of the leak. Following this, the principal investigator advised a more conservative approach, with it being safe to remove a drain when the reading was 0 for 12 h or more. There was concern on the part of one of the senior physicians as to whether this was truly necessary and indeed what the clinical significance of such low flow actually was. One of the physicians felt more confident with clamping the drain (when flow read zero) and following clamping the drain was successfully removed.
Another issue was the fact that the device only allowed the user to look back at 24 h’ worth of data. Although this was not an issue during the working week it was not possible to review weekend data. This has been raised with the company who plan to extend the time period it is possible to view data for on the device to up to 72 h. During the study there was a high turnover of nurses and junior doctors on the ward. The principal investigator carried out training for all staff likely to come into contact with the device on the ward. However, out of hours doctors from other specialties were asked to review patients on the ward who were not familiar with the device, which raised concerns.
Discussion
The 2010 British Thoracic Society (BTS) guidelines suggest that patients with a persistent air leak should be discussed with a thoracic surgeon after 3–5 days8 although the ideal timing for surgical intervention is unclear. Some advocate early surgery at day three9 and suggest that delayed referral and multiple interventions predispose to pleural sepsis, detrimentally affecting the clinical outcome.10 However, there is no good evidence that surgical intervention is necessary for every primary spontaneous pneumothorax before day 5. Although air-leaks will usually seal with conservative management14 this often slower in cases of secondary spontaneous pneumothorax14 and it may be difficult to predict clinically how long this will take.
Following cardiothoracic surgery, for example, postlobectomy, air leaks are common and have considerable cost implications.15 Although there is data to predict the patient groups who are likely to develop persistent air leaks,16 17 management of these patients can be challenging. The use of systems which can assist in the quantification of air leaks is not a new concept. Dernevik suggested that pleural manometry may have a place in management of pneumothorax and recommended connecting drains to a water column manometer to monitor pressure fluctuations in order to predict the presence of an ongoing air leak.18 In surgery, an analogue classification system, the RDC system (Robert David Cerfolio), has been used to grade air leaks post operatively. As its inventor recognises, even though such a system may have some benefits it is still open to intraobserver variation.19
Over recent years digital drainage systems have been used to quantify air leaks and it has been suggested that such devices may provide a useful adjunct to clinical management without the need for provocative clamping.20 Several studies have shown that the use of these devices has benefits in comparison to current practice. The use of digital systems has been associated with chest drains being removed sooner,12 21–23 (by around 2 days) than with in a conventional system.12 Patients also have a shorter inpatient stay21–23 by up to 1.5 days12 with an estimated saving of €750 per patient.12 We feel that the quantification of air leaks digitally may allow more robust prediction of the likely prognosis and allow early discourse between medical and surgical teams where the initial leak is high.
Agreement between surgeons about when to remove the chest drain postoperatively was higher using digital systems than analogue systems.11 Such systems appear to be safe and cost-effective22 and acceptable to both nursing staff24 and other clinicians.24 25 Patient satisfaction with these systems, which allow early mobilisation, is reported to be higher than conventional systems.20 26
Some devices (such as Thopaz by Medela) are able to provide portable suction which allows early mobilisation, reduced portable X-rays, decreased infection risk and better physiotherapy25 and potentially allow patients to be discharged with chest drains in situ on suction as described in two papers. The first is a case series of three postoperative patients in Spain which reported a successful outcome.27 There has only been one case report of this device being used in the community for pneumothorax28 of a Scottish patient with severe idiopathic interstitial lung disease who developed pneumothorax. She was successfully treated for 4 weeks in the community following an initial inpatient stay of 116 days. Eventually her drain was removed without complications.
To the best of our knowledge this is the first study of the Thopaz drainage system in medical patients with pneumothorax. This pilot study has indicated that the Thopaz digital drainage system may have a useful role in pneumothorax management. The system appears to be subjectively acceptable to patients and staff. Training of staff is important when using digital devices which will be unfamiliar to most staff caring for medical patients. This small pilot study did not identify any safety concerns. Retrospective analysis of graphical data appears to indicate that this might be useful to predict earlier chest drain removal and predict a persistent leak requiring surgical intervention. Where flow rates are falling rapidly and pressure wave amplitude is increasing it seems likely that the air leak will resolve without surgical intervention, whereas if the flow rate still has spikes prior to high activity with a low amplitude pressure wave the converse may be true. It is possible that the ability to make such decisions sooner would reduce the length of inpatient stay, although this study is not designed to look at this issue specifically. A criticism of the Thopaz system only allows review of prior 24 h of data on the device. A printout over several days seems to be desirable to help decision-making.
Our study has several limitations. First it had a heterogeneous group of patients. It is unclear as to which patients may benefit from the use of digital air leak monitoring over the conventional system in the management of their pneumothorax. Second, we recognise that this study of only 13 patients is not powered to draw robust conclusions about the benefit of digital devices or to clearly state when suction should be applied or indeed the exact timing of chest drain removal. Third, we recognise that the study was performed at a centre, which had thoracic surgery on site, and that this may influence the referral practice for surgical intervention.
Despite these limitations and potential bias, we believe that the use of digital devices from our preliminary findings should be subjected to a randomised controlled trial to determine if the described data parameters can be used to predict earlier chest drain withdrawal or prompt referral for surgical intervention. Although this has been studied in surgical populations it is our belief that this does not necessarily translate to medical patient groups who have a different mechanism of air leak and in whom healing may not occur by the same mechanism as in postoperative patients. We hope our findings will inform the criteria for inclusion in such a trial as well as providing guidance as to drainage parameters that might be used.
