Abstract
Ischaemic heart disease (IHD) remains the leading cause of death and disability worldwide. As a result, novel therapies are still needed to protect the heart from the detrimental effects of acute ischaemia–reperfusion injury, in order to improve clinical outcomes in IHD patients. In this regard, although a large number of novel cardioprotective therapies discovered in the research laboratory have been investigated in the clinical setting, only a few of these have been demonstrated to improve clinical outcomes. One potential reason for this lack of success may have been the failure to thoroughly assess the cardioprotective efficacy of these novel therapies in suitably designed preclinical experimental animal models. Therefore, the aim of this Position Paper by the European Society of Cardiology Working Group Cellular Biology of the Heart is to provide recommendations for improving the preclinical assessment of novel cardioprotective therapies discovered in the research laboratory, with the aim of increasing the likelihood of success in translating these new treatments into improved clinical outcomes.
Keywords: Cardioprotection, Myocardial infarction, Animal models, Ischaemia, Reperfusion
1. Introduction
Ischaemic heart disease (IHD) is the leading cause of death and disability worldwide. Therefore, novel therapies are required to protect the myocardium against the detrimental effects of acute ischaemia–reperfusion injury (I/R injury) in patients with IHD, so as to reduce lethal cardiac arrhythmias and cardiomyocyte death, preserve cardiac function and prevent the onset of heart failure, and improve patient survival.1
Despite a large number of novel cardioprotective strategies (both pharmacological and non-pharmacological) being discovered in the research laboratory, their translation into the clinical setting to improve patient outcomes has been largely disappointing. The reasons for this failure are multiple and can be divided into two main categories: (i) the failure to rigorously test the novel cardioprotective therapy in the preclinical setting before embarking into the clinical arena.2,3 This is the topic of the current European Society of Cardiology (ESC) Working Group (WG) Cellular Biology of the Heart Position Paper; and (ii) inadequate design of the clinical studies for testing the novel therapy. This subject has been extensively reviewed in our recently published ESC WG Cellular Biology of the Heart Position Papers4,5 and elsewhere.2,6 In brief, the major issues with the study design of previous clinical cardioprotection studies relate to following areas: the selection of the patient population; taking into account known confounders of cardioprotection (such as age, diabetes, and hypertension); the timing of the administration of the cardioprotective therapy; and choosing the relevant endpoints for assessing the efficacy of the cardioprotective therapy.
The National Heart and Lung Institute (NHLI) have convened a WG,7 to discuss the reasons for the failure to translate potential cardioprotective therapies into the clinic, and they made several recommendations, including the formation of an US network of research laboratories with expertise in small (mice, rat, and rabbit) and large (pig) acute I/R injury models. The remit of the NIH (National Institute of Health) CAESAR (Consortium for preclinicAl assESsment of cARdioprotective therapies) Cardioprotection Consortium (http://www.nihcaesar.org) is to systematically test a particular novel cardioprotective therapy using a multicentre randomized controlled study approach before venturing into the clinical setting. Interestingly, of the agents tested so far by the CAESAR Cardioprotection Consortium, sodium nitrite and sildenafil citrate have both failed to limit myocardial infarction (MI) size, despite prior studies reporting beneficial effects with these therapies.8,9
In the present ESC WG Cellular Biology of the Heart Position Paper, we review the current approaches used for determining which novel cardioprotective therapies discovered in the research laboratory are investigated in the clinical setting. We recommend new strategies for optimizing this process, in order to limit the novel cardioprotective therapies selected for testing in the clinical setting to those most likely to succeed in improving patient outcomes.
2. Why has the translation of novel cardioprotective therapies into improved clinical outcomes been so difficult?
It takes a triad of: (i) creative and innovative basic scientists who generate a novel mechanistic insight into the signal transduction of cardioprotective strategies, and identify novel signalling molecules, which may eventually be suited for drug development; (ii) enthusiastic physician scientists with a personal background in basic science and thorough clinical experience in the care of patients subjected to acute I/R injury, who can eventually perform proof-of-concept trials; and finally (iii) a dedicated pharmaceutical company with sufficient financial and logistic capabilities to develop a molecule of interest into a drug for the market. With respect to the largely disappointing translation of cardioprotection into clinical practice over the last decades, we have seen failures at every single level of the above triad and even more so in their interaction.
The basic science in the field of cardioprotection is increasingly being performed by non-physicians, oriented towards molecular and (sub-) cellular approaches and using largely reductionist in vitro cell models or at best rodent preparations: the strength of this development is the generation of truly novel mechanistic insights on a basic level of molecular and cellular biology, and the disadvantage is its ever increasing distance from clinical reality. Of note, the use of less reductionist and a more integrative large mammal model of regional myocardial I/R which are much closer to clinical reality is increasingly rare, in part secondary to animal welfare restrictions, but also secondary to expenses. Even when large mammal models are used, they most often do not take into consideration age, co-morbidities, and co-medications which a patient with an acute coronary syndrome would most likely have. The species of physician scientists who have both a personal background in basic science experimentation and clinical experience in the treatment of patients with acute I/R injury is increasingly rare and almost extinct. These are the people who would be enthusiastic about a novel basic mechanism and/or molecule and would potentially undertake a proof-of-concept small clinical trial to see whether or not this mechanism and/or molecule might also work in patients. And finally, a pharmaceutical company would need to have interest in a potential drug that might not translate into a blockbuster for daily intake over a long period of time but possibly just for one single treatment just before or at reperfusion of an acute MI (AMI). The interaction between the three potential partners has also been problematic in the past. Basic scientists have been overly optimistic and advocated the newly identified molecules for treating patients before even testing the translation to more integrative large mammal models of regional myocardial ischaemia/reperfusion. Also, pharmaceutical companies have prematurely embarked on novel signalling molecules for drug development, only to later find out that the experimental data were not so solid and non-controversial as initially assumed. An extremely important step in the translation of cardioprotection is the conduct of small proof-of-concept clinical trials. Such trials have more recently established proof-of-concept for protection by postconditioning and remote preconditioning in elective and emergency I/R settings;10–14 of note, this success was related to mechanical strategies or to established, no longer patent-protected drugs where there was no involvement of pharmaceutical industry.
3. Statistical considerations in research
A recurring observation in research is that the promise of impressive and significant results in preclinical studies tends to gradually evaporate during subsequent clinical trials. This frustrating phenomenon is hardly restricted to studies of cardioprotection. Indeed, a widely discussed publication of statistical simulations arrived at the disturbing conclusion that most current published research findings are, in fact, false.15 This is not due to fraud but is the expected outcome when many investigators in a large scientific field conduct numerous small studies, with a bias towards the publication of positive results and the presence of other biases such as flexibility in experimental design.15 While some of these problems can be difficult to overcome,15 a number of specific recommendations can be given for preclinical animal studies that should be completed before progressing to clinical trials.
First, of course, it is essential to adhere to the usual expectations of a well-designed experiment, including the use of contemporaneous controls, correct vehicle controls, and appropriate use of ANOVA for statistical analysis of multiple groups. It is important to be aware that while the arbitrarily chosen P-value of 0.05 is indicative of significance, it does not necessarily imply that reproducibility will occur at least 95% of the time.16
Wherever possible, the statistical power of an experiment should be calculated a priori, from previously existing data, and the required number of animals per group calculated before embarking on experiments. Not only are underpowered experiments less likely to detect significant effects, when they do, they are less likely to represent a true effect that will be reproducible. Despite the drive to reduce animal numbers for ethical reasons, it must be recognized that insufficiently powered experiments are themselves, wasteful, misleading, and hence unethical. The Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines17 specify the importance of calculating sample size and power, but such calculations assume an accurate measurement of the expected size of effects. A recent damning indictment of studies in the neuroscience field demonstrates that many studies are severely underpowered.18 It cannot be assumed that studies in the cardioprotection field would fare much better.
Other important recommendations are that exclusion criteria be defined in advance, rigorously adhered to, and reported in publications. The animals and treatments should be randomized, with the researcher blinded as to whether an individual animal is receiving vehicle or drug. The analysis should also be performed blinded to the treatment group. Any conflicts of interest must be reported.
Finally, it is the responsibility of the researcher to ensure that results as reported are unbiased and as accurate a representation of data as possible. Recent events in cardiovascular research have highlighted the importance of vigilance in this regard.
Of note, a useful ‘checklist’ of six points for evaluating published preclinical research includes: blinding of experiments, repetition of experiments, selection of results presented, presence of positive and negative controls, validation of reagents such as antibodies, and the use of appropriate statistical tests19 (Table 1). Previous studies have shown that when such guidelines are not adhered to, the odds of obtaining significant effects in animal studies can be approximately three-fold higher.20 It has been proposed that animal trials should be pre-registered, similar to clinical trials.15 While this approach is currently rare in basic science, possibilities do exist (e.g. The Open Science Framework21), and their expanded use might help to reduce a positive publication bias.
Table 1.
Six points ‘checklist’ for evaluating published preclinical research studies19
| 1. Were the experiments performed blinded? | ✓ |
| 2. Were basic experiments repeated? | ✓ |
| 3. Were all the results presented? | ✓ |
| 4. Were there positive and negative controls? | ✓ |
| 5. Were reagents validated? | ✓ |
| 6. Were statistical analysis tests appropriate? | ✓ |
One effective means of avoiding many of the above pitfalls is to ensure experiments are replicated by several independent groups. In fact, such a multicentre design was first used in the cardioprotection field in 2000, in order to examine the efficacy of an adenosine A1 receptor agonist in limiting infarct size in rabbits when administered at reperfusion. The results of this rigorous trial were negative.22 However, the disappointment of negative results must be weighed against the benefit of being able to refocus efforts on drugs, which are more likely to be clinically translatable.3 Subsequently, a workshop held in June 2003 by the NHLI recommended that an independent organization for the preclinical assessment of cardioprotective therapies be established. This has now been realized in a publically accessible infrastructure called CAESAR. The remit of this consortium is to test promising therapies in three species (in vivo acute I/R injury rodent, rabbit, and porcine models) across three centres.23 A logical approach would be to develop an equivalent European facility for preclinical testing of cardioprotective therapies.
