Abstract
A 51-year-old male with history of resected renal cell carcinoma and prior pulmonary embolism presented with tachypnea, tachycardia and progressive dyspnea on exertion. Chest computed tomography revealed bilateral acute pulmonary embolism. Transthoracic echocardiogram showed severe pulmonary hypertension with severe cor-pulmonale and presence of a large worm-like thrombus extending across the foramen ovale, entering both ventricles through the mitral and tricuspid valves. The risks of anti-coagulation, pharmacologic thrombolysis, and surgical thrombectomy, in a hemodynamically stable patient, posed a significant therapeutic dilemma. Ultimately, a collective decision was made to start anticoagulation, without incident. At 1 month follow up, complete resolution of the intracardiac thrombus, pulmonary hypertension, and cor-pulmonale were observed with full clinical recovery of the patient.
Keywords: Thrombus, paradoxical emboli, patent foramen ovale, pulmonary embolism, echocardiography
CASE REPORT
A 51- year- old obese male with right-sided renal cell carcinoma, resected 1.5 year ago, presented to our emergency department with tachypnea, tachycardia and progressive dyspnea on exertion. He had history of pulmonary embolism (PE) approximately one year prior to admission, treated with warfarin, which had been self-discontinued two months prior to admission. Initial heart rate was 97 bpm, blood pressure was 117/88 mm Hg and O2 saturation was 95% on room air. Physical examination was only remarkable for elevated jugular venous pressure. The electrocardiogram was unremarkable. Troponin I was mildly elevated at 0.13 ng/ml (normal <0.034).
Imaging Findings
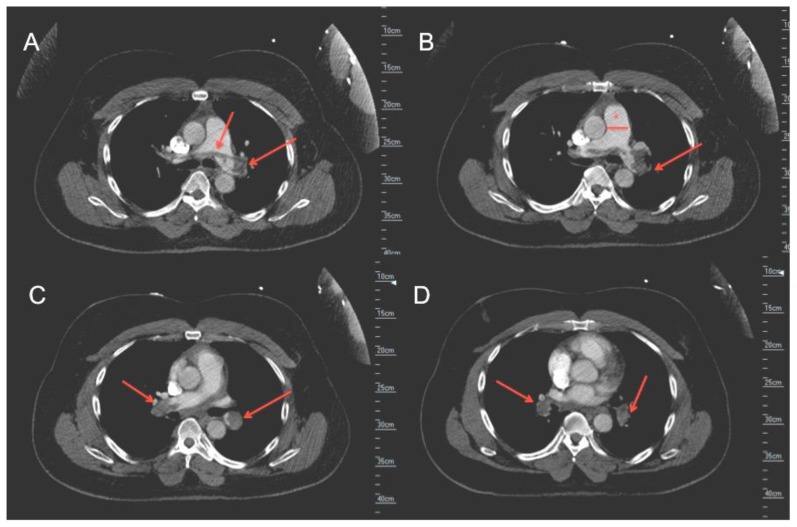
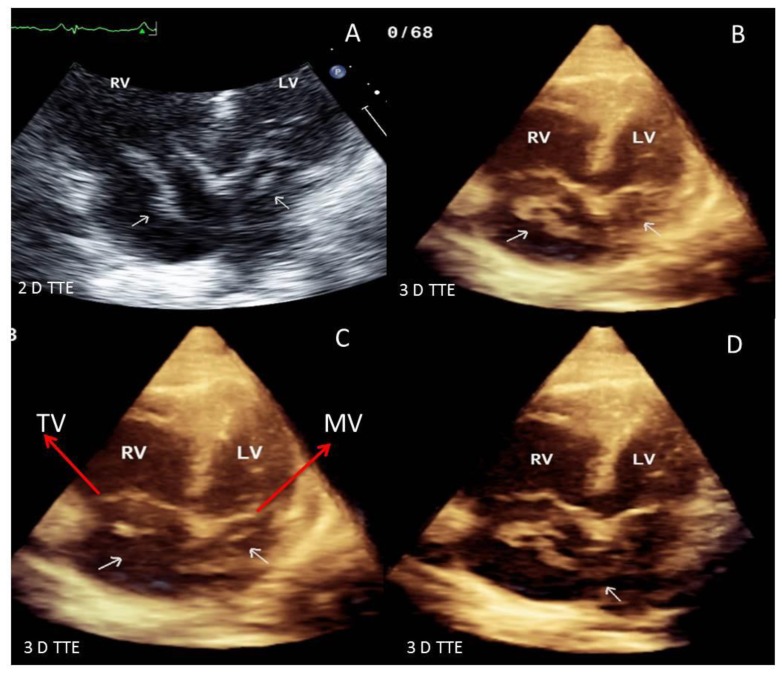
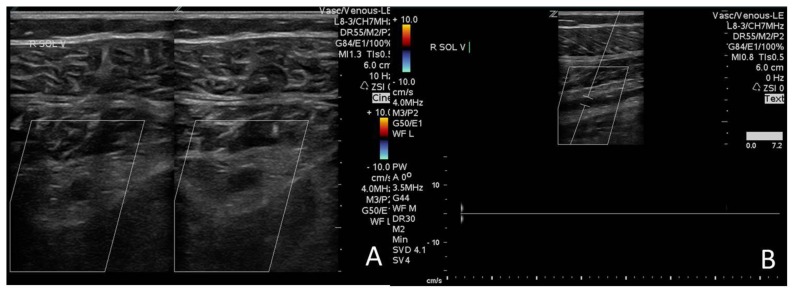
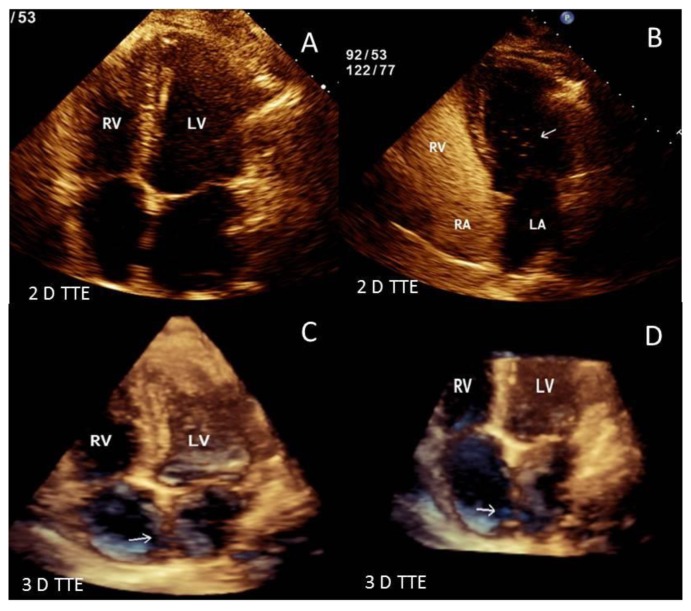
Contrast enhanced chest-computed tomography (CT), timed for the pulmonary artery, showed bilateral PE with saddle thrombus in the main pulmonary artery (Figure 1-A&B). A two- and three-dimensional transthoracic echocardiogram (TTE) performed the next day, revealed a large worm-like thrombus straddling across the foramen ovale (FO) and extending through both mitral and tricuspid valves into the respective ventricles (Figure 2 A–D). Severe right-sided chamber dilatation, severe right ventricular (RV) systolic dysfunction and severe pulmonary hypertension (pulmonary artery systolic pressure of 70–75 mm Hg) were also noted (Figure 3 A–D). Limited visualization of the cardiac chambers due to the non-electrocardiographic