Abstract
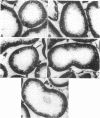
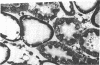
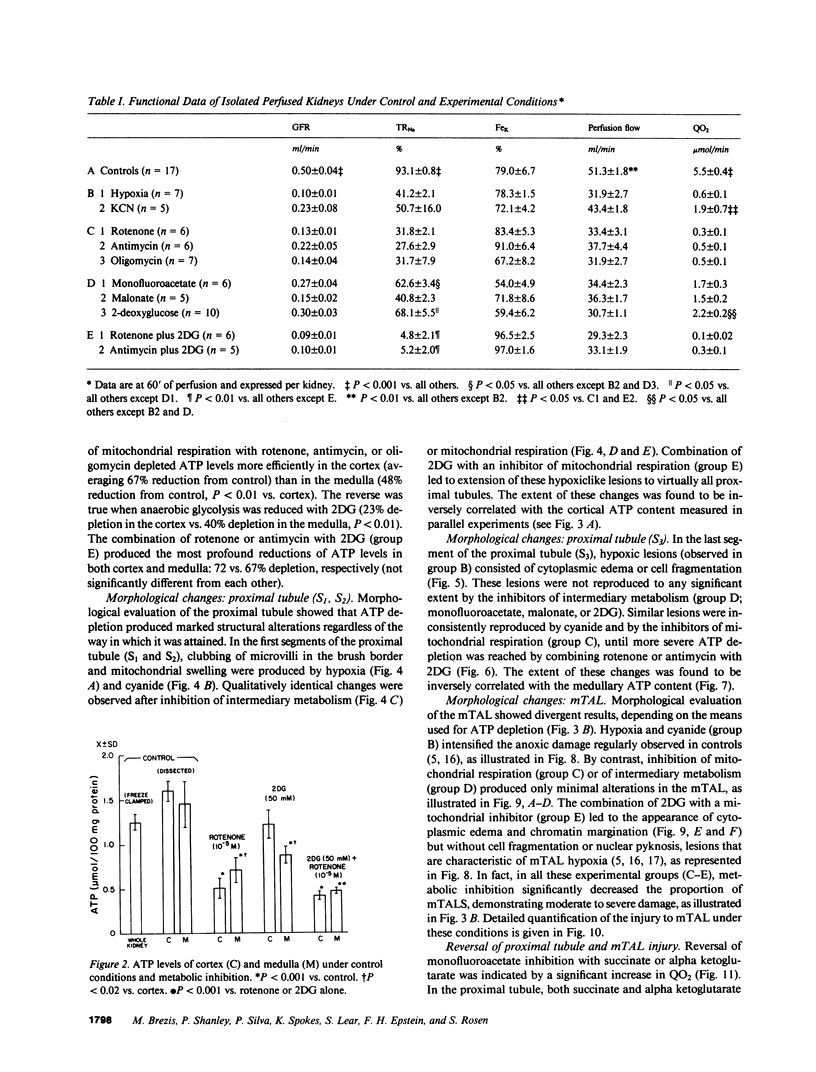
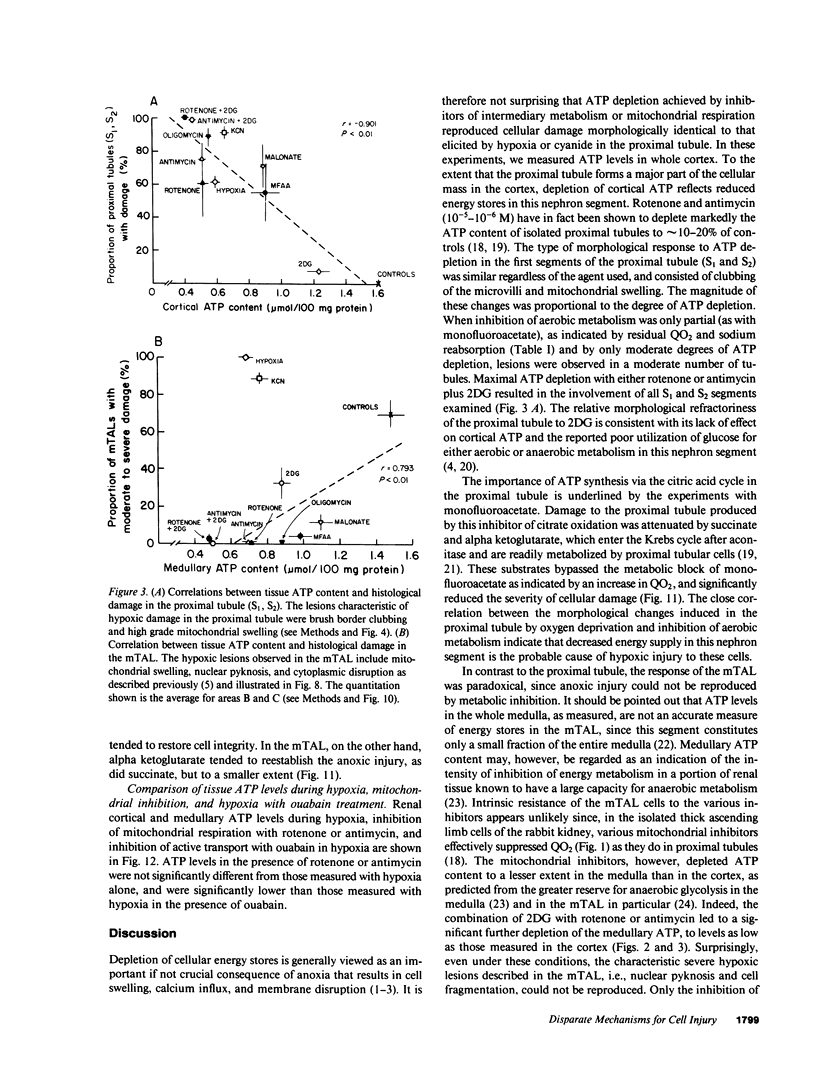
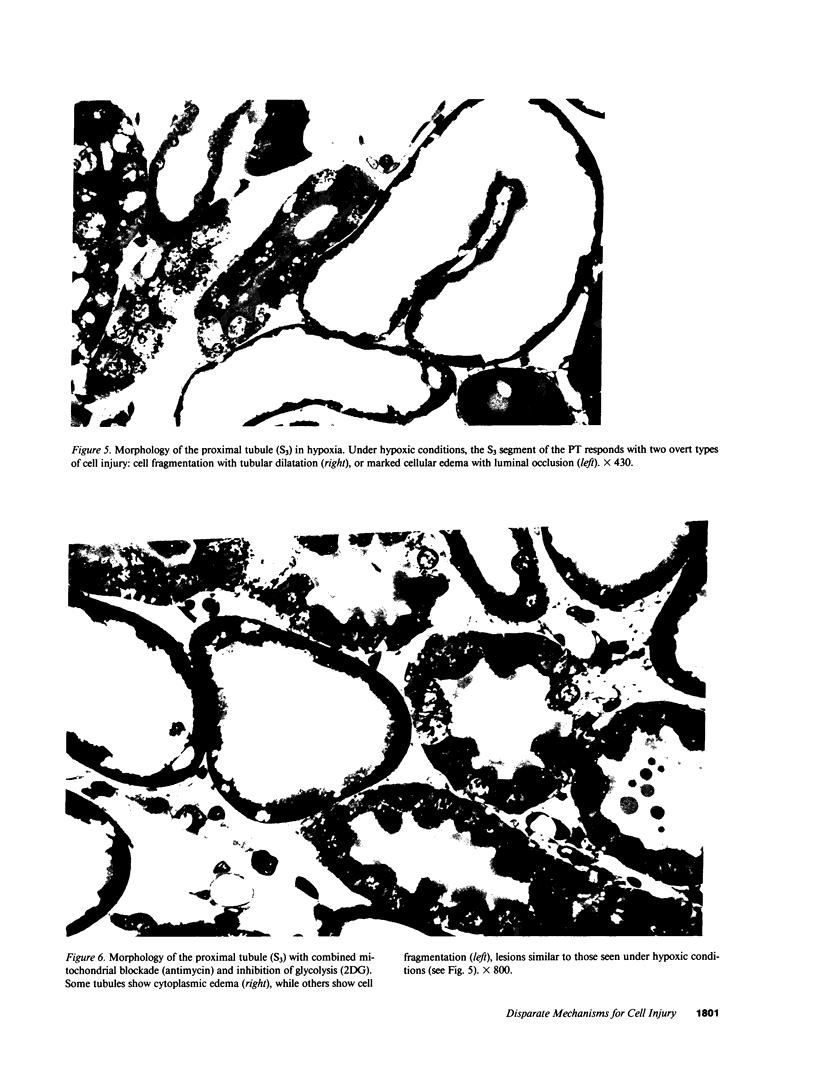
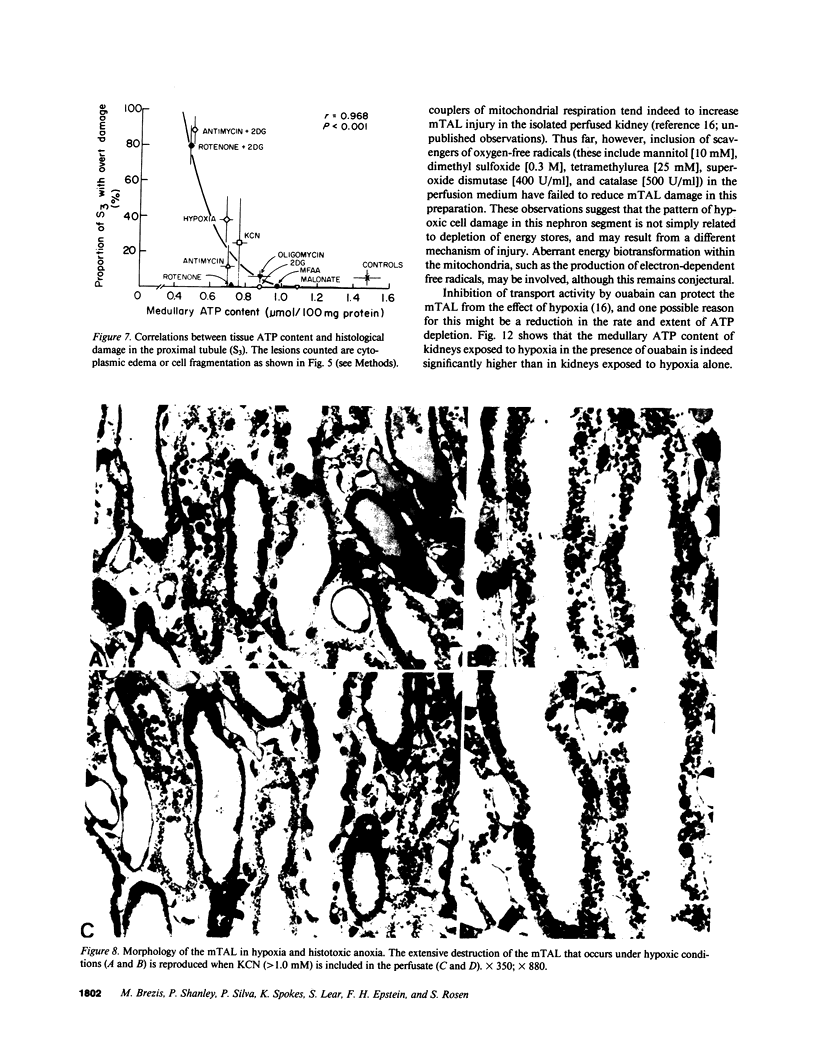
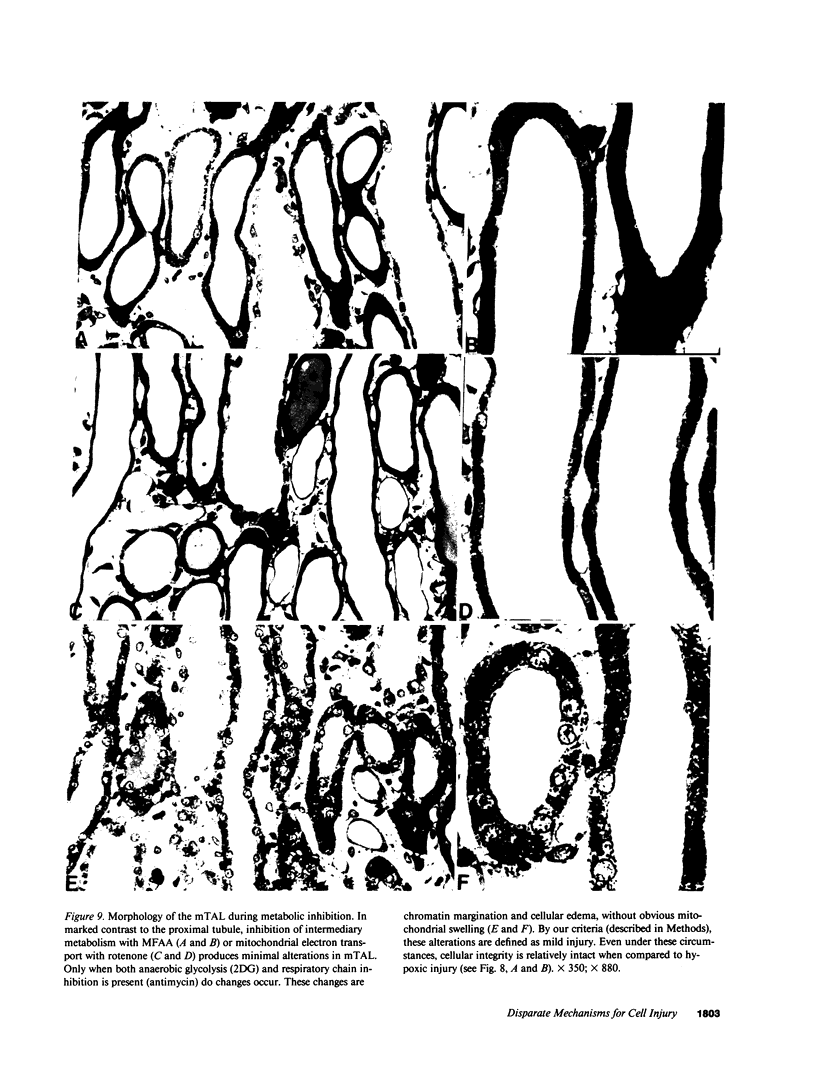
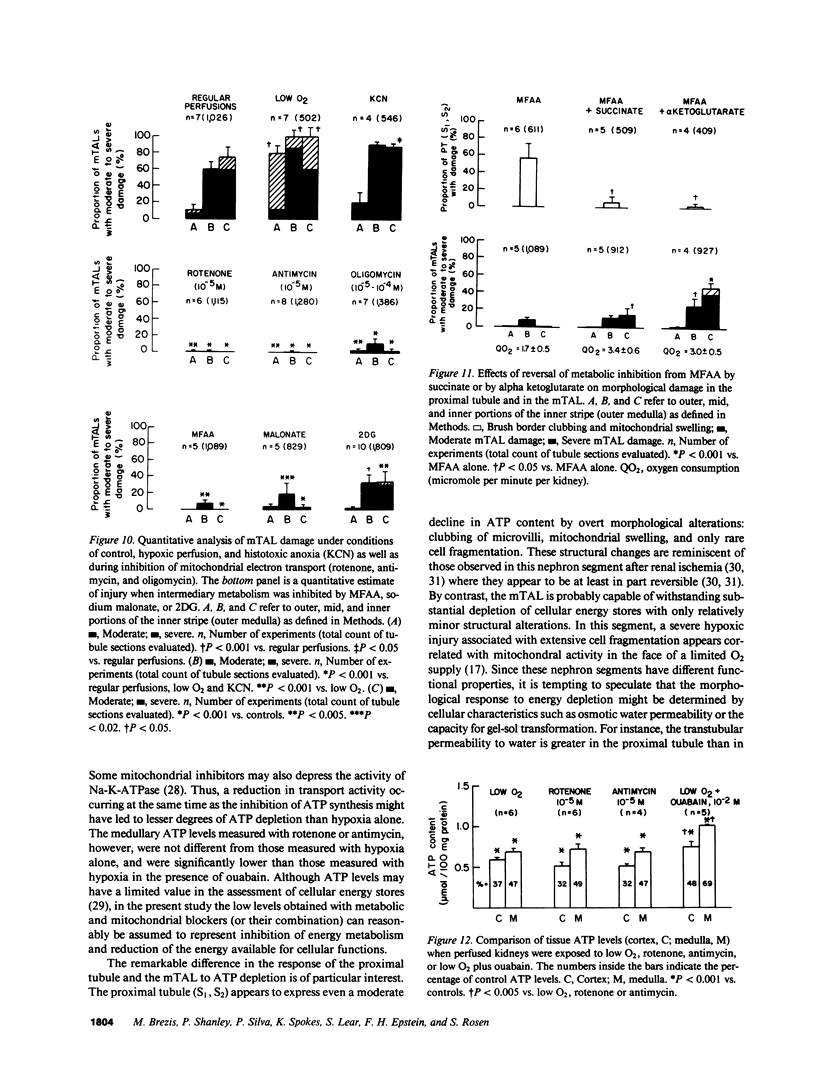
Hypoxic injury was evaluated morphologically in the proximal tubule and in the medullary thick ascending limb of isolated rat kidneys perfused for 90 min without O2 or with various metabolic inhibitors. Inhibition of mitochondrial respiration (with rotenone, antimycin, oligomycin) or of intermediary metabolism (with monofluoroacetate, malonate, 2-deoxyglucose) caused reduction in renal oxygen consumption, renal function, and ATP content comparable with those elicited by oxygen deprivation. Metabolic inhibition produced hypoxiclike injury in the first portions of the proximal tubule, S1 and S2 ("clubbing" of microvilli, mitochondrial swelling), and the extent of damage was correlated with the degree of ATP depletion. In the third portion of the proximal tubule, S3, hypoxiclike damage (cytoplasmic edema or fragmentation) occurred most consistently when both aerobic and anaerobic metabolism were inhibited simultaneously. In the medullary thick ascending limb, none of the metabolic or mitochondrial inhibitors used could reproduce the injury of oxygen deprivation. Thus, the proximal tubule and the thick ascending limb have markedly different responses to cellular energy depletion, suggesting disparate mechanisms for hypoxic injury along the nephron.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Vernon C. A. Reassessment of the role of ATP in vivo. J Theor Biol. 1970 Nov;29(2):301–326. doi: 10.1016/0022-5193(70)90024-x. [DOI] [PubMed] [Google Scholar]
- Berry C. A. Water permeability and pathways in the proximal tubule. Am J Physiol. 1983 Sep;245(3):F279–F294. doi: 10.1152/ajprenal.1983.245.3.F279. [DOI] [PubMed] [Google Scholar]
- Blumenthal S. S., Ware R. A., Kleinman J. G. Sodium gradient-driven transport processes in ATP-depleted renal tubules. Am J Physiol. 1983 May;244(5):C331–C335. doi: 10.1152/ajpcell.1983.244.5.C331. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Renal ischemia: a new perspective. Kidney Int. 1984 Oct;26(4):375–383. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest. 1984 Jan;73(1):182–190. doi: 10.1172/JCI111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Spokes K., Silva P., Epstein F. H. Transport-dependent anoxic cell injury in the isolated perfused rat kidney. Am J Pathol. 1984 Aug;116(2):327–341. [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983 Aug;245(2):R117–R134. doi: 10.1152/ajpregu.1983.245.2.R117. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am J Physiol. 