4. Improving the experimental models of acute I/R injury
Once a cardioprotective therapy has been proved to be safe and efficient in preclinical animal studies, it is estimated that only 20% of these are translated into the clinical setting for patient benefit.24 The translation from bench to bedside would certainly be improved if preclinical studies were more appropriately designed.24 A non-adapted experimental protocol, an inadequate choice of the animal model, the exclusion of confounding factors will all contribute to limiting the chance of a successful translation to humans.
4.1. Selecting the appropriate experimental model of acute I/R injury
When designing the experimental study for testing the novel cardioprotective therapy, careful selection of the appropriate in vitro (‘within glass’), ex vivo (‘outside of living’), or in vivo (‘within the living’) model of acute I/R injury is required.
4.1.1. Cell models of simulated acute I/R injury
The use of cell models of simulated acute I/R injury can allow one to overcome the ethical problems and costs (from time and resources) of experimenting on animals. They can be tightly controlled, can usually be undertaken quicker than ex vivo and in vivo experiments models of acute I/R injury, and can assess the direct effect of the novel cardioprotective therapy on the cardiomyocyte itself. However, they do not accurately represent the organism as a whole, and do not include the complex interactions of different cell types and/or effector systems.
The primary cell of the heart, which is damaged by acute I/R injury, is the cardiomyocyte. The primary adult cardiomyocyte is the most closely related to the adult heart in terms of characteristics and is most often harvested from mice, rats, or rabbits, but can also be obtained from guinea pig, dog, pig, and human. A number of different cells have been used in place of adult primary cardiomyocytes in experimental models of acute I/R injury, including HL-1 cells (derived from murine atrial myoblasts),25 H9C2 cells (a rat cardiomyoblast cell line),26 C2C12 cells (a murine myoblast cell line),27 (embryonic) stem cell-derived cardiomyocytes,28 and neonatal cardiomyocytes.29 Although these cells may possess some of the key features of adult cardiomyocytes such as an organized internal structure, cell–cell contacts, high oxygen consumption, and spontaneous beating, several key differences such as the ability to proliferate and signal transduction pathways might affect their use as surrogates for primary adult cardiomyocytes. Adult progenitor cell-derived cardiomyocytes are electrophysiologically more mature cardiomyocytes than those derived from young (foetal) progenitor cells,30 indicating that several stages of maturation exist in cardiomyocytes, and this could potentially affect the response to cardioprotection.
The simulated acute I/R injury model itself can vary, with the use of a variety of different insults to simulate acute I/R injury including metabolic inhibition (using cyanide),31 hypoxic pelleting of cells (using surface mineral oil),32,33 and oxidative stress (using hydrogen peroxide).34 However, these experimental models are unable to recapitulate the complex conditions of acute I/R injury.
For a cell model of simulated acute I/R injury, we recommend that primary adult cardiomyocytes should be used with ischaemia simulated by incubating the cells in ischaemic buffer (de-oxygenated and substrate-free) in an air-tight hypoxic chamber. Reperfusion can then be simulated by replacing the ischaemic buffer with normal oxygenated buffer and removing the cells from the hypoxic chamber. The simulated ischaemic and reperfusion times should be characterized for the cell type with the overall aim to induce 50% of cardiomyocyte death.
4.1.2. Ex vivo models of acute myocardial I/R injury
Ex vivo models of myocardial acute I/R injury using the isolated perfused heart are often used to assess the cardioprotective efficacy of a novel therapy, as they can be carried out relatively rapidly, and they often produce reproducible results in the absence of systematic factors in the blood. The Langendorff-perfused heart was first used at the end of the 19th century, and comprises mounting the isolated heart on the aortic cannula of a Langendorff perfusion apparatus and retrogradely perfusing the heart at a constant flow or constant pressure, using blood or oxygenated buffer (typically Krebs–Henseleit solution).35,36 This model is most often used for perfusing hearts from small animals such as mice, rats, rabbits, and guinea pigs, but it can also be used for larger animal hearts from pig and even human hearts.37,38 The ischaemic insult is induced regionally by coronary ligation (thereby mimicking a clinical AMI) or globally via partial or complete reduction of the flow (thereby mimicking the ischaemic conditions of cardiac bypass surgery).
The isolated working heart model of acute I/R injury allows one to perfuse the heart in the anterograde manner via the left atrium, filling the left ventricle and ejected via the aorta against a variable afterload. The advantages of this model over Langendorff perfusion include the ability to modulate both preload and afterload on the heart, and this model allows low-flow ischaemia.39
Although the ex vivo Langendorff and isolated working heart models are useful for assessing the cardioprotective efficacy of novel therapies, the complexity of the in vivo setting is not recapitulated by these models and, for this reason, they should only be used as an initial step in the preclinical assessment of novel cardioprotective therapies.40
4.1.3. In vivo heart models of acute I/R injury
In the majority of preclinical in vivo animal models of acute I/R injury, the main left coronary is occluded to induce complete regional ischaemia for 30–90 min depending on the animal species, followed by coronary reflow to induce myocardial reperfusion. The duration of reperfusion can vary depending on the particular animal model of acute I/R injury (see later). This experimental model of acute I/R injury best represents the clinical setting of a patient presenting with an acute ST-elevation myocardial infarction (STEMI), who is reperfused by primary percutaneous coronary intervention (PPCI).
A variety of methods have been employed to induce AMI. Left anterior descending coronary artery ligation following left thoracotomy is by far the most commonly used technique, although the model can be time-consuming and the recovery model is associated with a high mortality rate, varying from 20 to 50% according to the species.41
Closed-chest models of AMI, in which occlusion is performed by intracoronary devices (most frequently by catheterization and inflation of an angioplasty balloon), can be performed in rodents and large animals.42,43 These models have the advantage of not being associated with the surgical trauma and inflammation associated with the open-chest approach.
Experimental coronary microembolization using intracoronary infusion of microparticles can serve as a model of an acute coronary syndrome simulating the microvascular obstruction and dysfunction, which occurs in these patients.44 Repetitive intracoronary infusion of microparticles induces severe myocardial dysfunction and has been used as an experimental model of ischaemic heart failure.45 Intracoronary infusion of inert microspheres induces embolization of the coronary microcirculation, where the level of vascular obstruction depends on microsphere diameter. The initial response to coronary microembolization is an immediate decrease in coronary blood flow, which is followed by a reactive hyperaemia response.46 Since coronary microembolization induces permanent coronary microvascular obstruction, patchy MI with a subsequent inflammatory reaction ensues.47 Infiltration of leucocytes goes along with increased expression of tumour necrosis factor alpha (TNFα),48 and a signal transduction of nitric oxide, sphingosine, and reactive oxygen species formation with subsequent oxidative modification of the contractile machinery induces contractile dysfunction.49,50 On the other hand, the enhanced expression of TNFα following coronary microembolization can protect from subsequent MI,51 and this may partially explain the protection observed clinically with pre-infarction angina.52,53
In summary, if using a cell-based model of simulated acute I/R injury to assess the novel cardioprotective therapy, primary adult cardiomyocytes should be used where possible. Ex vivo isolated perfused heart models of acute I/R injury may be used to ‘screen’ the novel therapy where applicable, but it is essential to demonstrate cardioprotective efficacy in small and large in vivo animal models of acute I/R injury.
4.1.4. Specialist models of acute myocardial I/R injury
There are other clinical settings in which the heart is subjected to acute I/R injury such as cardiac bypass surgery, cardiac arrest (CA), and cardiac transplantation, which are not represented in the preclinical setting using the above acute I/R injury models. In this case, more specialized preclinical animal I/R injury models are required, which better reflect these clinical settings.
4.1.4.1. Cardiopulmonary bypass surgery model
The clinical setting of cardiopulmonary bypass (CPB) surgery provides a controlled setting for investigating the efficacy of novel cardioprotective strategies against perioperative myocardial injury, the extent of which can be measured using serum cardiac enzymes. However, this model of acute I/R injury is complicated by several factors: (i) the presence of other cardioprotective strategies such as cardioplegia and hypothermia; (ii) the presence of concomitant medication which may potentially be cardioprotective such as inhaled anaesthetics, nitrates, and opioids; and (iii) the heart is subjected to a variety of different forms of injury including global I/R injury, direct injury from handling of the heart, coronary microembolization, and inflammatory injury from the CPB circuit. Therefore, in order to simulate the clinical setting of cardiac bypass surgery, a specific I/R injury model of CPB may be better suited to test novel cardioprotective therapies.54 In this regard, there are rat models of CPB which are well-established and have the following advantages when compared with larger animal models: rats are relatively cheap, easy to survive, and do not require intensive care units (ICUs) after surgery. More realistic CPB models of global myocardial I/R injury can be achieved in large animals (such as dogs or pigs) with median sternotomy, aortic cross-clamping, and direct perfusion with cardioplegic solution leading to CA.55 However, such animal models are expensive and require the presence of ICU for recovery studies, and the evaluation of organ damage is difficult to standardize.
