Abstract
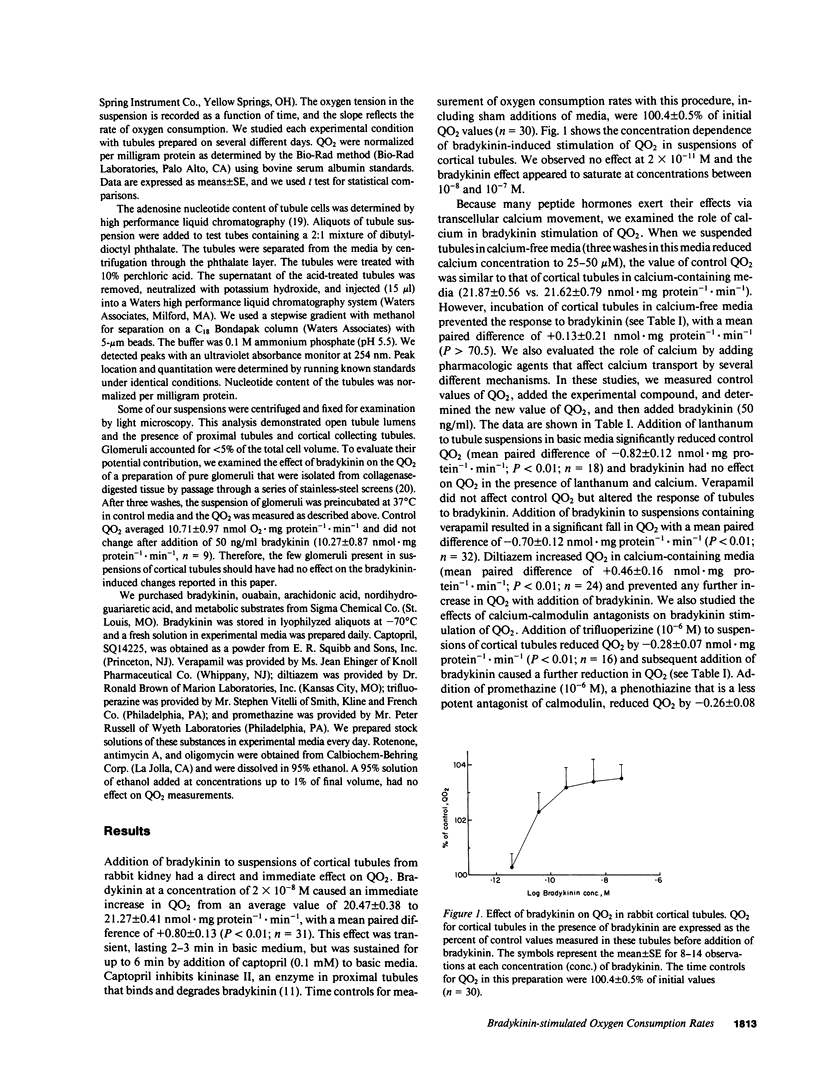
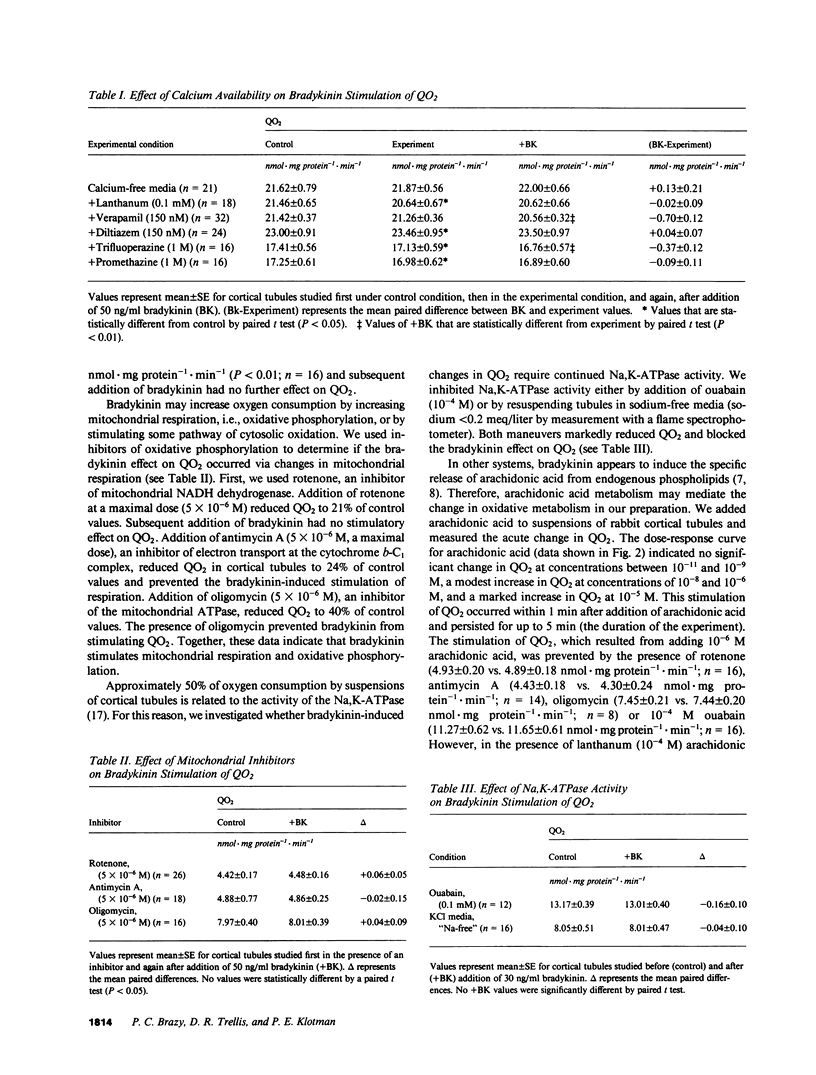
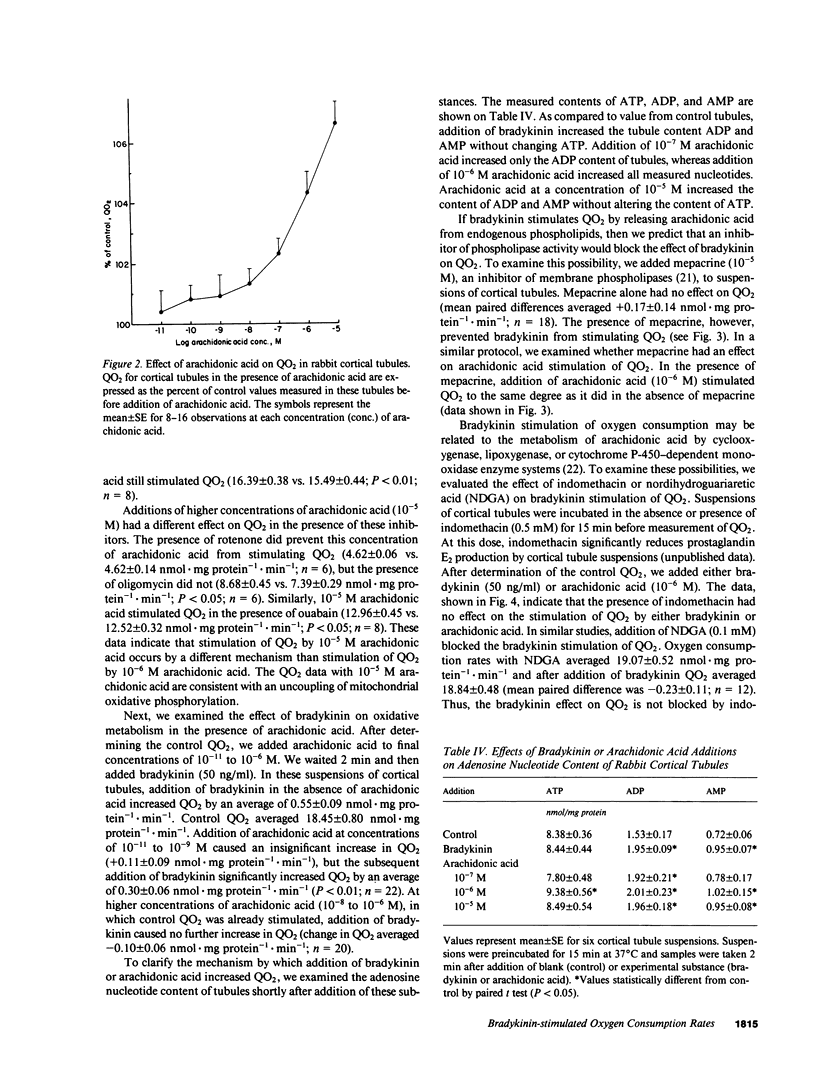
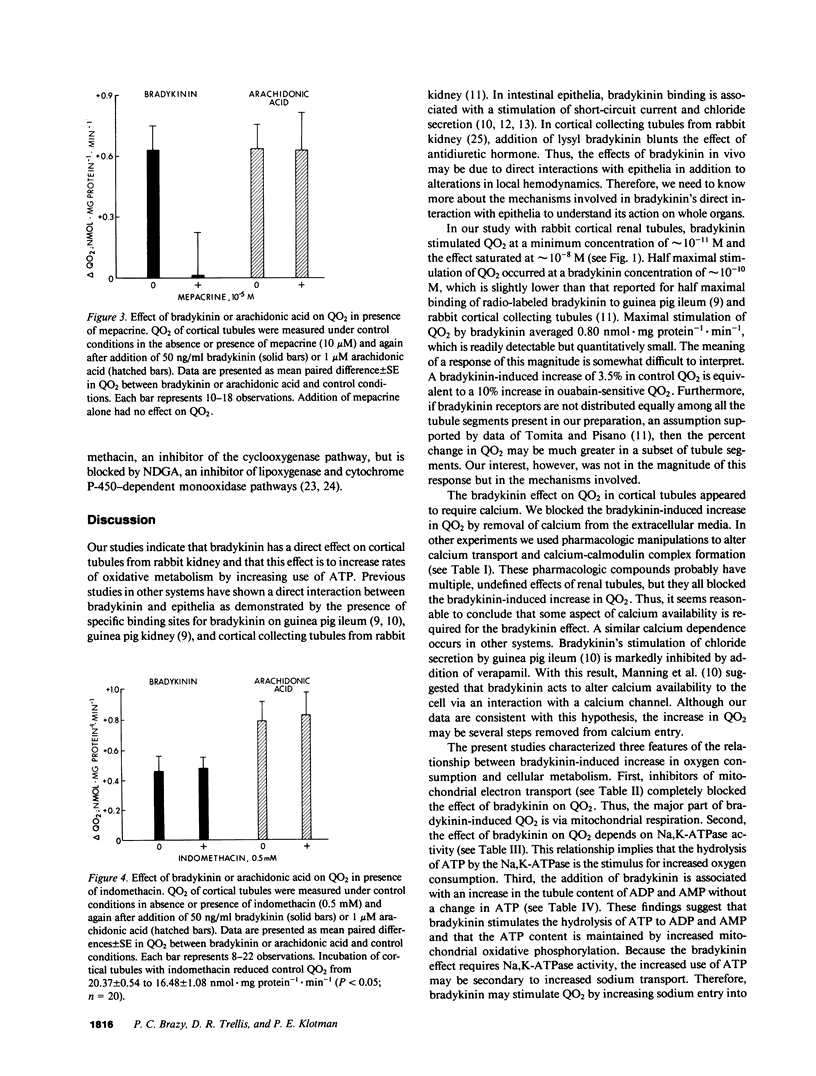
Vasoactive peptides may have direct effects on both renal vasculature and renal tubules. In this study, we examined the direct and immediate effects of bradykinin on oxygen consumption by suspensions of cortical tubules from rabbit kidney. Bradykinin (10(-11) to 10(-7) M) stimulated oxygen consumption rates (QO2) in a dose-dependent manner with a maximal increase of +0.80 +/- 0.13 nmol X mg protein-1 X min-1. This stimulation was prevented by calcium-free media or by the addition of inhibitors of calcium transport, calcium-calmodulin complex formation, Na,K-ATPase activity, mitochondrial respiration, and phospholipase activity. Addition of bradykinin increased the ADP and AMP contents of cortical tubules without changing the ATP content. These data indicate that bradykinin stimulates ATP use and Na,K-ATPase activity. We also examined the effects of exogenous arachidonic acid on QO2 in cortical tubules. Acute additions of arachidonic acid stimulated QO2 at low concentrations (10(-8) to 10(-6) M) and uncoupled mitochondrial respiration at high concentrations (10(-5) M). The effect of arachidonic acid on adenosine nucleotide content was dose-dependent and indicated increased use of ATP. Bradykinin increased QO2 in the presence of low concentrations of arachidonic acid (10(-11) to 10(-9) M), but had no further effect on QO2 in the presence of