Abstract
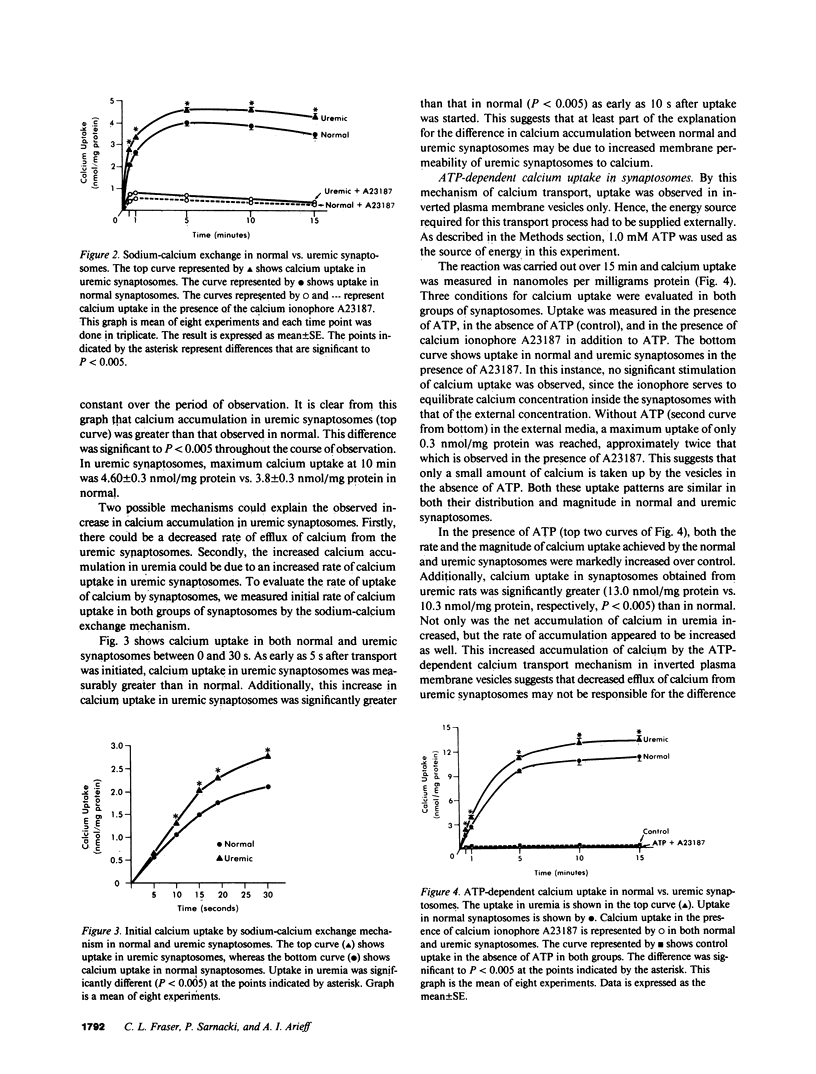
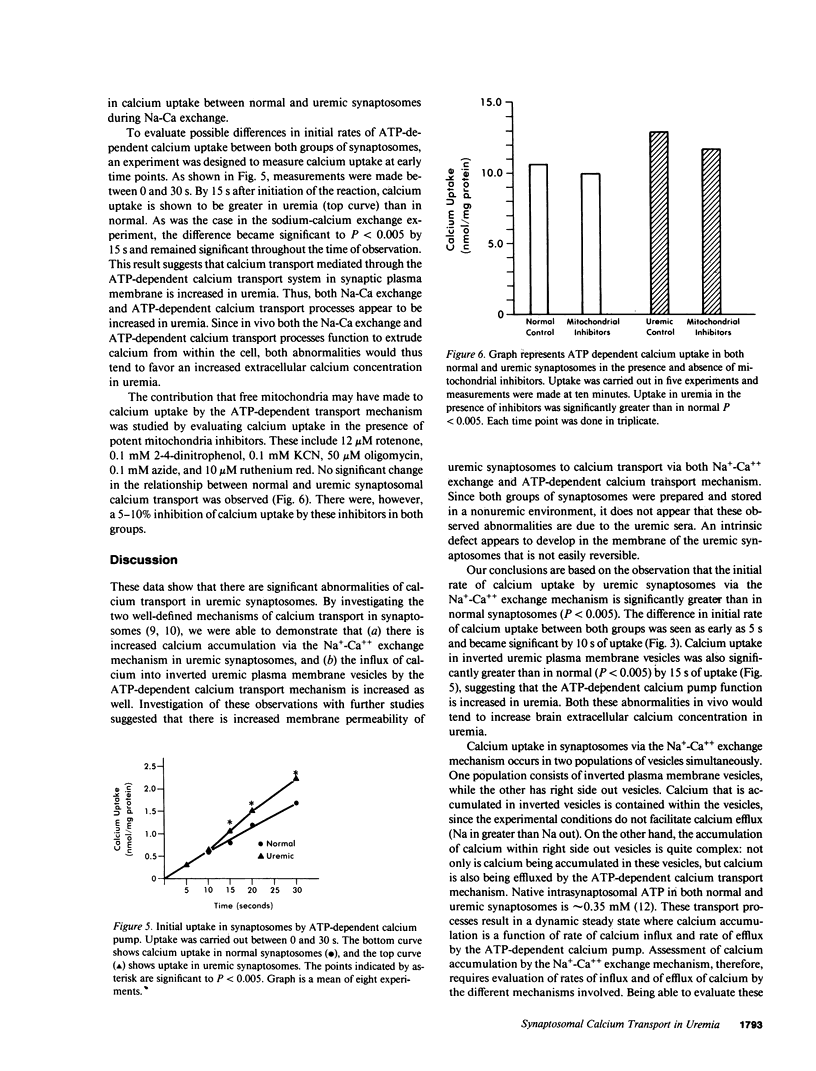
Brain calcium is elevated in patients and laboratory animals with uremia. The significance of this finding is unclear. We evaluated calcium transport in brain of both normal and acutely uremic rats (blood urea nitrogen = 250 mg/dl) by performing studies in synaptosomes from rat brain cerebral cortex. Synaptosomes are vesicular presynaptic nerve endings from brain that contain mitochondria and are metabolically active. Two mechanisms of calcium transport were evaluated using radioactive 45Ca++ as a tracer. Both mechanisms were evaluated in the absence of exogenously administered parathyroid hormone (PTH). We first evaluated Na+-Ca++ exchange in vesicles that were loaded with NaCl in an external media containing 10 microM CaCl2. Both initial rates of calcium transport and equilibrium levels of calcium accumulation in synaptosomes prepared from uremic rats were significantly greater (P less than 0.005) than in normal. To assess calcium efflux, ATP-dependent calcium uptake (1 mM ATP) was studied in inverted plasma membrane vesicles loaded with KCl. In the uremic synaptosomes, a significant increase (P less than 0.005) in ATP-dependent calcium uptake was observed as compared with the normal. These studies show that (a) Calcium accumulation via the Na+-Ca++ exchanger is increased in synaptosomes prepared from uremic rat brain. (b) Calcium influx into inverted plasma membrane vesicles from uremic rats via the ATP-dependent calcium transport mechanism is increased when compared with normal. (c) The increased calcium accumulation in uremia by both Na+-Ca++ exchange and ATP-dependent calcium transport mechanism appears to be a result of increased synaptosomal membrane permeability to calcium. Both these abnormalities of calcium transport in uremia would tend to increase brain extracellular calcium in vivo. The defects observed in uremia do not appear to be readily reversible, and the relationship to PTH is presently unclear. These abnormalities may affect neurotransmission in the uremic state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Nicholls D. G. Calcium transport by intact synaptosomes. Influence of ionophore A23187 on plasma-membrane potential, plasma-membrane calcium transport, mitochondrial membrane potential, respiration, cytosolic free-calcium concentration and noradrenaline release. Eur J Biochem. 1981 Mar 16;115(1):67–73. [PubMed] [Google Scholar]