gated prior chest CT scan (per PE protocol), precluded assessment of intracardiac thrombus. Although the patient had remote history of renal cell carcinoma with partial right nephrectomy, no evidence of recurrence on chest, abdominal and pelvic CT was identified. A lower extremity venous Doppler study demonstrated a focal, acute, right soleal deep venous thrombosis (DVT) (Figure 4).
Figure 1.
51 year old male with saddle pulmonary embolus. Findings: Axial images from contrast enhanced computed tomography (CT) scan of chest in venous phase showing saddle pulmonary embolus (arrows indicating pulmonary artery embolus at the bifurcation of the main pulmonary artery and into the right and left main pulmonary artery branches, asterisk indicates dilated main pulmonary artery) Technique: GE-High definition CT scanner, 1 mm slice thickness, 120 KVP, 500 mAmp, Iohexol, 60 cc contrast dose).
Figure 2.
51 year old male with straddling thrombus in the patent foramen ovale (PFO) and impending paradoxical embolism. Findings: 2D and 3D-TTE images showing a large serpiginous thrombus (arrows) straddling across the inter-atrial septum and extending through the mitral and tricuspid valves into the left and right ventricles. Technique: IE-33 Philips echocardiography machine, 2 MHz transducer. [Abbreviations: RV: right ventricle; LV: left ventricle; 3 D: three dimensional; 2 D: two dimensional, TTE: Transthoracic echocardiogram: TEE: Trans-esophageal echocardiogram; PFO: Patent foramen ovale]
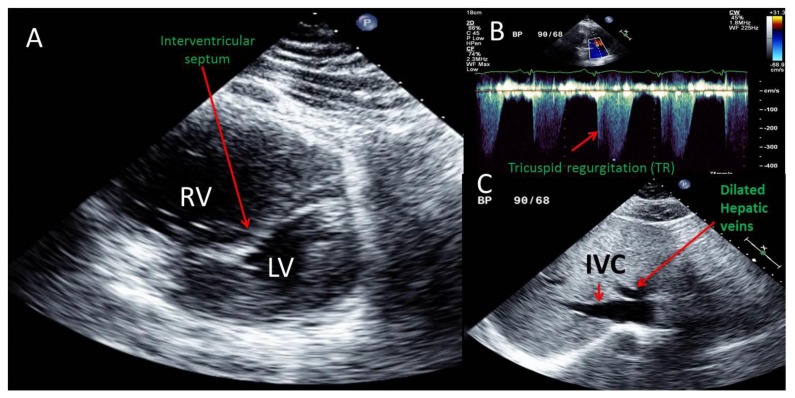
Figure 3.
A 51 year old male with saddle pulmonary embolus and impending paradoxical embolism. Transthoracic echocardiographic (TTE) images are shown. Findings: A. Parasternal short axis view showing a severely dilated right ventricle (RV) severely compressing and deforming the left ventricle (LV) into a D shape due to marked deformity of the interventricular septum during late systolic and throughout diastole indicative of severe right ventricular pressure and volume overload.
B. Continuous wave Doppler of tricuspid regurgitation obtained from an off axis apical view demonstrating tricuspid regurgitation (TR) with a peak velocity 3.7 m/sec which using the simplified Bernoulli equation would correspond to an RV systolic pressure of 54 mmHg. Note that TR peak velocity is higher with every other beat and represent an equivalent of pulsus alternans due to severe RV systolic dysfunction. With the addition of an estimated right atrial pressure of 15–20 mmHg, the estimated pulmonary artery systolic pressure was 70–75 mmHg.