4.1.4.2. Cardiac arrest models
Although timely cardiopulmonary resuscitation (CPR) can result in 25–50% of patients achieving restoration of spontaneous circulation (ROSC), only 2–10% of patients survive without any neurological deficit. Given the poor outcome in this patient group, novel cardioprotective therapies are required to protect the heart against acute I/R injury, reduce arrhythmias and cardiomyocyte death, and preserve left ventricular (LV) systolic function. Apart from brain injury, one major reason for this poor outcome post-CPR is due to the post-CA syndrome, which comprises in part systemic I/R injury and post-CPR myocardial dysfunction which can be present in 50% of successfully resuscitated patients. In patients presenting with a CA, the heart is subjected to global ischaemic injury and following timely CPR, and if restoration of ROSC is successful, the heart is subjected to global reperfusion injury, the effects of which are cardiomyocyte death, and post-CPR myocardial dysfunction.
There are both small (murine and rat) and large animal (rabbit and pig) models of acute I/R injury, which are able to simulate CA, successful CPR, and ROSC. In these models, CA is induced using either an electrode to cause ventricular fibrillation or anoxia (stopping ventilation) and is left untreated for several minutes, then CPR is started (ventilations and external cardiac compressions) for a period of time, followed by defibrillation to achieve ROSC. In these models, the most common endpoints of cardioprotection include death, cerebral function, myocardial injury, and LV systolic function. These animal CA models have been used to investigate a number of cardioprotective therapies, including inhaled anaesthetics56–58 and cyclosporine A.59,60
4.1.4.3. Cardiac transplantation models
When the heart is removed from the donor patient, it is subjected to acute global ischaemic injury, although this is prevented by cardioplegic solution and hypothermia. The heart remains ischaemic until it is implanted into the recipient heart where it is subjected to acute global reperfusion injury. Acute myocardial I/R injury sustained during cardiac transplantation can contribute to graft vasculopathy and failure.61 Therefore, novel cardioprotective strategies are required to protect the transplanted heart from the detrimental effects of acute I/R injury in order to preserve graft function. In this regard, a number of preclinical studies have investigated a variety of cardioprotective interventions applied to the donor heart including pharmacological agents (i.e. sodium–hydrogen exchange inhibitors) and mechanical interventions (conditioning) [reviewed in ref. (61–63)].
4.1.4.4. Computational models
In silico (‘in silicon’) systems, in which mathematical models of a physiological or pharmacological system are developed and tested on a computer, are a hybrid of in vivo/ex vivo and in vitro techniques.64 Like in vivo techniques, in silico experiments are designed to mimic the behaviour of organisms in their entirety.65 However, like in vitro experiments, they do not require actual experimentation on animal subjects, and conditions can be better controlled.65 To date, several studies have reported a number of analyses and protocols in relation to myocardial ischaemia and reperfusion (reviewed in Ferrero et al.66). Future work should focus on broadening the applicability of these modelling techniques outside of the realm of pharmacology, and towards novel cardioprotective strategies.
In summary, there exist small and large animal models of CPB, CA, and cardiac transplantation for investigating novel cardioprotective therapies, which better represent the patient subjected to acute I/R injury in these clinical settings.
4.2. Selecting the appropriate experimental protocol
After selecting the appropriate model of ischaemia and reperfusion, careful consideration needs to be taken in the design of the experimental protocol.
4.2.1. Choice of anaesthetic
Although volatile anaesthetics are currently the preferred drug of choice to anaesthetize animals in preclinical experiments, their use in cardioprotective studies has been limited, due to their inherent cardioprotective properties.19,67,68 Ex vivo, the use of sodium pentobarbitone remains the anaesthetic of choice for isolated heart studies as isoflurane and sevoflurane reduce MI size and improve functional parameters, when administered prior to ischaemia.69 When conducting in vivo experiments, the choice of the anaesthetic agent is critical as it may directly affect the measurement of different endpoints. As an example, the use of some anaesthetics may modify the heart rate in such a way that it may compromise the capture of data by echocardiography.70,71
4.2.2. Duration of ischaemia
In most experimental models of AMI, the duration of the index ischaemic episode is selected so as to generate 50% cell death or an MI size of 50% of the area-at-risk (AAR). This allows the experimental acute I/R injury model to determine whether the novel cardioprotective therapy is beneficial or detrimental. However, the response of the heart to I/R injury varies depending on the animal model used. In rodent models of AMI, 30–40 min of either regional or global myocardial ischaemia is sufficient to induce an MI size of 50% of the AAR, whereas in porcine and primate MI models, which are more representative of the human physiology in terms of ischaemic time, 50–90 min of myocardial ischaemia is required to achieve equivalent levels of infarction.72–74 Crucially, in patients presenting with an AMI who are treated by reperfusion therapy, the time from onset of symptoms to reperfusion can vary from 30 min to 12 h, with evidence of MI (troponin release) developing after 30 min.40,75 Even then, the MI size in patients is highly variable and in theory, it can range between 5 and 40% of the LV volume depending on the site of the acute coronary occlusion. However, in clinical cardioprotection studies in which both MI size and the AAR have been measured using cardiac MRI, mean MI size as a % LV volume has been reported as 15–25%, and the mean infarct size as a % of the AAR has been found to be 39–70%.10,74,76
4.2.3. Duration and mode of reperfusion
Because this review focuses on novel cardioprotective therapies for preventing acute I/R injury, we do not review experimental models of permanent coronary artery ligation, which are most often used to investigate post-MI heart failure. The reperfusion time used in experimental models of acute I/R injury varies with the model. In some cases, short periods of reperfusion lasting only 2–3 h are used at the end of which the experiment is terminated and MI size determined. Although this particular model allows rapid assessment of the cardioprotective efficacy of the novel therapy, it does not adequately represent the clinical setting in which MI assessment may occur several days after reperfusion. Therefore, in order to accurately assess the cardioprotective efficacy of the novel therapy, extended periods of reperfusion should be used where possible, although for these prolonged periods of reperfusion, the animal has to be recovered from anaesthesia, making this approach technically more demanding. Long-term cardioprotective efficacy is often assessed at 2–4 weeks post-MI, an experimental approach that allows one to also determine the effect of the novel cardioprotective therapy on post-MI LV remodelling.1 The availability of echocardiography and small and large animal cardiac MRI (MRI) allow one to serially assess the cardioprotective efficacy of the novel therapy on MI size, cardiac function, and post-MI remodelling over time.40
4.2.4. Drug administration
An important aspect to consider when testing the efficacy of a novel cardioprotective therapy is to ensure that the timing of the drug administration takes into account the clinical setting of the acute I/R injury. If myocardial reperfusion injury in PPCI-treated STEMI patients is the intended clinical target, then it would be essential to demonstrate that the novel cardioprotective therapy can limit MI size when administered just prior or at the onset of reperfusion. Conversely, if the therapy is intended for use in clinical settings in which the onset of acute I/R injury can be reliably anticipated such as in elective PPCI or coronary artery bypass graft surgery, then it would be essential to show that the novel cardioprotective therapy is effective when administered prior to the index ischaemic event. Most importantly, the use of appropriate vehicle/sham control groups needs to be included in the study, especially when invasive mechanical therapeutic interventions are being considered.
4.2.5. Endpoints for assessing cardioprotection
A number of different endpoints have been measured to assess the cardioprotective efficacy of a novel therapy. The most robust and critical endpoint is to demonstrate a reduction in cardiomyocyte death, which can be easily measured by cell viability (in cell models of simulated acute I/R insult) or myocardial infarct size (in ex vivo and in vivo models of acute I/R insult). Other endpoints which are often used include arrhythmias, LV geometry and function, and so forth, all of which are surrogate endpoints that can be used to confirm cardioprotection.
4.2.5.1. Myocardial infarct size
It is essential to demonstrate that a novel cardioprotective therapy can reduce cardiomyocyte death induced by acute I/R injury. Following a sustained episode of lethal I/R, the major determinants of MI size are the AAR of infarction, the duration of ischaemia, and the presence of collaterals.62 Therefore, providing the index ischaemic time is fixed, significant collateralization has been excluded or accounted for, and MI size should be expressed as the percentage of the AAR. In most animal I/R injury studies, MI size and the AAR are delineated using dual staining with triphenyltetrazolium chloride (TTC) and Evan's Blue [for more details on MI size measurement techniques, see review in ref. (35,77)]. Although this technique is well established and widely used, it is important to keep in mind that it has some limitations as it relies on the wash-out of dehydrogenases, and therefore the histological determination of MI size may be a more reliable and robust technique. A complimentary approach to measuring MI size by either TTC or histology is to assess MI size using cardiac enzymes.
4.2.5.2. Cardiac enzyme release
The measurement of cardiac enzyme release either in the serum (in vivo studies) or perfusate (isolated heart studies) allows a dynamic assessment of cardiomyocyte death as reperfusion progresses, providing an insight into the potential mechanisms underlying I/R injury. It also provides an alternative assessment of cardioprotective efficacy for a novel therapy, which compliments MI size measured by TTC or histology. In the past, creatine kinase and lactate dehydrogenase (LDH) have been used, but more recently troponins T and I have been used to measure myocardial necrosis in experimental I/R injury models.78,79 In the post-ischaemic reperfused isolated rat heart, there appear to be two distinct peaks of LDH release, one occurring in the first few minutes of reperfusion, and the second taking place later between 30 and 120 min reperfusion, potentially reflecting different phases of reperfusion injury—of note, these peaks were differentially attenuated by the cardioprotective therapy.79 The potential existence of this phenomenon in vivo and the mechanism underlying the two different peaks of reperfusion injury need to be investigated as the kinetic of cardiac enzyme release may differ between in vitro and in vivo conditions.