higher concentrations of arachidonic acid (10(-8) to 10(-6) M). Bradykinin stimulation of QO2 was not prevented by inhibition of cyclooxygenase activity with indomethacin but was prevented by inhibition of lipoxygenase-like activity with nordihydroguariaretic acid. These results suggest that the bradykinin effect on QO2 may be mediated by arachidonic acid release and subsequent metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Mandel L. J., Soltoff S. P., Storey J. M. Coupling of active ion transport and aerobic respiratory rate in isolated renal tubules. Proc Natl Acad Sci U S A. 1980 Jan;77(1):447–451. doi: 10.1073/pnas.77.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Blasingham M. C., Nasjletti A. Contribution of renal prostaglandins to the natriuretic action of bradykinin in the dog. Am J Physiol. 1979 Sep;237(3):F182–F187. doi: 10.1152/ajprenal.1979.237.3.F182. [DOI] [PubMed] [Google Scholar]
- Carretero O. A., Scicli A. G. The renal kallikrein-kinin system. Am J Physiol. 1980 Apr;238(4):F247–F255. doi: 10.1152/ajprenal.1980.238.4.F247. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Margolius H. S. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982 Apr;75(4):587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri N. R., Schwartzman M., Ibraham N. G., Chander P. N., McGiff J. C. Arachidonic acid metabolism in a cell suspension isolated from rabbit renal outer medulla. J Pharmacol Exp Ther. 1984 Nov;231(2):441–448. [PubMed] [Google Scholar]