C. Subcostal view illustrating a plethoric inferior vena cava (IVC) with minimal collapse to inspiration indicating an estimated right atrial pressure of 15–20 mmHg. Also, note plethora of the hepatic veins also consistent with significantly elevated right atrial pressure. Technique: IE_33 Philips echocardiography machine, 2 MHz transducer
Figure 4.
A 51 year old male with saddle pulmonary embolism and soleal vein thrombosis. Venous ultrasound examination of the right leg shows A. Cross-sectional views of the right soleal vein. On the left image note the distended soleal vein filled by thrombus. On the right image note the distended soleal being non-compressible due to thrombus. B. Long axis view of the right soleal vein with Doppler showing no flow due to vessel thrombosis. Technique: Philips ultrasound machine. 4 MHz transducer
Management
In hemodynamically stable patients at risk for recurrent fatal pulmonary and systemic embolism, the management strategy is not clearly defined. This is due to the significant risks associated with thrombolytics and surgical thrombectomy compared to anticoagulation. In our patient there was a clear potential for arterial and pulmonary embolization due to the left and right atrial highly mobile thrombus. The significant pulmonary hypertension and severe right ventricular dysfunction raised concerns of the patient’s ability to tolerate repeat pulmonary embolism, risk of thrombolytics, or difficult weaning from cardiopulmonary bypass with thrombectomy. Similarly, no literature or guidelines are available on different anticoagulation strategies other than heparin followed by warfarin in patients with impending paradoxical embolism. After consultative consensus, conservative therapy with heparin 1150 units/hr and warfarin 5 mg po once a day was instituted. Patient remained hemodynamically stable and was discharged home after 5 days with therapeutic INR.
Follow up
At one month, follow up TTE revealed complete resolution of biatrial thrombus with normalization of RV size and function and pulmonary artery pressures. A saline contrast study confirmed the presence of a patent foramen ovale with right to left shunt during Valsalva maneuver (Figure 5 A–D). To date, patient remains clinically stable on warfarin therapy.
Figure 5.
51 year old male with saddle pulmonary embolism and patent foramen ovale with intracardiac thrombus managed with anti-coagulation. TTE images from 1 month follow up. Findings: A. 2D-TTE four chamber view at 1 month follow up showing complete resolution of the large straddling and biatrial thrombus with normalization of the right heart chambers size and function as well as pulmonary artery pressure. B. Follow-up 2D-TTE four chamber view showing a positive saline contrast study with right to left shunt- consistent with a patent foramen ovale (arrow points toward immediate appearance of bubbles from the right atrium through PFO to the left sided cardiac chambers) C,D. Follow-up 3D-TTE four chamber views further defining the inter-atrial septum and bi-atria with no evidence of thrombus which was clearly seen in the images 1 month ago. Technique: IE-33 Philips echocardiography machine, 2 MHz transducer.
DISCUSSION
Etiology & demographics
Paradoxical embolism [1] is characterized by a venous thrombus entering the systemic arterial circulation through a right to left shunt. The shunt can be intra-cardiac: i.e. patent foramen ovale [PFO], atrial septal defect [ASD], ventricular septal defect [VSD]; or extra-cardiac: i.e. pulmonary arteriovenous (AV) fistula. The venous thrombus can originate in lower extremity veins, pelvic veins, in the atria during atrial fibrillation or flutter, in the atria in patients with central venous catheters, in an atrial septal aneurysm or from the edges of a PFO. Impending paradoxical embolism (IPE) is characterized by a thrombus trapped and in transit through a right to left shunt, i.e. a thrombus seen in a PFO but without clinical evidence of systemic arterial emboli.
Conheim [2] reported the first suspected case of paradoxical embolism in 1877. The first case of impending paradoxical embolism was published in 1985 initially documented by transesophageal echocardiography (TEE) and confirmed at surgery [3]. With improvements in both invasive and non-invasive imaging techniques, the diagnosis in living patients has been facilitated [4–6]
Loscalzo et al [7] presented a review of 30 patients with paradoxical embolism. 66% and 43% of their patients respectively had evidence of PE and DVT. DVT was attributed to post-operative state, obesity, myocardial infarction and congestive heart failure. Systemic arterial embolism was peripheral vs. cerebral in 49% vs. 37% of the patients. PFO was found to be the etiology for right to left shunt in 72% of the patients, while in 10%, 10% and 3% had ASD, pulmonary AV fistulas and VSDs respectively. Reports have been published of PFO with a straddling thrombus, some with and some without evidence of systemic embolism. Fauveau E et al [8] published a nice review of 93 such cases and added 4 new cases to this report. The mean age of these patients was 58 ± 15 years and 42% were male. Impending embolism was identified in 56% while 44% indeed had paradoxical embolism.
We searched PubMed, since the publication of this comprehensive review of, from 2009 to 2013 and we found few more cases. A brief review of these cases of ‘impending paradoxical embolus’ through thrombus bestriding the PFO is presented in Table 1[9–20]. Of note, all except 2 had presence of DVT, 2 patients had confirmation of cerebral emboli and one each had renal and coronary emboli.
Table 1.