4.2.5.3. Functional endpoints
Functional endpoints such as LV development pressure and LV ejection fraction are sometimes used to assess the cardioprotective efficacy of novel therapies. However, reduced contractile function in the setting of myocardial I/R is difficult to interpret, as it may reflect adaptation to ischaemia as in hibernating myocardium,80,81 reversible injury as in stunning,63 irreversible injury from infarction, or remodelling post-MI.1 Therefore, functional data should not be considered in isolation and should be used to compliment measured MI size. In the isolated heart, the use of an intraventricular balloon connected to a transducer/chart recorder will give useful functional parameters including heart rate and LV developed pressure.35 Insertion of a catheter can capture real-time right or LV pressure–volume loops, and these can be used in vitro or in vivo to measure the beat-to-beat variations in cardiac systolic and diastolic function. An ECG can assess arrhythmias and echocardiography can monitor cardiac dimensions and contractile function.
4.2.5.4. Long-term cardioprotection
The long-term cardioprotective effects of novel therapies can be investigated in terms of final MI size and post-MI LV remodelling as measured by LV dimensions and function using either cardiac echocardiography or MRI. There is also an opportunity to assess the effects of a novel cardioprotective therapy on post-MI survival.
In summary, MI size is the most reliable and robust endpoint for assessing the cardioprotective efficacy of novel therapies. The duration of the index ischaemia used should be sufficient to cause 50% cardiomyocyte death or an MI size of 50% of the AAR. Extended periods of reperfusion (24–72 h) are preferred as these allow one to evaluate the long-term cardioprotective efficacy of the novel therapy. Careful consideration of the intended clinical application of the new treatment should be given when timing the administration of the novel cardioprotective therapy in the preclinical setting.
4.3. Selecting the appropriate animal model
Preclinical animal AMI models are not always predictive, and in some cases they poorly represent the clinical setting.2 Inadequate selection of the animal species could importantly contribute to spurious or inconsistent results, as well as unnecessary animal use. The lack of prediction and conflicting findings are related, in some instances, to species-dependent sensitivity to myocardial I/R injury—that does not necessarily reflect disease in humans—which in turn, is modulated by anatomical factors, metabolism, idiosyncratic toxicity reactions, immunological responses, as well as co-morbidities and their routine medications.
4.3.1. Species, strain, and genetically modified animals
The common use of rat and murine AMI models in the research field of cardioprotection is driven more by practicality and cost, rather than by relevance to the clinical setting. However, some cardioprotective strategies that have proved to protect in experimental conditions using initially a rat/mouse model have been translated to humans in small proof-of-concept clinical studies, making the use of these models relevant to delineate concepts and cellular signalling events. It is important to keep in mind that reproducibility of cardioprotective strategies may also differ between strains in the same species. Genetic divergence between different inbred mouse strains leads to variations in ex vivo and in vivo cardiac function82 and also infarct size.34 These physiological differences, and also different responses to the environment and stress from one strain of mice to another strain of mice, may explain variations in MI sizes when mice are subjected to the exactly same protocol of I/R.34,83
The use of mice is the preferred animal model for genetic manipulation. However, many knockout animals have not been fully characterized, and the manipulation of one gene may lead to multiple pathophysiological adaptations which are not controlled by the researchers. Therefore, the conclusions of these studies should be interpreted with caution.24 When studying novel cardioprotective therapies in genetically modified animals, the choice of the littermate wild-type control over the original strain mouse is also critical as a small genetic variation often incorrectly considered as irrelevant by the researchers, may affect the outcome of the study. Hence, six cycles of ischaemia and reperfusion as an ischaemic postconditioning cardioprotective strategy in the isolated mouse heart model subjected to ischaemia–reperfusion reduced the infarct size in C57BL/6 pure mice, but failed to protect in C57BL/6 floxed signal transducer and activator of transcription (STAT-3) mice commonly used as littermate controls in studies with STAT-3 knockout mice.83
4.3.2. Small-to-large animals
Animal size is a critical determinant of the experimental strategy and may be a limiting factor in itself for certain procedures (i.e. technical challenge for the instrumentation of coronary vessels in small animals, unaffordable costs derived from the wider biodistribution of some engineered compounds in large animals). Small-sized animals, typically mice and rats, and to a lesser extent guinea pigs and rabbits, are extensively used in cardioprotection studies, mainly due to their lower cost, shorter gestation time, easier handling, and well-characterized native or manipulated genetic profile.84 This practice is facilitated by the availability of novel technological tools that allow the use of rodents for in vivo cardiac echocardiography and MRI.85 In contrast, the use of large animals (primarily pigs and dogs, and more exceptionally sheep and non-human primates) is limited by their high cost, difficult maintenance, requirement of skilled personnel, and increased individual and social ethical concerns. As a general rule, there is an inverse relationship between body weight and heart rate across animal species due to size-dependent differences in a metabolic demand.86,87 Consequently, oxygen consumption and cardiac contractile activity are higher in small animals, resulting in increased free radical production, reduced contraction-relaxation times, and differences in calcium handling and myofilament protein isoforms.87,88 In rodents, sarco/endoplasmic reticulum calcium ATPase (SERCA) is responsible for the sequestration of 92% of cytosolic calcium and only the remaining 8% is extruded by the sarcolemmal sodium/calcium exchange (NCX),88 whereas in humans SERCA and NCX account for 76 and 24% of calcium normalization, respectively.89 These differences may be particularly relevant when testing pharmacological cardioprotective strategies during the first minutes of reperfusion, in which cell death develops mainly as a consequence of cytosolic calcium overload and rapid sarcoplasmic reticulum-driven calcium oscillations.90 However, large body size does not warrant anatomical resemblance with the human cardiovascular system. The extent of collateralization and atherosclerotic resistance/blood lipid profile confer non-comparable native cardioprotection after an ischaemic insult. A well-developed coronary collateral circulation is present in dogs and cats (and guinea pigs), whereas it is negligible in pigs and primates (and rodents).91 Similarly, most dog strains are relatively resistant to develop atherosclerotic disease.84 Although selective breeding for production purposes have distanced adult swine heart/body weight relationship from that present in humans, pigs are considered the better suited animals for cardioprotection studies,92 because they share with humans similar coronary circulation anatomy and aortic histology, as well as vulnerability to develop spontaneous or diet-induced vulnerable atherosclerotic plaques.93 Also, and unlike other animals, pigs may develop coronary restenosis after gradual occlusion.94 Primates are notoriously resistant to MI and develop only 17% infarct size of the AAR after 90 min coronary occlusion.95 Finally, it is important to take into account that some of the most powerful cardioprotective strategies like pre- and postconditioning have been proved to be more dependent on age, co-morbidities, and drugs than on animal size.96,97
4.3.3. Gender and age
Most of the experimental studies confirm the clinical observations that female hearts have an increased resistance to I/R injury compared with male hearts [for review, see ref. (96,98–100)]. Cardioprotective strategies such as ischaemic pre- and postconditioning also appear to be influenced by the gender of the animal, and menopause may affect resistance towards I/R injury during aging in females.98 Similarly, while pre- and postconditioning attenuate I/R injury in young animal hearts, most studies suggest a loss of protection in aged hearts [for review, see ref. (97,101,102)].
4.3.4. Co-morbidities
Hyperlipidaemia was the first cardiovascular risk factor or co-morbidity to be associated with the loss of cardioprotection by preconditioning in rabbits and rats.103,104 Since then, it has been well established that, in addition to hyperlipidaemia, most of the major co-morbidities of IHD as well as their co-medications may lead to the loss or attenuation of cardioprotection by ischaemic or pharmacological conditioning [see, for extensive review, ref. (96,97)]. However, most experimental studies on cardioprotection are still undertaken in healthy juvenile animal models, although IHD in humans is a complex disorder caused by or associated with cardiovascular risk factors and co-morbidities, including hypertension, hyperlipidaemia, diabetes, and aging. These risk factors induce fundamental alterations in cellular signalling cascades that affect the severity of I/R injury and the responses to cardioprotective interventions. In the hypertensive heart with or without LV hypertrophy, while cardioprotection by preconditioning is still present, reduction in MI size by ischaemic postconditioning appears to be lost.97,105,106 Most of the preclinical studies, together with some small scale clinical studies, have shown that hyperlipidaemia per se, but not the development of atherosclerosis, leads to a significant aggravation of myocardial I/R injury and to attenuation of the cardioprotective effect of both early and late preconditioning and postconditioning [reviewed in ref. (96,97)]. The majority of both preclinical and clinical data suggest that the chronically diabetic heart is more susceptible to I/R injury, and that the cardioprotective effect of ischaemic and pharmacological preconditioning and postconditioning is impaired [reviewed in ref. (97,107)]. Table 2 provides a summary of the effect of co-morbidities on the response to I/R injury and cardioprotection.
Table 2.
Effect of different co-morbidities on the susceptibility to myocardial I/R injury and the response to cardioprotection elicited by ischaemic preconditioning, ischaemic postconditioning, and remote ischaemic conditioning in experimental animal MI models. Modified from Ref 96
| Co-morbidity | I/R injury | Ischaemic preconditioning | Ischaemic postconditioning | Remote ischaemic conditioning |
|---|---|---|---|---|
| Aging | Increased | Attenuated response | Attenuated response | Not tested |
| Hypertension | No difference | No difference | Attenuated response | Not tested |
| Hyperlipidaemia | Increased | Attenuated response | Attenuated response | Not tested |
| Diabetes | Increased | Attenuated response | Attenuated response | Attenuated response |
| Kidney failure | Increased | No difference | No difference | No difference |
In summary, because co-morbidities can affect the cardioprotective efficacy of novel therapies, testing in co-morbid animal MI models should be part of the preclinical assessment of novel cardioprotective therapies.