- Flamenbaum W., Gagnon J., Ramwell P. Bradykinin-induced renal hemodynamic alterations: renin and prostaglandin relationships. Am J Physiol. 1979 Dec;237(6):F433–F440. doi: 10.1152/ajprenal.1979.237.6.F433. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Figueiredo J. F., Maack T., Windhager E. E. Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol. 1981 Jun;240(6):F558–F568. doi: 10.1152/ajprenal.1981.240.6.F558. [DOI] [PubMed] [Google Scholar]
- Gullans S. R., Brazy P. C., Soltoff S. P., Dennis V. W., Mandel L. J. Metabolic inhibitors: effects on metabolism and transport in the proximal tubule. Am J Physiol. 1982 Aug;243(2):F133–F140. doi: 10.1152/ajprenal.1982.243.2.F133. [DOI] [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Hull-Ryde E. A., Cummings R. G., Lowe J. E. Improved method for high energy nucleotide analysis of canine cardiac muscle using reversed-phase high-performance liquid chromatography. J Chromatogr. 1983 Jul 8;275(2):411–417. doi: 10.1016/s0378-4347(00)84388-1. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Manning D. C., Stewart J. M., Snyder S. H. [3H]Bradykinin receptor binding in mammalian tissue membranes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2630–2634. doi: 10.1073/pnas.78.4.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim K., Hassid A., Sun F., Dunn M. J. Lipoxygenase activity in rat kidney glomeruli, glomerular epithelial cells, and cortical tubules. J Biol Chem. 1982 Sep 10;257(17):10294–10299. [PubMed] [Google Scholar]