Clinical presentation and management of identified recent cases (2009–2013) of impending paradoxical embolism indexed in PubMed.
| Reference | Demographics | Hemodynamics | DVT | Treatment | Systemic Embolism | Follow up |
|---|---|---|---|---|---|---|
| Mintz,2013, J Emerg Med[9] | 54 year old AA M | BP: 144/99 mmHg, Pulse: 87/min RR: 19/min 97% on RA |
Yes, Right LE |
|
Yes, renal infarct TPA was given, patient discharged on Coumadin | No events |
| Faustino, 2012, BMJ Case Rep[10] | 42 year old F | BP: 100/69 mm Hg Pulse: 110/min 95% on RA PASP: 83 mm Hg |
Yes, Right LE |
|
Cerebral embolism (multiple small strokes on MRI) | Not given Stable at discharge |
| Forkman,2012, Clin Res Cardiol[11] | 77 year old F | BP: 150/80 mm Hg Pulse: 105/min RR: 25/min TTE RVSP: 50 mmHg |
Yes, Left LE |
|
Not documented | Stable at discharge No follow up given |
| Turfan, 2012, Heart Lung Circ[12] | 72 year old F | BP: 60/40 mm Hg 80% on RA |
Yes, B/L LE |
|
Not documented | Stable at discharge No follow up given |
| Chow, 2012, J Clinic Ultrasound[13] | 35 year old F | Not given | History of DVT |
|
Left middle cerebral artery stroke | Stable at discharge No events at 4 months follow up |
| Shah, 2011, J Card Surg[14] | 78 year old M | 88% on RA Pulse: 103/min Tachypnea |
Yes, Right LE |
|
Not documented | Stable at discharge No follow up given |
| Citro, 2010, J Cardiovasc Med[15] | 69 year old F | BP: 10/70 mm Hg Pulse: 110/min PASP: 81 mm Hg |
None |
|
Not documented | Stable at discharge No events at 1 year follow up |
| Fontanella, 2010, Kardiol[16] | 81 year old F | BP: 130/80 mm Hg Pulse: 100/min 86% on RA PaO2: 57 mm Hg RVSP: 50 mm Hg |
Yes, Right LE |
|
Not documented | Stable at discharge No events at 1 year follow up |
| Ruiz-Bailen, 2009, Interact Cardiovasc Thorac Surg[17] | 81 year old F | BP: 78/42 mm Hg Pulse: 115/min Tachypnea PASP: 90 mm Hg |
Yes, Right LE |
|
Not documented | Stable at discharge No events at 1 month follow up |
| Younker, 2009, Anesthesiology[18] | 46 year old M | BP: 110/70 to 85/50 mm Hg Pulse: 85 - 45/min 100%-88% on RA Followed by cardiac arrest during anesthesia induction |
Yes, Left LE |
|
Not documented | Stable at discharge No follow up given |
| Mascarenhas, 2009, J Am Coll Cardiol[19] | 66 year old F | Not documented | Not documented |
|
Not documented | Not documented |
| Dietz, 2013 J of Cardiol Cases [20] | 54 years old M | Not documented | Yes, Left LE |
|
Yes, Left anterior descending coronary artery embolism leading to acute STEMI | Stable at 3 weeks follow up. |
Abbreviations: AA: African American: DVT: deep venous thrombosis, LE: Lower extremity; PFO: Patent foramen ovale; IPE: Impending paradoxical embolism; MRI: Magnetic resonance imaging; M: Male; F: Female; BP: Blood pressure; RR: respiratory rate; TTE: transthoracic echocardiogram; RVSP: right ventricular systolic pressure; IVC: Inferior vena cava; PASP: Pulmonary artery systolic pressure; RA: Room air; B/L: Bilateral; UF: unfractionated; RA: right atrium; LA: left atrium; PA: pulmonary artery; IVC: inferior vena cava filter; STEMI: ST elevation Myocardial infarction
Clinical & imaging findings
In patients presenting with DVT or PE in the presence of right to left shunt, it is important to have a high index of suspicion for silent left-sided emboli. Elevated right-sided pressures or pulmonary hypertension (PH) in the above setting greatly increases the risk of paradoxical systemic emboli. PH is commonly seen in patients with chronic obstructive airway disease (COPD) and in patients with obstructive sleep apnea (OSA). An unusual case of elevated right-sided pressure from tricuspid stenosis due to thrombus obstructing the tricuspid valve has also been reported [21]. Temporary increase in right-sided pressures with cough, vomiting, defecation or shivering in a patient with straddling thrombus in the PFO, increase the likelihood of dislodgement.