4.3.5. Concomitant medication
Many cardioprotective drugs—when administered alone prior to ischaemia and/or reperfusion—reduce irreversible myocardial injury; i.e. attenuate infarct size and potentially reduce patient morbidity and mortality (Table 3). However, in clinical reality, more than one drug is needed to adequately treat patients and therefore, drug–drug interactions must be taken into account. For example, the beneficial effects of angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin receptor (AT)1-receptor antagonists are attenuated when cyclooxygenase (COX) inhibitors are simultaneously applied,108 and the beneficial effects of metformin in diabetic patients is lost when co-administered with the potassium adenosine triphosphate channel blocker glyburide, as is the protection by remote ischaemic preconditioning in diabetic patients receiving sulfonylureas.109 Aspirin at low dose does not affect MI size, whereas at high dose—blocking COX—interferes with cardioprotective interventions.110 Platelet inhibition by clopidogrel, prasugrel, or ticagrelor has been shown to reduce infarct size in animal models and some data exist to also prove clinical efficacy.111 The mechanisms involved have been suggested to involve nitric oxide (clopidogrel) or adenosine (ticagrelor). Potentially, many more interactions will occur in patients with multiple medications. In healthy hearts, most drugs when applied acutely or chronically will interfere with endogenous cardioprotection, except for ACE inhibitors and AT1-receptor antagonists, which lower the threshold to achieve endogenous cardioprotection. However, in hearts from animals or patients suffering co-morbidities, some drugs might restore the otherwise lost possibility to induce endogenous cardioprotection [for extensive review on cardioprotection and co-medication, see review in ref. (96,97)].
Table 3.
Effect of different concomitant medication on the susceptibility to myocardial I/R injury and the response to cardioprotection elicited by ischaemic preconditioning, ischaemic postconditioning, and remote ischaemic conditioning in experimental animal MI models. Modified from Ref 96
| Drug class | I/R injury | Ischaemic preconditioning (co-morbidity) | Ischaemic postconditioning (co-morbidity) | Remote ischaemic conditioning |
|---|---|---|---|---|
| Acute nitrate Nitrate tolerant |
Decreased Increased |
No tested Attenuated response |
Not tested Attenuated response |
Not tested No difference |
| Acute statin Chronic statin |
Decreased/no difference Decreased/no difference |
Attenuated response/enhanced response (hyperlipid) No difference |
No difference Attenuated response |
Not tested Not tested |
| Beta-blocker | Decreased (drug-dependent) | Attenuated response/no difference/enhanced response (stenosis) | Attenuated response/no difference/enhanced response (stenosis) | Attenuated response |
| ACE inhibitor | Decreased | Enhanced response (DM) | Attenuated response | Not tested |
| AT1 antagonist | Decreased | Enhanced response (LVH) | Not tested | Not tested |
| Diabetic drugs | ||||
| Metformin | Decreased | Not tested | Not tested | Not tested |
| KATP blocker | No difference | Attenuated response (drug-dependent) | Attenuated response (drug-dependent) | Attenuated |
| DPP-4 inhibitor | Decreased | Not tested | Not tested | Not tested |
| GLP-1 analogue | Decreased | Not tested | Not tested | Not tested |
| Insulin | Decreased | Not tested | Not tested | Not tested |
| COX inhibitor | Decreased/no difference | Attenuated response | Attenuated response | Not tested |
In some studies, the effect of the concomitant medication was tested using an animal MI model with co-morbidities such as diabetes (DM), hyperlipidaemia (hyperlipid), left ventricular hypertrophy (LVH), or chronic ischaemia (stenosis). DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
4.4. Other confounding factors
In addition to the effect modifiers outlined in previous sections, several other factors have now been identified that likely influence the success of cardioprotective strategies and should therefore be considered potential confounders in the translational process.
4.4.1. Circadian rhythms
The majority of preclinical experiments do not take into account the effect of the time of day on the efficacy of the novel cardioprotective therapy. Both cardiovascular physiology and pathophysiology are profoundly influenced by circadian rhythms, biorhythms that persist with a period of ∼24 h, consistent with our regular day/night rhythm.112 A central ‘master’ clock is located in the brain and orchestrates time-of-day-dependent function of nearby and distant tissues via neurohumoral signals. Each adult mammalian cell also has its own ‘peripheral’ clock that can regulate circadian variations in cell function autonomously.113 In the heart this translates into, among others, a circadian variation in tolerance to I/R injury resulting in circadian variation in MI size and cardiac function at least in mice undergoing experimental AMI.114 MI size and LV function after reperfused STEMI in humans are also correlated with the time-of-day onset of symptoms even when corrected for confounders,115,116 although this notion is not unequivocal.117 Importantly, the amplitude of circadian variation but also the capacity of animals to adapt to externally induced changes in day–night rhythms are reduced with age.118 Thus, when considering the use of aged animals, one has to realize that the circadian pattern of young animals cannot simply be extrapolated to their older counterparts. Co-morbidities such as diabetes can further disturb circadian rhythms as has been demonstrated in patients.112,119
Notably, for practical reasons, most animal facilities follow a ‘lights on at daytime, lights off at night-time’ setting. Thus, preclinical experiments are typically performed at human daytime, which represents the subjective night for nocturnal animals such as rodents and may add an additional difference between preclinical and clinical success of a tested cardioprotective strategy. In addition, seasonal variation is present in humans120 but not in animals housed under standard conditions, although seasonal variation can be mimicked through light settings.121 Experimental design for preclinical cardioprotection studies should therefore at least report and ideally match for the circadian phase in which the injury and therapeutic intervention were initiated.
4.4.2. Depression and stress
Depression and psychological stress are commonly present in patients with IHD and are independently associated with increased cardiovascular morbidity and mortality.122 In experimental models, depression alters cardioprotective strategies,123 and researchers usually avoid exposing their animals to stress prior to experiments as it often affects the beneficial outcome of pharmacological and mechanical cardioprotective strategies. Although this strategy leads to a higher sensitivity for the result of the tested intervention, it reduces its translational applicability in a manner similar to the somatic co-morbidities. Especially when testing strategies that interfere directly or indirectly with stress-inducible pathways, such as the adrenaline–cortisol axis, one should consider that the natural response in patients may mimic or counteract, respectively, the experimental intervention.
4.4.3. Environmental factors
Smoking, air pollution, oxidative stress, temperature, noise, and likely other yet unknown factors can induce or inhibit signalling pathways in the cardiomyocyte and other relevant cell types. Besides causing failure to translate preclinical to clinical results, this issue may already present at an earlier stage as a reproducibility problem between laboratories. For example, Kaljusto et al.124 aimed to identify a robust postconditioning protocol in rat and mouse heart and found that postconditioning in rat hearts in vivo was protective in one laboratory, but not the other laboratory applying the same protocol. Some efforts have been made to characterize or even therapeutically translate the mechanisms by which these environmental aspects may influence the damage induced by I/R injury and the therapeutic effects of cardioprotection.125,126 The development of systems biology approaches may possibly in the future provide additional means of taking into account these environmental and psychological factors that are currently very hard to correct for. The problem with potential confounding effects in the laboratory environment may be overcome by multicentre testing of the novel cardioprotective therapy.
In summary, careful attention must be given to the animal species selected to assess the novel cardioprotective therapy. A variety of factors (including age, gender, co-morbidities, concomitant medication, circadian rhythms, and environmental stress) may confound the intrinsic response to acute I/R injury and confound the cardioprotective efficacy of the novel therapy under investigation. These factors should therefore be taken into account when preclinically assessing the clinical potential of the novel cardioprotective therapy.
4.5. Looking towards the future
Having endured for many years the laments of being ‘lost in translation’,40 the field of cardioprotection appears, at long last, to be back on the map. There have been positive results reducing infarct size using both the beta-blocker metoprolol and the insulin secretagogue exenatide.10,74,127
The results of early preclinical studies investigating the ability of beta-blockers to protect the heart had been mixed.128 However, a more recent study in pigs was able to take advantage of delayed-enhancement cardiac MRI in order to accurately quantify the extent of myocardial necrosis in vivo, and used a clinically relevant study design with metoprolol administered after the onset of ischaemia but before the onset of reperfusion.129,130 The positive results of this study enabled the investigators to progress to the METOCARD-CNIC clinical study, which proved successful in reducing infarct size and increasing LV ejection fraction in patients with anterior Killip class II or less STEMI undergoing PPCI.10
Similarly, exenatide was first shown to be cardioprotective during reperfusion in isolated rat hearts.131 Importantly, this was subsequently confirmed in a pig model by an independent group,132 before progressing to an appropriately designed study,133 which demonstrated improved myocardial salvage in patients undergoing PPCI who received exenatide.74
Other cardioprotective drugs such as cyclosporine A,11 as well as other modalities such as remote ischaemic preconditioning,13,134,135 have undergone extensive preclinical testing and have now made the translation to small proof-of-concept studies and larger studies. With optimism re-instilled, the outcome of these studies is awaited with much interest.
However, it must be borne in mind that these positive data are from small proof-of-concept clinical studies, and large adequately powered multicentre studies are required to confirm their cardioprotective effect, and to determine whether they can actually improve long-term relevant clinical outcomes such as cardiac death and hospitalization for heart failure.