- LARDY H. A., PRESSMAN B. C. Effect of surface active agents on the latent ATPase of mitochondria. Biochim Biophys Acta. 1956 Sep;21(3):458–466. doi: 10.1016/0006-3002(56)90182-2. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Manning D. C., Snyder S. H., Kachur J. F., Miller R. J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982 Sep 16;299(5880):256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- Misra R. P. Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol. 1972 Aug;58(2):135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7375–7378. doi: 10.1073/pnas.78.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch M. W., Kachur J. F., Miller R. J., Field M., Stoff J. S. Bradykinin-stimulated electrolyte secretion in rabbit and guinea pig intestine. Involvement of arachidonic acid metabolites. J Clin Invest. 1983 May;71(5):1073–1083. doi: 10.1172/JCI110857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Wyche A., Bronson S. D., Holmberg S., Morrison A. R. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [PubMed] [Google Scholar]
- Oliw E. H., Lawson J. A., Brash A. R., Oates J. A. Arachidonic acid metabolism in rabbit renal cortex. Formation of two novel dihydroxyeicosatrienoic acids. J Biol Chem. 1981 Oct 10;256(19):9924–9931. [PubMed] [Google Scholar]
- Schuster V. L., Kokko J. P., Jacobson H. R. Interactions of lysyl-bradykinin and antidiuretic hormone in the rabbit cortical collecting tubule. J Clin Invest. 