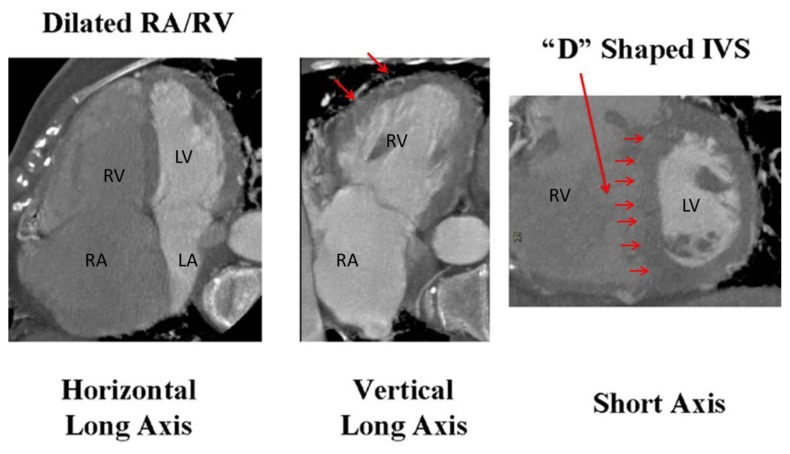
Non-invasive cardiovascular imaging helps identify the presence of PFO, intra-cardiac thrombus, PE, DVT as well as, presence of PH. TTE with Doppler allows us to estimate pulmonary arterial pressures (PAP). In the absence of pulmonary flow obstruction, the tricuspid regurgitation (TR) peak velocity is linearly correlated with increased in systolic PAP (SPAP) against the gold standard, invasive cardiac catheterization [22]. Similarly, peak diastolic and end-diastolic velocities of pulmonary regurgitation (PR) correlate with mean PAP (MPAP) and pulmonary arterial end-diastolic pressure (PA-EDP) respectively[22]. Pulmonary vascular resistance (PVR) is estimated by dividing TR peak velocity by the time velocity integral of RV outflow tract [22]. According to European Society of Cardiology guidelines, PH is likely present in the presence of TR velocity >3.4 m/sec and SPAP >50 mm Hg with and without additional signs of PH [23]. The other signs of PH include: enlarged right sided cardiac chambers, increased interventricular septal thickness, reduced global RV systolic function and flattening of the interventricular septum during late systole and early diastole, but can persists throughout diastole when volume overload from secondary moderate or worse tricuspid and pulmonary regurgitation are present [24]. On contrast-enhanced chest CT, the signs of PH include: Enlargement of main pulmonary artery, RV hypertrophy and dilatation, RV dysfunction (identified if gated cardiac scan is performed) and bronchial artery dilatation. Cardiac CT and magnetic resonance imaging (CMR) also help evaluate for presence of anomalous pulmonary venous return, intracardiac shunt or pulmonary AV malformation [25]. CT also helps to diagnose pulmonary causes of pulmonary hypertension. [26] [Figure 6]. Doppler ultrasonography of peripheral veins helps identify presence of venous thrombus and doppler arterial examination can help localize arterial embolism.
Figure 6.
Contrast enhanced Computed tomography (CT) scan (ECG gated) indicating signs of pulmonary hypertension. A) 4 chamber view showing dilated RA and RV. B) 2 chamber view indicating right ventricular hypertrophy (arrows), C) Short axis view at the mid ventricular level showing markedly enlarged right ventricle and D shaped septum indicating right ventricular pressure overload. Adapted with permission from [26] [Abbreviations: RV: right ventricle; LV: left ventricle; RA: right atrium; LA: Left atrium; IVS: Interventricular septum
Treatment & prognosis
General consensus is lacking regarding optimal treatment of impending paradoxical embolism, particularly in a hemodynamically stable patient [27]. In patients with acute pulmonary embolism with severe hypoxia, significant right heart dysfunction, or hemodynamic instability, thrombolytics, extracorporeal membrane oxygenation (ECMO), or surgical thrombectomy [5] can be considered. Similarly, thrombolytics or thrombectomy can be entertained when there is evidence of peripheral arterial embolism.
In stable patients with impending paradoxical embolism, however, treatment options include: IV and oral anticoagulation vs. anticoagulation with intravenous (IV) thrombolytics [28–31] vs. thrombectomy/embolectomy [32–35] with or without PFO/ASD closure (surgical or percutaneous). Although the risk exists for thrombus fragmentation and embolization when IV thrombolytics are employed, the true incidence of this complication is unknown.
In Fauveau’s [8] case series, inclusive of patients with impending systemic embolism, 55 patients were treated surgically, 21 with heparin, and 11 with thrombolysis. Their mortality rates were 13%, 14%, and 36% respectively. However, a bias was noted towards treatment with heparin in older patients and in patients with stroke, while patients who received thrombolytics were more likely to have hemodynamic compromise. Bonvinie et al [36], reported the mortality rate of thrombolytic treatment between 16% and 22%.
In the recent systematic review of 154 patients by Myers et al [37], the 30-day mortality rate of impending paradoxical embolism was found to be 18.4%. On multivariate analysis, none of the treatment choices (surgery, thrombolytics or anticoagulation) had a significant survival advantage. Surgically treated patients were found to have a nonsignificant trend towards decreased mortality (OR, 0.65, p=0.65) while patients treated with thrombolytics had a nonsignificant trend towards increased mortality (OR, 1.62, p =0.47), but had fewer systemic embolic events (OR 0.13, p=0.02). The combined endpoint of systemic emboli and death was significantly lower with surgery (OR, 0.26, p=0.001). It appears that when immediate surgery is not available to a hemodynamically unstable patient, thrombolysis may be a viable option [38].
In our review of recent 13 cases (Table 1) of impending systemic embolism, four of which eventually had systemic emboli, 4 received thrombolytics, 6 underwent surgical thrombectomy, and 3 received anticoagulation.