5. Recommendations
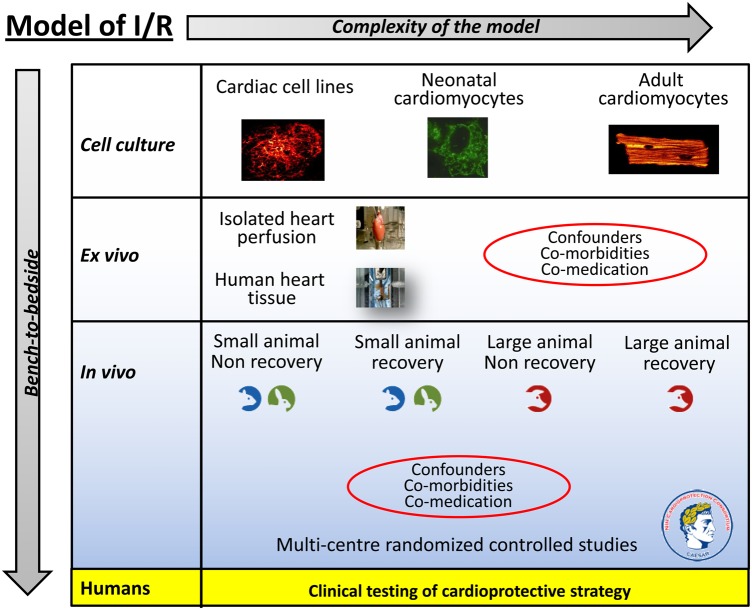
In this ESC WG Position Paper, we have discussed several areas in which the current approach to the preclinical assessment of novel cardioprotective therapies can be improved. In Figure 1, we provide an illustrated overview of the different experimental approaches that exist in the preclinical assessment of novel cardioprotective therapies with an increase in complexity of the I/R injury model as one moves from cell-based studies, ex vivo isolated heart studies, to in vivo large animal recovery AMI models. Adding to this complexity is the need to take into account the effect of confounding factors such as co-morbidities (such as age, diabetes, hypertension, dyslipidaemia, etc.) and concomitant medication (such as morphine, nitrates, sulfonylureas, nicorandil, etc.) in the preclinical assessment.
Figure 1.
The experimental I/R injury model used for testing the cardioprotective strategy in the preclinical setting needs to increase in complexity the closer one gets to the clinical setting. On top of this is the need to take into account confounders of cardioprotection such as co-morbidities and co-medication. Hopefully, rigorous testing of the cardioprotective strategy using this scheme as a guide should increase the likelihood of translating the therapy into the clinical setting (more detail is given in the text).
To this translational pathway, we would like to propose the setting up of an European network of research centres of different animal AMI models to test one particular novel cardioprotective therapy using a multicentre randomized controlled study approach, in a similar manner to the proposed CAESAR network in the USA (http://www.nihcaesar.org).
In summary, we would like to make the following recommendations when assessing the clinical potential of a novel cardioprotective therapy discovered in the research laboratory:
The novel cardioprotective therapy has been demonstrated to offer unequivocable efficacy (in terms of MI size reduction) in all experimental I/R injury models tested, including a large animal I/R injury model.
The novel cardioprotective therapy has been demonstrated to be effective in the presence of one of more co-morbidities (age, diabetes, and dyslipidaemia) and/or concomitant medication (known to potentially have an impact on cardioprotection).
The novel cardioprotective therapy should be investigated in preclinical studies within a research network of centres using a multicentre, randomized controlled, double-blinded, and study approach.
6. Conclusions
The translation of novel cardioprotective therapies from the research laboratory into the clinical setting to improve clinical outcomes in IHD patients has been challenging. Despite the discovery of hundreds of novel cardioprotective therapies in the preclinical laboratory setting, none of these have been incorporated into routine clinical practice for patient benefit. This has been due, in part, to the failure to rigorously assess the novel cardioprotective therapy in the preclinical setting before embarking on patient studies. In this ESC WG Position Paper, we provide recommendations for optimizing this process in order to improve the translation of novel cardioprotective therapies discovered in the research laboratory into improved clinical outcomes for IHD patients.
Conflict of interest: none declared.
Funding
Funding to pay the Open Access publication charges for this article was provided by University College London.
References
- 1.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM, National Heart Lung and Blood Institute NIoH. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- 5.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R Working Group of Cellular Biology of Heart of European Society of Cardiology. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 7.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 8.Kukreja R, Tang X, Lefer DJ, Steenbergen C, Jones S, Guo Y, Li Q, Kong M, Stowers H, Hunt G, Tokita Y, Wu W, Ockaili R, Salloum F, Book MJ, Du J, Bhushan S, Goodchild T, Chang C, Bolli R. Adminstration of sildenafil at reperfusion fails to reduce infarct size: results from the CAESAR cardioprotection consortium. FASEB J. 2014;28:LB650. [Google Scholar]
- 9.Lefer DJ, Jones S, Steenbergen C, Kukreja R, Guo Y, Tang X, Li Q, Ockaili R, Salloum F, Kong M, Polhemus DJ, Bhushan S, Goodchild T, Chang C, Book MJ, Du J, Bolli R. Sodium nitrite fails to limit myocardial infarct size: results from the CAESAR cardioprotection consortium. FASEB J. 2014;28:LB645. [Google Scholar]
- 10.Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de Prado A, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 11.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 12.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 13.Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 14.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 15.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson VE. Revised standards for statistical evidence. Proc Natl Acad Sci USA. 2013;110:19313–19317. doi: 10.1073/pnas.1313476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 19.Begley CG. Six red flags for suspect work. Nature. 2013;497:433–434. doi: 10.1038/497433a. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis JP. Extrapolating from animals to humans. Sci Transl Med. 2012;4:151ra126. doi: 10.1126/scitranslmed.3004631. [DOI] [PubMed] [Google Scholar]
- 21. The Open Science Framework. https://osf.io .
- 22.Baxter GF, Hale SL, Miki T, Kloner RA, Cohen MV, Downey JM, Yellon DM. Adenosine A1 agonist at reperfusion trial (AART): results of a three-center, blinded, randomized, controlled experimental infarct study. Cardiovasc Drugs Ther. 2000;14:607–614. doi: 10.1023/a:1007850527878. [DOI] [PubMed] [Google Scholar]
- 23.Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther. 2011;16:332–339. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- 24.Perrin S. Preclinical research: make mouse studies work. Nature. 2014;507:423–425. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan R, Salloum FN, Fisher BJ, Ownby ED, Kukreja RC, Fowler AA., III Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–H1580. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 26.Fu J, Lin G, Wu Z, Ceng B, Wu Y, Liang G, Qin G, Li J, Chiu I, Liu D. Anti-apoptotic role for C1 inhibitor in ischemia/reperfusion-induced myocardial cell injury. Biochem Biophys Res Commun. 2006;349:504–512. doi: 10.1016/j.bbrc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 27.Yuan D, Huang J, Yuan X, Zhao J, Jiang W. Zinc finger protein 667 expression is upregulated by cerebral ischemic preconditioning and protects cells from oxidative stress. Biomed Rep. 2013;1:534–538. doi: 10.3892/br.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorbe A, Varga ZV, Paloczi J, Rungarunlert S, Klincumhom N, Pirity MK, Madonna R, Eschenhagen T, Dinnyes A, Csont T, Ferdinandy P. Cytoprotection by the NO-donor SNAP against ischemia/reoxygenation injury in mouse embryonic stem cell-derived cardiomyocytes. Mol Biotechnol. 2014;56:258–264. doi: 10.1007/s12033-013-9704-2. [DOI] [PubMed] [Google Scholar]
- 29.Luo T, Chen B, Zhao Z, He N, Zeng Z, Wu B, Fukushima Y, Dai M, Huang Q, Xu D, Bin J, Kitakaze M, Liao Y. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res Cardiol. 2013;108:342. doi: 10.1007/s00395-013-0342-4. [DOI] [PubMed] [Google Scholar]
- 30.Cavalheiro RA, Marin RM, Rocco SA, Cerqueira FM, da Silva CC, Rittner R, Kowaltowski AJ, Vercesi AE, Franchini KG, Castilho RF. Potent cardioprotective effect of the 4-anilinoquinazoline derivative PD153035: involvement of mitochondrial K(ATP) channel activation. PLoS ONE. 2010;5:e10666. doi: 10.1371/journal.pone.0010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strijdom H, Genade S, Lochner A. Nitric oxide synthase (NOS) does not contribute to simulated ischaemic preconditioning in an isolated rat cardiomyocyte model. Cardiovasc Drugs Ther. 2004;18:99–112. doi: 10.1023/B:CARD.0000029027.50796.84. [DOI] [PubMed] [Google Scholar]
- 32.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161–163. doi: 10.1016/j.yjmcc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Lim NR, Thomas CJ, Silva LS, Yeap YY, Yap S, Bell JR, Delbridge LM, Bogoyevitch MA, Woodman OL, Williams SJ, May CN, Ng DC. Cardioprotective 3′,4′-dihydroxyflavonol attenuation of JNK and p38(MAPK) signalling involves CaMKII inhibition. Biochem J. 2013;456:149–161. doi: 10.1042/BJ20121538. [DOI] [PubMed] [Google Scholar]
- 35.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Hill AJ, Laske TG, Coles JA, Jr, Sigg DC, Skadsberg ND, Vincent SA, Soule CL, Gallagher WJ, Iaizzo PA. In vitro studies of human hearts. Ann Thorac Surg. 2005;79:168–177. doi: 10.1016/j.athoracsur.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 37.Schuster A, Grunwald I, Chiribiri A, Southworth R, Ishida M, Hay G, Neumann N, Morton G, Perera D, Schaeffter T, Nagel E. An isolated perfused pig heart model for the development, validation and translation of novel cardiovascular magnetic resonance techniques. J Cardiovasc Magn Reson. 2010;12:53. doi: 10.1186/1532-429X-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 39.Depre C. Isolated working heart: description of models relevant to radioisotopic and pharmacological assessments. Nucl Med Biol. 1998;25:711–713. doi: 10.1016/s0969-8051(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 40.Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech. 2010;3:35–38. doi: 10.1242/dmm.003855. [DOI] [PubMed] [Google Scholar]