- Akmal M., Goldstein D. A., Multani S., Massry S. G. Role of uremia, brain calcium, and parathyroid hormone on changes in electroencephalogram in chronic renal failure. Am J Physiol. 1984 May;246(5 Pt 2):F575–F579. doi: 10.1152/ajprenal.1984.246.5.F575. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. S. Enzyme localization in the inner and outer membranes of rat liver mitochondria. Biochem Biophys Res Commun. 1968 Jun 28;31(6):901–907. doi: 10.1016/0006-291x(68)90537-8. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Kendrick N. C., Schweitzer E. S. Calcium buffering in presynaptic nerve terminals. I. Evidence for involvement of a nonmitochondrial ATP-dependent sequestration mechanism. J Gen Physiol. 1978 Jul;72(1):15–41. doi: 10.1085/jgp.72.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Cooper J. D., Lazarowitz V. C., Arieff A. I. Neurodiagnostic abnormalities in patients with acute renal failure. J Clin Invest. 1978 Jun;61(6):1448–1455. doi: 10.1172/JCI109064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho O. P., Carvalho A. P., Carvalho C. A. Effect of monovalent cations on Na+/Ca2+ exchange and ATP-dependent Ca2+ transport in synaptic plasma membranes. J Neurochem. 1983 Sep;41(3):670–676. doi: 10.1111/j.1471-4159.1983.tb04793.x. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Drown C., Rafalowska U., Silver I. A. Synaptosomes from rat brain: morphology, compartmentation, and transmembrane pH and electrical gradients. J Neurochem. 1981 Jun;36(6):2063–2072. doi: 10.1111/j.1471-4159.1981.tb10835.x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Ca pump driven by ATP in squid axons. Nature. 1978 Jul 27;274(5669):390–392. doi: 10.1038/274390a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Characterization of the ATP-dependent calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1977 Jun;69(6):795–813. doi: 10.1085/jgp.69.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan H. M., Mackler B. Electron transport systems of yeast. 3. Preparation and properties of cytochrome oxidase. J Biol Chem. 1966 Apr 25;241(8):1694–1697. [PubMed] [Google Scholar]
- Fraser C. L., Sarnacki P., Arieff A. I. Abnormal sodium transport in synaptosomes from brain of uremic rats. J Clin Invest. 1985 Jun;75(6):2014–2023. doi: 10.1172/JCI111920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Gill D. L., Chueh S. H., Whitlow C. L. Functional importance of the synaptic plasma membrane calcium pump and sodium-calcium exchanger. J Biol Chem. 1984 Sep 10;259(17):10807–10813. [PubMed] [Google Scholar]
- Gill D. L., Grollman E. F., Kohn L. D. Calcium transport mechanisms in membrane vesicles from guinea pig brain synaptosomes. J Biol Chem. 1981 Jan 10;256(1):184–192. [PubMed] [Google Scholar]