1984 Jun;73(6):1659–1667. doi: 10.1172/JCI111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M., Liberman E., Raz A. Bradykinin and angiotensin II activation of arachidonic acid deacylation and prostaglandin E2 formation in rabbit kidney. Hormone-sensitive versus hormone-insensitive lipid pools of arachidonic acid. J Biol Chem. 1981 Mar 10;256(5):2329–2333. [PubMed] [Google Scholar]
- Schwartzman M., Raz A. Prostaglandin generation in rabbit kidney. Hormone-activated selective lipolysis coupled to prostaglandin biosynthesis. Biochim Biophys Acta. 1979 Feb 26;572(2):363–369. doi: 10.1016/0005-2760(79)90052-3. [DOI] [PubMed] [Google Scholar]
- Stein J. H., Congbalay R. C., Karsh D. L., Osgood R. W., Ferris T. F. The effect of bradykinin on proximal tubular sodium reabsorption in the dog: evidence for functional nephron heterogeneity. J Clin Invest. 1972 Jul;51(7):1709–1721. doi: 10.1172/JCI106972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., Pisano J. J. Binding of [3H]bradykinin in isolated nephron segments of the rabbit. Am J Physiol. 1984 May;246(5 Pt 2):F732–F737. doi: 10.1152/ajprenal.1984.246.5.F732. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Hai N. D. Selective inhibition by mepacrine of the release of "rabbit aorta contracting substance" evoked by the administration of bradykinin. J Pharm Pharmacol. 1972 Feb;24(2):159–161. doi: 10.1111/j.2042-7158.1972.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Willis L. R., Ludens J. H., Hook J. B., Williamson H. E. Mechanism of natriuretic action of bradykinin. Am J Physiol. 1969 Jul;217(1):1–5. doi: 10.1152/ajplegacy.1969.217.1.1. [DOI] [PubMed] [Google Scholar]