- 41.Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res. 2007;74:29–38. doi: 10.1016/j.cardiores.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Crisostomo V, Maestre J, Maynar M, Sun F, Baez-Diaz C, Uson J, Sanchez-Margallo FM. Development of a closed chest model of chronic myocardial infarction in Swine: magnetic resonance imaging and pathological evaluation. ISRN Cardiol. 2013;2013:781762. doi: 10.1155/2013/781762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 45.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 46.Skyschally A, Schulz R, Erbel R, Heusch G. Reduced coronary and inotropic reserves with coronary microembolization. Am J Physiol Heart Circ Physiol. 2002;282:H611–H614. doi: 10.1152/ajpheart.00797.2001. [DOI] [PubMed] [Google Scholar]
- 47.Dorge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279:H2587–H2592. doi: 10.1152/ajpheart.2000.279.6.H2587. [DOI] [PubMed] [Google Scholar]
- 48.Dorge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 49.Canton M, Skyschally A, Menabo R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J. 2006;27:875–881. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 50.Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Buchert A, Kruger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 51.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 52.Rezkalla SH, Kloner RA. Ischemic preconditioning and preinfarction angina in the clinical arena. Nat Clin Pract Cardiovasc Med. 2004;1:96–102. doi: 10.1038/ncpcardio0047. [DOI] [PubMed] [Google Scholar]
- 53.Heusch G. Nitroglycerin and delayed preconditioning in humans: yet another new mechanism for an old drug? Circulation. 2001;103:2876–2878. doi: 10.1161/01.cir.103.24.2876. [DOI] [PubMed] [Google Scholar]
- 54.Jungwirth B, de Lange F. Animal models of cardiopulmonary bypass: development, applications, and impact. Semin Cardiothorac Vasc Anesth. 2010;14:136–140. doi: 10.1177/1089253210370491. [DOI] [PubMed] [Google Scholar]
- 55.You XM, Nasrallah F, Darling E, Robins M, Nieman G, Searles B. Rat cardiopulmonary bypass model: application of a miniature extracorporeal circuit composed of asanguinous prime. J Extra Corpor Technol. 2005;37:60–65. [PMC free article] [PubMed] [Google Scholar]
- 56.Knapp J, Bergmann G, Bruckner T, Russ N, Bottiger BW, Popp E. Pre- and postconditioning effect of sevoflurane on myocardial dysfunction after cardiopulmonary resuscitation in rats. Resuscitation. 2013;84:1450–1455. doi: 10.1016/j.resuscitation.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Meybohm P, Gruenewald M, Albrecht M, Muller C, Zitta K, Foesel N, Maracke M, Tacke S, Schrezenmeir J, Scholz J, Bein B. Pharmacological postconditioning with sevoflurane after cardiopulmonary resuscitation reduces myocardial dysfunction. Crit Care. 2011;15:R241. doi: 10.1186/cc10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meybohm P, Gruenewald M, Albrecht M, Zacharowski KD, Lucius R, Zitta K, Koch A, Tran N, Scholz J, Bein B. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS ONE. 2009;4:e7588. doi: 10.1371/journal.pone.0007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cour M, Loufouat J, Paillard M, Augeul L, Goudable J, Ovize M, Argaud L. Inhibition of mitochondrial permeability transition to prevent the post-cardiac arrest syndrome: a pre-clinical study. Eur Heart J. 2011;32:226–235. doi: 10.1093/eurheartj/ehq112. [DOI] [PubMed] [Google Scholar]
- 60.Gill RS, Manouchehri N, Liu JQ, Lee TF, Cho WJ, Thiesen A, Churchill T, Bigam D, Cheung PY. Cyclosporine treatment improves cardiac function and systemic hemodynamics during resuscitation in a newborn piglet model of asphyxia: a dose-response study. Crit Care Med. 2012;40:1237–1244. doi: 10.1097/CCM.0b013e3182387d2b. [DOI] [PubMed] [Google Scholar]
- 61.Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front Biosci. 2008;13:221–235. doi: 10.2741/2672. [DOI] [PubMed] [Google Scholar]
- 62.Beyersdorf F. The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovasc Res. 2009;83:262–268. doi: 10.1093/cvr/cvp110. [DOI] [PubMed] [Google Scholar]
- 63.Jacobs S, Rega F, Meyns B. Current preservation technology and future prospects of thoracic organs. Part 2: heart. Curr Opin Organ Transplant. 2010;15:156–159. doi: 10.1097/MOT.0b013e328337343f. [DOI] [PubMed] [Google Scholar]
- 64.Colquitt RB, Colquhoun DA, Thiele RH. In silico modelling of physiologic systems. Best Pract Res Clin Anaesthesiol. 2011;25:499–510. doi: 10.1016/j.bpa.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Szabo G, Soans D, Graf A, J Beller C, Waite L, Hagl S. A new computer model of mitral valve hemodynamics during ventricular filling. Eur J Cardiothorac Surg. 2004;26:239–247. doi: 10.1016/j.ejcts.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Ferrero JM, Trenor B, Romero L. Multiscale computational analysis of the bioelectric consequences of myocardial ischaemia and infarction. Europace. 2014;16:405–415. doi: 10.1093/europace/eut405. [DOI] [PubMed] [Google Scholar]
- 67.Zaugg M. Is protection by inhalation agents volatile? Controversies in cardioprotection. Br J Anaesth. 2007;99:603–606. doi: 10.1093/bja/aem276. [DOI] [PubMed] [Google Scholar]
- 68.Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 69.Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Lairez O, Lonjaret L, Ruiz S, Marchal P, Franchitto N, Calise D, Fourcade O, Mialet-Perez J, Parini A, Minville V. Anesthetic regimen for cardiac function evaluation by echocardiography in mice: comparison between ketamine, etomidate and isoflurane versus conscious state. Lab Anim. 2013;47:284–290. doi: 10.1177/0023677213496236. [DOI] [PubMed] [Google Scholar]
- 71.Stein AB, Tiwari S, Thomas P, Hunt G, Levent C, Stoddard MF, Tang XL, Bolli R, Dawn B. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res Cardiol. 2007;102:28–41. doi: 10.1007/s00395-006-0627-y. [DOI] [PubMed] [Google Scholar]
- 72.Wayman NS, McDonald MC, Chatterjee PK, Thiemermann C. Models of coronary artery occlusion and reperfusion for the discovery of novel antiischemic and antiinflammatory drugs for the heart. Methods Mol Biol. 2003;225:199–208. doi: 10.1385/1-59259-374-7:199. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Dorado D, Theroux P, Elizaga J, Galinanes M, Solares J, Riesgo M, Gomez MJ, Garcia-Dorado A, Fernandez Aviles F. Myocardial reperfusion in the pig heart model: infarct size and duration of coronary occlusion. Cardiovasc Res. 1987;21:537–544. doi: 10.1093/cvr/21.7.537. [DOI] [PubMed] [Google Scholar]
- 74.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 75.Ferraro S, Biganzoli E, Marano G, Santagostino M, Boracchi P, Panteghini M, Bongo AS. New insights in the pathophysiology of acute myocardial infarction detectable by a contemporary troponin assay. Clin Biochem. 2013;46:999–1006. doi: 10.1016/j.clinbiochem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 76.White SK, Frohlich GM, Sado D, Maestrini V, Fontana M, Treibel TA, Tehrani S, Bulluck H, Flett AS, Meier P, Moon J, Yellon DM, Hausenloy D. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2014 doi: 10.1016/j.jcin.2014.05.015. doi:10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Csonka C, Kupai K, Kocsis GF, Novak G, Fekete V, Bencsik P, Csont T, Ferdinandy P. Measurement of myocardial infarct size in preclinical studies. J Pharmacol Toxicol Methods. 2010;61:163–170. doi: 10.1016/j.vascn.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009;23:327–331. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- 79.Povlsen JA, Lofgren B, Dalgas C, Jespersen NR, Johnsen J, Botker HE. Frequent biomarker analysis in the isolated perfused heart reveals two distinct phases of reperfusion injury. Int J Cardiol. 2014;171:9–14. doi: 10.1016/j.ijcard.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 80.Heusch G. Hibernating myocardium. Physiol Rev. 1998;78:1055–1085. doi: 10.1152/physrev.1998.78.4.1055. [DOI] [PubMed] [Google Scholar]
- 81.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–H999. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 82.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genomics. 2010;42A:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Somers SJ, Lacerda L, Opie L, Lecour S. Age, genetic characteristics and number of cycles are critical factors to consider for successful protection of the murine heart with postconditioning. Physiol Res. 2011;60:971–974. doi: 10.33549/physiolres.932129. [DOI] [PubMed] [Google Scholar]
- 84.Badimon L, Casani L, Vialhur G. Models for the study of atherosclerosis and thrombosis. In: Michael Conn P, editor. Animal Models for the Study of Human Disease. Oregon, USA: Elsevier; 2013. [Google Scholar]
- 85.Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. 2014;141:235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ostergaard G, Hansen HN, Ottesen JL. Physiological, hematological and clinical chemistry parameters, including conversion factors. In: Hau J, Shapiro SJ, editors. Handbook of Laboratory Animal Science. 3rd edn. Boca Raton, FL: CRC Press; [Google Scholar]