Differential Diagnoses
The differential diagnosis of impending systemic embolism in patients with DVT or PE includes cardiomyopathy with right and left sided mural thrombi, atrial fibrillation with intracardiac thrombi, right ventricular myocardial infarction with a right ventricular thrombus, central venous catheter with associated atrial thrombi, left or right -sided non-infective (Libman-Sacks or thrombotic vegetations) or infective endocarditis, atrial myxoma, or papillary fibroelastoma. In the presence of systemic embolism in a patient with venous thrombosis, mechanism of paradoxical embolism, via intra-cardiac or intrapulmonary shunting needs to be determined. This can be accomplished by either non-invasive (contrast echocardiography, CT or MRI) or invasive (cardiac catheterization) means. Contrast agents and provocative maneuvers i.e. Valsalva or cough can improve the diagnostic yield for diagnosing shunt with both non-invasive imaging modalities (Contrast 2D with or without 3 D transthoracic or trans-esophageal echocardiography or contrast cardiac CT or cardiac magnetic resonance imaging with provocative maneuvers) or invasive imaging modalities (e.g. invasive right heart catheterization with oxymetries). [39,40].
Future studies required in this area
To our knowledge, this is the first case of large pulmonary emboli complicated by severe cor-pulmonale with a large thrombus straddling the PFO and extending into both ventricles in addition to a saddle pulmonary emboli. The success of anticoagulation alone in our case also supports a conservative approach to similar patients. However, the only definitive way to establish the ideal therapy is to perform an appropriately stratified, randomized controlled trial on patients with IPE. Such a trial would obviously have to be a multi-center study, due to the small incidence of IPE.
TEACHING POINT
Acute pulmonary embolism with impending paradoxical systemic embolism is an entity that may be recognized more frequently due to the availability of echocardiography in most of clinical centers. Despite numerous case reports, optimal management of impending paradoxical emboli is unknown. Anticoagulation therapy might be an effective and safe therapeutic alternative in patients with impending paradoxical systemic embolism.
Table 2.
Summary table for impending paradoxical embolism
| Etiology | Patent foramen ovale, atrial septal defect, ventricular septal defect, pulmonary arterio-venous malformation |
| Incidence | Rare, not completely known rate. |
| Gender ratio | 1:1.5 (M:F) [10] |
| Age predilection | 58±15 years (45–75 years) [10], |
| Risk factors | Postoperative state, obesity, hypercoagulable conditions including malignancy, myocardial infarction, prolonged immobilization, congestive heart failure. |
| Treatment | Anticoagulation with heparin or Bivalirudin, intravenous thrombolytics, surgical thrombectomy and embolectomy along with surgical closure of right of left shunt e.g. patent foramen ovale or atrial septal defect) followed by oral anticoagulants |
| Prognosis | Depends on the presence of hemodynamic collapse. 18% mortality was noted by Myers et al[2]. Amongst survivors the following can happen: systemic embolism, cardiac arrest, cardiogenic shock, right heart failure, acute myocardial ischemia or infarct, seizures, stroke and peripheral vascular ischemia. |
| Findings on imaging |
|
Abbreviations: TTE: Transthoracic echocardiogram; TEE: Transesophageal echocardiogram; CT: Computed tomography; PFO: patent foramen ovale; ASD: Atrial septal defect: VSD: Ventricular septal defect: CMR: Cardiac magnetic resonance.
Table 3.
Differential diagnosis table of intracardiac masses with simultaneous venous thrombosis
| Differential Diagnosis | X-Ray | Ultrasound (US) | Echocardiogram | Computed Tomography (CT) | Magnetic Resoance Imaging (MRI) |
|---|---|---|---|---|---|
| Paradoxical embolism | Enlarged right sided cardiac chambers | DVT and/or arterial thrombosis |
|
|
|
| Cardiomyopathy with right and left heart thrombi |
|
|
|
|
|
| Atrial fibrillation with intracardiac thrombi |
|
|
|
|
|
| Cardiac tumors including biatrial myxomas, papillary fibroelastoma and metastatic cardiac tumor | Cardiac chambers usually normal, may appear dilated
|
|
|
|
|
| MI with LV thrombus and simultaneous DVT | Cardiac chambers may appear dilated
|
|
|
|
|
| Right heart thrombus associated with central lines, PICC lines, Swan Ganz catheters and pacemakers/defibrillators in patient who has atrial septal defect or patent foramen ovale | Cardiac chambers may appear dilated. Leads/catheters visualized on x ray |
|
|
|
|
| Left sided non-infective and infective endocarditis |
|
|
|
|
|
Abbreviations: MI: Myocardial infarction; DVT: Deep venous thrombosis; TTE: Transthoracic echocardiogram; TEE: Trans-esophageal echocardiogram; SSFP: Steady State Free precession images; US: Ultrasound: CT: Computed tomography; MRI: Magnetic resonance Imaging: PFO: Patent foramen ovale; ASD: Atrial septal defect; VSD: Ventricular septal defect.