- Guisado R., Arieff A. I., Massry S. G., Lazarowitz V., Kerian A. Changes in the electroencephalogram in acute uremia. Effects of parathyroid hormone and brain electrolytes. J Clin Invest. 1975 Apr;55(4):738–745. doi: 10.1172/JCI107984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd J. W., Jones L. R., Mahler H. R., Moore W. J. Isolation and partial characterization of rat brain synaptic plasma membranes. J Neurochem. 1974 Feb;22(2):281–290. doi: 10.1111/j.1471-4159.1974.tb11591.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. K., WHITTAKER V. P. LACTATE DEHYDROGENASE AS A CYTOPLASMIC MARKER IN BRAIN. Biochem J. 1963 Sep;88:404–409. doi: 10.1042/bj0880404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors M. A., Bowden C. L., Ross D. H. Kinetic characterization of Ca2+ transport in synaptic membranes. J Neurochem. 1981 Aug;37(2):381–387. doi: 10.1111/j.1471-4159.1981.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Cheng L., Liang C. T., Sacktor B. Sodium gradient-dependent calcium uptake in renal basolateral membrane vesicles. Effect of parathyroid hormone. J Biol Chem. 1984 Sep 10;259(17):10827–10833. [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Keen P., White T. D. The permeability of pinched-off nerve endings to sodium, potassium and chloride and the effects of gramicidin. J Neurochem. 1971 Jun;18(6):1097–1103. doi: 10.1111/j.1471-4159.1971.tb12038.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis L. D., Pontén U., Siesjö B. K. Arterial acid-base changes in unanaesthetized rats in acute hypoxia. Respir Physiol. 1973 Dec;19(3):312–321. doi: 10.1016/0034-5687(73)90035-2. [DOI] [PubMed] [Google Scholar]
- Mahoney C. A., Arieff A. I. Central and peripheral nervous system effects of chronic renal failure. Kidney Int. 1983 Aug;24(2):170–177. doi: 10.1038/ki.1983.141. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Calcium transport and porton electrochemical potential gradient in mitochondria from guinea-pig cerebral cortex and rat heart. Biochem J. 1978 Mar 15;170(3):511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Pastuszko A., Wilson D. F., Erecińska M., Silver I. A. Effects of in vitro hypoxia and lowered pH on potassium fluxes and energy metabolism in rat brain synaptosomes. J Neurochem. 1981 Jan;36(1):116–123. doi: 10.1111/j.1471-4159.1981.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Rahamimoff H., Spanier R. Sodium-dependent calcium uptake in membrane vesicles derived from rat brain synaptosomes. FEBS Lett. 1979 Aug 1;104(1):111–114. doi: 10.1016/0014-5793(79)81094-7. [DOI] [PubMed] [Google Scholar]
- Requena J., Mullins L. J. Calcium movement in nerve fibres. Q Rev Biophys. 1979 Aug;12(3):371–460. doi: 10.1017/s0033583500005473. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Swanson P. D. Sodium-dependent and calcium-dependent calcium transport by rat brain microsomes. Biochim Biophys Acta. 1981 Oct 20;648(1):13–27. doi: 10.1016/0005-2736(81)90120-6. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]