- 87.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 88.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piacentino V, III, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res. 2012;94:168–180. doi: 10.1093/cvr/cvs116. [DOI] [PubMed] [Google Scholar]
- 91.Shaper W, Bernotat-Danielowski S, Nienaber C, Schaper J. Collateral circulation. In: Fozzard HA, Haber E, Jennings RB, Katz AM, editors. The Heart and Cardiovascular System. New York: Raven Press; 1992. [Google Scholar]
- 92.Heusch G, Skyschally A, Schulz R. The in-situ pig heart with regional ischemia/reperfusion—ready for translation. J Mol Cell Cardiol. 2011;50:951–963. doi: 10.1016/j.yjmcc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol. 2011;2011:907575. doi: 10.1155/2011/907575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen YT, Fallon JT, Iwase M, Vatner SF. Innate protection of baboon myocardium: effects of coronary artery occlusion and reperfusion. Am J Physiol. 1996;270:H1812–H1818. doi: 10.1152/ajpheart.1996.270.5.H1812. [DOI] [PubMed] [Google Scholar]
- 96.Ferdinandy P, Hausenloy D, Heusch G, Baxter G, Schulz R. Interaction of risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014 doi: 10.1124/pr.113.008300. 66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 97.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 98.Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. Gender differences in cardiac ischemic injury and protection—experimental aspects. Exp Biol Med (Maywood) 2009;234:1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- 99.Canali E, Masci P, Bogaert J, Bucciarelli Ducci C, Francone M, McAlindon E, Carbone I, Lombardi M, Desmet W, Janssens S, Agati L. Impact of gender differences on myocardial salvage and post-ischaemic left ventricular remodelling after primary coronary angioplasty: new insights from cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2012;13:948–953. doi: 10.1093/ehjci/jes087. [DOI] [PubMed] [Google Scholar]
- 100.Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009;104:469–483. doi: 10.1007/s00395-009-0040-4. [DOI] [PubMed] [Google Scholar]
- 101.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 102.Przyklenk K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28:331–343. doi: 10.2165/11587190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 103.Ferdinandy P, Szilvassy Z, Horvath LI, Csont T, Csonka C, Nagy E, Szentgyorgyi R, Nagy I, Koltai M, Dux L. Loss of pacing-induced preconditioning in rat hearts: role of nitric oxide and cholesterol-enriched diet. J Mol Cell Cardiol. 1997;29:3321–3333. doi: 10.1006/jmcc.1997.0557. [DOI] [PubMed] [Google Scholar]
- 104.Szilvassy Z, Ferdinandy P, Nagy I, Jakab I, Koltai M. The effect of continuous versus intermittent treatment with transdermal nitroglycerin on pacing-induced preconditioning in conscious rabbits. Br J Pharmacol. 1997;121:491–496. doi: 10.1038/sj.bjp.0701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Penna C, Tullio F, Moro F, Folino A, Merlino A, Pagliaro P. Effects of a protocol of ischemic postconditioning and/or captopril in hearts of normotensive and hypertensive rats. Basic Res Cardiol. 2010;105:181–192. doi: 10.1007/s00395-009-0075-6. [DOI] [PubMed] [Google Scholar]
- 106.Wagner C, Ebner B, Tillack D, Strasser RH, Weinbrenner C. Cardioprotection by ischemic postconditioning is abrogated in hypertrophied myocardium of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2013;61:35–41. doi: 10.1097/FJC.0b013e3182760c4d. [DOI] [PubMed] [Google Scholar]
- 107.Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol. 2012;11:67. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jalowy A, Schulz R, Dorge H, Behrends M, Heusch G. Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J Am Coll Cardiol. 1998;32:1787–1796. doi: 10.1016/s0735-1097(98)00441-0. [DOI] [PubMed] [Google Scholar]
- 109.Kottenberg E, Thielmann M, Kleinbongard P, Frey UH, Heine T, Jakob H, Heusch G, Peters J. Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol Scand. 2014;58:453–462. doi: 10.1111/aas.12278. [DOI] [PubMed] [Google Scholar]
- 110.Przyklenk K, Heusch G. Late preconditioning against myocardial stunning. Does aspirin close the ‘second window’ of endogenous cardioprotection? J Am Coll Cardiol. 2003;41:1195–1197. doi: 10.1016/s0735-1097(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 111.Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M. Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol. 2012;107:275. doi: 10.1007/s00395-012-0275-3. [DOI] [PubMed] [Google Scholar]
- 112.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du Pre BC, Van Veen TA, Young ME, Vos MA, Doevendans PA, Van Laake LW. Circadian rhythms in cell maturation. Physiology (Bethesda) 2014;29:72–83. doi: 10.1152/physiol.00036.2013. [DOI] [PubMed] [Google Scholar]
- 114.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–976. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 117.Ammirati E, Maseri A, Cannistraci CV. Still need for compelling evidence to support the circadian dependence of infarct size after ST-elevation myocardial infarction. Circ Res. 2013;113:e43–e44. doi: 10.1161/CIRCRESAHA.113.301908. [DOI] [PubMed] [Google Scholar]
- 118.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Russcher M, Koch BC, Nagtegaal JE, van Ittersum FJ, Pasker-de Jong PC, Hagen EC, van Dorp WT, Gabreels B, Wildbergh TX, van der Westerlaken MM, Gaillard CA, Ter Wee PM. Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): a randomized controlled trial. Br J Clin Pharmacol. 2013;76:668–679. doi: 10.1111/bcp.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia EZ, Xu ZX, Cai HZ, Guo CY, Li L, Zhu TB, Wang LS, Cao KJ, Ma WZ, Yang ZJ. Time distribution of the onset of chest pain in subjects with acute ST-elevation myocardial infarction: an eight-year, single-center study in China. PLoS ONE. 2012;7:e32478. doi: 10.1371/journal.pone.0032478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Otsuka T, Kawai M, Togo Y, Goda R, Kawase T, Matsuo H, Iwamoto A, Nagasawa M, Furuse M, Yasuo S. Photoperiodic responses of depression-like behavior, the brain serotonergic system, and peripheral metabolism in laboratory mice. Psychoneuroendocrinology. 2014;40:37–47. doi: 10.1016/j.psyneuen.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 122.Frasure-Smith N, Lesperance F. Depression and cardiac risk: present status and future directions. Heart. 2010;96:173–176. doi: 10.1136/hrt.2009.186957. [DOI] [PubMed] [Google Scholar]
- 123.Zhuo C, Wang Y, Wang X, Wang Y, Chen Y. Cardioprotection by ischemic postconditioning is abolished in depressed rats: role of Akt and signal transducer and activator of transcription-3. Mol Cell Biochem. 2011;346:39–47. doi: 10.1007/s11010-010-0589-0. [DOI] [PubMed] [Google Scholar]
- 124.Kaljusto ML, Mori T, Mohammad Husain Rizvi S, Galagudza M, Frantzen ML, Valen G, Vaage J. Postconditioning in rats and mice. Scand Cardiovasc J. 2006;40:334–341. doi: 10.1080/14017430601007587. [DOI] [PubMed] [Google Scholar]
- 125.Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman MS, Halestrap AP. Temperature preconditioning of isolated rat hearts—a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–1161. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meyer G, Andre L, Tanguy S, Boissiere J, Farah C, Lopez-Lauri F, Gayrard S, Richard S, Boucher F, Cazorla O, Obert P, Reboul C. Simulated urban carbon monoxide air pollution exacerbates rat heart ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H1445–H1453. doi: 10.1152/ajpheart.01194.2009. [DOI] [PubMed] [Google Scholar]
- 127.Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 128.Pizarro G, Fernandez-Friera L, Fuster V, Fernandez-Jimenez R, Garcia-Ruiz JM, Garcia-Alvarez A, Mateos A, Barreiro MV, Escalera N, Rodriguez MD, de Miguel A, Garcia-Lunar I, Parra-Fuertes JJ, Sanchez-Gonzalez J, Pardillos L, Nieto B, Jimenez A, Abejon R, Bastante T, de Vega VM, Cabrera JA, Lopez-Melgar B, Guzman G, Garcia-Prieto J, Mirelis JG, Zamorano JL, Albarran A, Goicolea J, Escaned J, Pocock S, Iniguez A, Fernandez-Ortiz A, Sanchez-Brunete V, Macaya C, Ibanez B. Long term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial. J Am Coll Cardiol. 2014;63:2356–2362. doi: 10.1016/j.jacc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 129.Ibanez B, Prat-Gonzalez S, Speidl WS, Vilahur G, Pinero A, Cimmino G, Garcia MJ, Fuster V, Sanz J, Badimon JJ. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation. 2007;115:2909–2916. doi: 10.1161/CIRCULATIONAHA.106.679639. [DOI] [PubMed] [Google Scholar]
- 130.Ibanez B, Cimmino G, Prat-Gonzalez S, Vilahur G, Hutter R, Garcia MJ, Fuster V, Sanz J, Badimon L, Badimon JJ. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int J Cardiol. 2011;147:428–432. doi: 10.1016/j.ijcard.2009.09.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 132.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 133.Hausenloy DJ, Yellon DM. Taking lizard saliva to heart. Eur Heart J. 2012;33:1426–1430. doi: 10.1093/eurheartj/ehr382. [DOI] [PubMed] [Google Scholar]
- 134.Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, Evans R, Robertson S, Knight R, Ariti C, Clayton T, Yellon DM, Investigators ET. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol. 2012;101:339–348. doi: 10.1007/s00392-011-0397-x. [DOI] [PubMed] [Google Scholar]
- 135.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]