ABBREVIATIONS
- ASD
Atrial Septal Defect
- AV
Arterio-venous
- Bnp
Brain Natriuretic Peptide
- CMP
Cardiomyopathy
- CT
Computed Tomography
- DVT
Deep Venous Thrombosis
- ECMO
Extracorporeal Membrane Oxygenation
- FO
Foraman Ovale
- IPE
Impending Paradoxial Embolism
- IVS
Interventricular Septum
- LA
Left Atrium
- LV
Left Ventricle
- MI
Myocardial Infarction
- MRI
Magnetic Resonance Imaging
- PFO
Patent Foramen Ovale
- RA
Right Atium
- RV
Right Ventricle
- TEE
Trans-esophageal Echocardgiogram
- TTE
Trans Thoracic Echocardiogram
- VSD
Ventricular Septal Defect
REFERENCES
- 1.Corrin B. Paradoxical Embolism. Br Heart j. 164:26–549. doi: 10.1136/hrt.26.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connheim J. Vorlesung uber Allgemeine Pathologie. Vol. 1. Berlin: Hirschwald; 1877. Thrombose und Embolie; p. 134. [Google Scholar]
- 3.Nellessen U, Daniel WG, Matheis G, Oelert H, Depping K, Lichtlen PR. Impending paradoxical embolism from atrial thrombus: correct diagnosis by transesophageal echocardiography and prevention by surgery. J Am Coll Cardiol. 1985;5(4):1002–4. doi: 10.1016/s0735-1097(85)80449-6. [DOI] [PubMed] [Google Scholar]
- 4.Caes FL, Van Belleghem YV, Missault LH, et al. Surgical treatment of impending paradoxical embolism through patient foramen ovale. Ann Thorac Surg. 1995;59:1559. doi: 10.1016/0003-4975(94)00892-b. [DOI] [PubMed] [Google Scholar]
- 5.Falk V, Walther T, Krankenberg H, Mohr FW. Trapped thrombus in a patent foramen ovale. Thorac Cadiovasc Surg. 1997:45–90. doi: 10.1055/s-2007-1013695. [DOI] [PubMed] [Google Scholar]
- 6.Meacham RR, 3rd, Headley AS, Bronze MS, et al. Impending paradoxical embolism. Arch Intern Med. 1998;158:438. doi: 10.1001/archinte.158.5.438. [DOI] [PubMed] [Google Scholar]
- 7.Loscalzo J. Paradoxical embolism: clinical presentation, diagnostic strategies, and therapeutic options. Am Heart J. 1986 Jul;112(1):141–5. doi: 10.1016/0002-8703(86)90692-7. [DOI] [PubMed] [Google Scholar]
- 8.Fauveau E, Cohen A, Bonnet N, Gacem K, Lardoux H. Surgical or medical treatment for thrombus straddling the patent foramen ovale: impending paradoxical embolism? Report of four clinical cases and literature review. Arch Cardiovasc Dis. 2008 Oct;101(10):637–44. doi: 10.1016/j.acvd.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Mintz R, Brody K. Impending Paradoxical Embolism: A Case Report. J Emerg Med. 2013 Feb 6; doi: 10.1016/j.jemermed.2012.11.046. pii: S0736-4679(12)01570-3. [DOI] [PubMed] [Google Scholar]
- 10.Faustino A, Costa G, Providência R, Paiva L. Impending paradoxical embolism with a thrombus crossing a patent foramen ovale. BMJ Case Rep. 2012 Nov;27:2012. doi: 10.1136/bcr-2012-006662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forkmann M, Tugtekin SM, Strasser R, Schrötter H. Impending paradoxical thromboembolism: thrombus caught in transit. A case report. Clin Res Cardiol. 2012 Jun;101(6):497–8. doi: 10.1007/s00392-012-0418-4. [DOI] [PubMed] [Google Scholar]
- 12.Turfan M, Vatankulu MA, Murat SN, Oksuz F, Duran M, Ornek E. Thrombolytic treatment of simultaneous pulmonary embolism and impending paradoxical embolism through a patent foramen ovale: a different thrombolytic regimen. Heart Lung Circ. 2012 Apr;21(4):225–8. doi: 10.1016/j.hlc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Chow V, Wang W, Wilson M, Yiannikas J. Thrombus in transit within a patent foramen ovale: an argument for consideration of prophylactic closure? J Clin Ultrasound. 2012 Feb;40(2):115–8. doi: 10.1002/jcu.20820. [DOI] [PubMed] [Google Scholar]
- 14.Shah DK, Ritter MJ, Sinak LJ, Miller JA, Sundt TM., 3rd Paradoxical embolus caught in transit through a patent foramen ovale. J Card Surg. 2011 Mar;26(2):151–3. doi: 10.1111/j.1540-8191.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 15.Citro R, Panza A, Bottiglieri G, et al. Surgical treatment of impending paradoxical embolization associated with pulmonary embolism in a patient with heterozygosis of factor V Leiden. J Cardiovasc Med (Hagerstown) 2010 Jul 15; doi: 10.2459/JCM.0b013e32833893f9. [DOI] [PubMed] [Google Scholar]
- 16.Fontanella B, Vizzardi E, Bordonali T, D’Aloia A, Chiari E, Dei Cas L. Pulmonary embolism complicated by impending paradoxical embolism - a case report and a review of literature. Kardiol Pol. 2010 Mar;68(3):314–6. [PubMed] [Google Scholar]
- 17.Ruiz-Bailen M, Ramos-Cuadra JA, Machado-Casas J, Rucabado-Aguilar L. Successful administration of alteplase in a venous thromboembolism crossing through a patent foramen ovale. Interact CardioVasc Thorac Surg. 2009;9:712–714. doi: 10.1510/icvts.2009.209064. [DOI] [PubMed] [Google Scholar]
- 18.Younker D, Reeves-Viets JL, Gopinath SP, Tsai PI, Moon TL, Cuzick LM. Cardiac arrest upon induction of general anesthesia: transesophageal echocardiography-assisted diagnosis of impending paradoxical embolus. Anesthesiology. 2009 Sep;111(3):679–80. doi: 10.1097/ALN.0b013e3181b27a85. [DOI] [PubMed] [Google Scholar]