1979 May;236(5):F423–F433. doi: 10.1152/ajprenal.1979.236.5.F423. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Silva P., Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflugers Arch. 1981 Mar;389(3):263–270. doi: 10.1007/BF00584788. [DOI] [PubMed] [Google Scholar]
- Farber J. L. Biology of disease: membrane injury and calcium homeostasis in the pathogenesis of coagulative necrosis. Lab Invest. 1982 Aug;47(2):114–123. [PubMed] [Google Scholar]
- Guder W. G., Ross B. D. Enzyme distribution along the nephron. Kidney Int. 1984 Aug;26(2):101–111. doi: 10.1038/ki.1984.143. [DOI] [PubMed] [Google Scholar]
- Gullans S. R., Brazy P. C., Soltoff S. P., Dennis V. W., Mandel L. J. Metabolic inhibitors: effects on metabolism and transport in the proximal tubule. Am J Physiol. 1982 Aug;243(2):F133–F140. doi: 10.1152/ajprenal.1982.243.2.F133. [DOI] [PubMed] [Google Scholar]
- Györy A. Z., Kinne R. Energy source for transepithelial sodium transport in rat renal proximal tubules. Pflugers Arch. 1971;327(3):234–260. doi: 10.1007/BF00586861. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Seiler M. W. Pathophysiology of Tamm-Horsfall protein. Kidney Int. 1979 Sep;16(3):279–289. doi: 10.1038/ki.1979.130. [DOI] [PubMed] [Google Scholar]
- Jones N. F., Welt L. G. Adenosinetriphosphate in rat renal papilla: effects of vasopressin and of ischemia. Am J Physiol. 1967 Apr;212(4):939–944. doi: 10.1152/ajplegacy.1967.212.4.939. [DOI] [PubMed] [Google Scholar]
- Klein K. L., Wang M. S., Torikai S., Davidson W. D., Kurokawa K. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 1981 Jul;20(1):29–35. doi: 10.1038/ki.1981.100. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Danielson R. A., Saidel G. M., Post R. S. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int. 1977 Nov;12(5):313–323. doi: 10.1038/ki.1977.118. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Carrière S., Cardinal J., Giocondi M. C. Fluidity of brush border and basolateral membranes from human kidney cortex. Am J Physiol. 1983 Aug;245(2):F227–F231. doi: 10.1152/ajprenal.1983.245.2.F227. [DOI] [PubMed] [Google Scholar]
- Needleman P., Passonneau J. V., Lowry O. H. Distribution of glucose and related metabolites in rat kidney. Am J Physiol. 1968 Sep;215(3):655–659. doi: 10.1152/ajplegacy.1968.215.3.655. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Silva P., Hallac R., Spokes K., Epstein F. H. Relationship among gluconeogenesis, QO2, and Na+ transport in the perfused rat kidney. Am J Physiol. 1982 May;242(5):F508–F513. doi: 10.1152/ajprenal.1982.242.5.F508. [DOI] [PubMed] [Google Scholar]
- Trimble M. E., Bowman R. H. Renal Na+ and K+ transport: effects of glucose, palmitate, and alpha-bromopalmitate. Am J Physiol. 1973 Nov;225(5):1057–1062. doi: 10.1152/ajplegacy.1973.225.5.1057. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]