Conclusion
In conclusion, we feel that this small pilot study has shown that digital devices in pneumothorax may be beneficial tool for decision-making. The device studied appeared safe, well tolerated and acceptable to staff and patients. We suggest that a randomised controlled trial is needed to gain a better understanding of how flow and pressure patterns in medical patients with pneumothorax indicates the eventual outcome and how this can guide the clinician in the management of these patients.
Acknowledgments
The authors thank Dr Lucy Schomberg.
Footnotes
Contributors: GT has designed the study, sought ethical approval, gathered data and written the current paper. AD has supported the design and execution of the study and coauthored the paper.
Competing interests: GT has received payment for presenting data at a Medela company training day.
Patient consent: Obtained.
Ethics approval: London-Dulwich NRES Committee Health Research Authority.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gupta D, Hansell A, Nichols T et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschopp JM, Rami-Porta R, Noppen M et al. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637–50. [DOI] [PubMed] [Google Scholar]
- 3.Baumann MH, Strange C. The clinician's perspective on pneumothorax management. Chest 1997;112:822–8. [DOI] [PubMed] [Google Scholar]
- 4.Baumann MH, Strange C, Heffner JE et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590–602. [DOI] [PubMed] [Google Scholar]
- 5.Soulsby T. British Thoracic Society guidelines for the management of spontaneous pneumothorax: do we comply with them and do they work? J Accid Emerg Med 1998;15:317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeoh JH, Ansari S, Campbell IA. Management of spontaneous pneumothorax—a Welsh survey. Postgrad Med J 2000;76:496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry MT, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58(Suppl 2):ii39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65(Suppl 2):ii18–31. [DOI] [PubMed] [Google Scholar]
- 9.Granke K, Fischer CR, Gago O et al. The efficacy and timing of operative intervention for spontaneous pneumothorax. Ann Thorac Surg 1986;42:540–2. [DOI] [PubMed] [Google Scholar]
- 10.Waller DA, McConnell SA, Rajesh PB. Delayed referral reduces the success of video-assisted thoracoscopic surgery for spontaneous pneumothorax. Respir Med 1998;92:246–9. [DOI] [PubMed] [Google Scholar]
- 11.Varela G, Jiménez MF, Novoa NM et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28–31. [DOI] [PubMed] [Google Scholar]
- 12.Pompili C, Brunelli A, Salati M et al. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interact Cardiovasc Thorac Surg 2011;13:490–3. [DOI] [PubMed] [Google Scholar]
- 13.Brunellia A, Salatia M, Pompilia C et al. Regulated tailored suction vs regulated seal: a prospective randomized trial on air leak duration. Eur J Cardiothorac Surg 2013;43:899–904. [DOI] [PubMed] [Google Scholar]
- 14.Chee CB, Abisheganaden J, Yeo JK et al. Persistent air-leak in spontaneous pneumothorax—clinical course and outcome. Respir Med 1998;92:757–61. [DOI] [PubMed] [Google Scholar]
- 15.Varela G, Jiménez MF, Novoa N et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329–33. [DOI] [PubMed] [Google Scholar]
- 16.Cerfolio RJ, Bass CS, Pask AH et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727–30. [DOI] [PubMed] [Google Scholar]
- 17.Abolhoda A, Liu D, Brooks A et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507–10. [DOI] [PubMed] [Google Scholar]
- 18.Dernevik L. [Use pleural drainage optimally! Current systems are quick and easy to manage]. Lakartidningen 1999;96:5227–30. [PubMed] [Google Scholar]
- 19.Cerfolio RJ, Bryant AS, Singh S et al. The management of chest tubes in patients with a pneumothorax and an air leak after pulmonary resection. Chest 2005;128:816–20. [DOI] [PubMed] [Google Scholar]
- 20.Anegg U, Lindenmann J, Matzi V et al. AIRFIX: the first digital postoperative chest tube airflowmetry—a novel method to quantify air leakage after lung resection. Eur J Cardiothorac Surg 2006;29:867–72. [DOI] [PubMed] [Google Scholar]
- 21.Filosso PL, Ruffini E, Solidoro P et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative costs and complications? J Cardiovasc Surg (Torino) 2010;51:429–33. [PubMed] [Google Scholar]
- 22.Brunelli A, Salati M, Refai M et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2009;37:56–60. [DOI] [PubMed] [Google Scholar]
- 23.Cerfolio RJ, Bryant AS. The quantification of postoperative air leaks. Multimed Man Cardiothorac Surg 2009;(409):mmcts.2007.003129. [DOI] [PubMed] [Google Scholar]
- 24.Dernevik L, Belboul A, Radberg G. Initial experience with the world's first digital drainage system. The benefits of recording air leaks with graphic representation. Eur J Cardiothorac Surg 2007;31:209–13. [DOI] [PubMed] [Google Scholar]
- 25.Rathinam S, Bradley A, Cantlin T et al. Thopaz Portable Suction Systems in Thoracic Surgery: an end user assessment and feedback in a tertiary unit. J Cardiothorac Surg 2011;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mier JM, Molins L, Fibla JJ. [The benefits of digital air leak assessment after pulmonary resection: prospective and comparative study]. Cir Esp 2010;87:385–9. [DOI] [PubMed] [Google Scholar]
- 27.Mier JM, Fibla JJ, Molins L. The benefits of digital thoracic drainage system for outpatients undergoing pulmonary resection surgery. Rev Port Pneumol 2011;17:225–7. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins WS, Hall DP, Dhaliwal K et al. The use of a portable digital thoracic suction Thopaz drainage system for the management of a persistent spontaneous secondary pneumothorax in a patient with underlying interstitial lung disease. BMJ Case Rep 2012;2012:pii: bcr0220125881. [DOI] [PMC free article] [PubMed] [Google Scholar]