- 19.Mascarenhas V, Kalyanasundaram A, Nassef LA, Lico S, Qureshi A. Simultaneous massive pulmonary embolism and impending paradoxical embolism through a patent foramen ovale. J Am Coll Cardiol. 2009 Apr 14;53(15):1338. doi: 10.1016/j.jacc.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 20.Dietz DM, Cleveland JD, Chewning KG, Dent JM, Kern JA, Keeley EC. Impending paradoxical embolism presenting as myocardial infarction. J Cardiol Cases. 2013 May 1;7(5):e145–e148. doi: 10.1016/j.jccase.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicol C, Danov V, Struck E. Paradoxical embolism in the presence of right-to-left shunt due to tricuspid occlusion. Ann Thorac Surg. 1995 Oct;60(4):1111–2. doi: 10.1016/0003-4975(95)00473-x. [DOI] [PubMed] [Google Scholar]
- 22.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–7. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 23.Galiè N, Hoeper MM, Humbert M, et al. ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong WF, Ryan T. Feigenbaum’s Echocardiography. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 25.King MA, Ysrael M, Bergin CJ. Chronic thromboembolic pulmonary hypertension: CT findings. AJR Am J Roentgenol. 1998;170:955–960. doi: 10.2214/ajr.170.4.9530043. [DOI] [PubMed] [Google Scholar]
- 26.Choi TY, Oudiz RJ. Cardiac CT imaging Diagnosis of Cardiovascular Disease. 2nd ed. Springer; 2010. Assessment of Pulmonary vascular disease; pp. 223–232. [Google Scholar]
- 27.Willis SL, Welch TS, Scally JP, et al. Impending paradoxical embolism presenting as a pulmonary embolism, transient ischemic attack, and myocardial infarction. Chest. 2007;132(4):1358–1360. doi: 10.1378/chest.07-0100. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves M, Maloney D, Gribbin B, Westaby S. Impending paradoxical embolism: a case report and literature review. Eur Heart J. 1994;15(9):1284–5. doi: 10.1093/oxfordjournals.eurheartj.a060667. [DOI] [PubMed] [Google Scholar]
- 29.Daley L, Deitcher SR, Bojar RM, Pandian NG. Urokinase resolution of impending paradoxic embolus in transit: evaluation by multiplane transesophageal echocardiography. Am Heart J. 1994;128(6 Pt 1):1239–41. doi: 10.1016/0002-8703(94)90758-7. [DOI] [PubMed] [Google Scholar]
- 30.Sardesai SH, Marshall RJ, Mourant AJ. Paradoxical systemic embolisation through a patent foramen ovale. Lancet. 1989;1(8640):732–3. doi: 10.1016/s0140-6736(89)92253-8. [DOI] [PubMed] [Google Scholar]
- 31.Zerio C, Canterin FA, Pavan D, Nicolosi GL. Spontaneous closure of a patent foramen ovale and disappearance of impending paradoxical embolism after fibrinolytic therapy in the course of massive pulmonary embolism. Am J Cardiol. 1995;76(5):422–4. doi: 10.1016/s0002-9149(99)80118-0. [DOI] [PubMed] [Google Scholar]
- 32.Maroto LC, Molina L, Carrascal Y, Rufilanchas JJ. Intracardiac throm- bus trapped in a patent foramen ovale. Eur J Cardiothorac Surg. 1997;12(5):807–10. doi: 10.1016/s1010-7940(97)00106-1. [DOI] [PubMed] [Google Scholar]
- 33.Cheng TO. Impending paradoxical embolism: a rare but important diagnosis. Br Heart J. 1991;66(3):258. doi: 10.1136/hrt.66.3.258-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuppuswamy M, Kourliouros A, Sutherland G, Sarsam M. Complete surgical correction for impending paradoxical embolism with pulmonary embolism, tricuspid regurgitation, and atrial flutter. Heart Surg Forum. 2008 Dec;11(6):E378–9. doi: 10.1532/HSF98.20081089. [DOI] [PubMed] [Google Scholar]
- 35.Aboyans V, Lacroix P, Ostyn E, Cornu E, Laskar M. Diagnosis and management of entrapped embolus through a patent foramen ovale. Eur J Cardiothorac Surg. 1998;14(6):624–8. doi: 10.1016/s1010-7940(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 36.Bonvini RF, Robert-Ebadi H, Fontana P, Fassa A, Myers P, Licker M, et al. Impending paradoxical embolism. When and how to treat? Ann Cardiol Angéiol. 2008;57:234–237. doi: 10.1016/j.ancard.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Myers PO, Bounameaux H, Panos A, Lerch R, Kalangos A. Impending paradoxical embolism: systematic review of prognostic factors and treatment. Chest. 2010;137:164–170. doi: 10.1378/chest.09-0961. [DOI] [PubMed] [Google Scholar]
- 38.Misfeld M, Hanke T. eComment: Systemic thrombolysis with alteplase in impending paradoxical embolism. Interact Cardiovasc Thorac Surg. 2009 Oct;9(4):714. doi: 10.1510/icvts.2009.209064A. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka J, Izumo M, Fukuoka Y, et al. Comparison of two-dimensional versus real-time three-dimensional transesophageal echocardiography for evaluation of patent foramen ovale morphology. Am J Cardiol. 2013 Apr 1;111(7):1052–6. doi: 10.1016/j.amjcard.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Kim YJ, Hur J, Shim CY, et al. Patent foramen ovale: diagnosis with multidetector CT--comparison with transesophageal echocardiography. Radiology. 2009 Jan;250(1):61–7. doi: 10.1148/radiol.2501080559. [DOI] [PubMed] [Google Scholar]