Abstract
We report an interesting case of a 19 year old female with findings on MRI suggestive of viral encephalitis. An extensive workup was negative for infectious causes and she was subsequently diagnosed with anti-NMDA encephalitis. Anti-NMDA encephalitis is a highly lethal but treatable form of autoimmune encephalitis that has recently been characterized. It is frequently found in young women and associated with an underlying teratoma. Although rare, this diagnosis should be considered in young females for whom a rapid onset of encephalitis cannot be explained by more common causes.
Keywords: Anti-NMDA, NMDA receptor, encephalitis, MRI, teratoma
CASE REPORT
A 19 year old female with no significant past medical history was in her usual state of good health when she went on a camping trip. Five days after arriving home the patient began to display unusual behavior. Initially, she was speaking much slower than normal and reported hearing echoes. Seven days after arriving home the patient had a witnessed tonic-clonic seizure. She was taken to an outside hospital where she was found to have a fever and leukocytosis. An initial CT was reported as negative (Figure 1). Urinalysis was positive for bacteria and she was discharged with antibiotics.
Figure 1.
19 year old female with anti-NMDA encephalitis. Normal non-contrast CT (140 kV, modulated mA, 2.5 mm slice thickness, display width 400HU, display level 40HU) of the brain with no evidence for hemorrhage, loss of gray-white differentiation, mass or hydrocephalus.
Subsequently she was admitted to a different outside hospital with persistent and worsening cognitive abnormalities. A lumbar puncture was performed for cerebrospinal fluid (CSF) collection which showed 29 white blood cells (Normal: 0–5/μL) with a lymphocytic predominance. Protein was mildly elevated at 79 mg/ml (Normal: 15–45 mg/dL). An MRI of the brain was performed and reported as normal. She was started on ceftriaxone and acyclovir due to a clinical suspicion of herpes encephalitis.
CSF was sent for further laboratory analysis including tests for detecting herpes simplex virus, coccidioidomycosis, west nile virus, lyme disease along with aerobic and anaerobic cultures. These tests were negative. The patient was transferred to our hospital at this time.
On admission to our hospital a repeat MRI was performed. There was diffuse cortical T2/FLAIR hyperintensity within the right lateral and anterior temporal lobe (Figure 2). There was no abnormal contrast enhancement, but there was restricted diffusion involving the area of T2/FLAIR hyperintensity (Figure 3). An encephalitis, most likely secondary to herpes infection, was the presumed diagnosis.
Figure 2.
19 year old female with anti-NMDA encephalitis. T2 TSE axial images (3T MRI, TR 4000, TE 137) demonstrating gyral enlargement and hyperintensity in the right temporal lobe laterally (a) and anteriorly (b) as shown by the solid arrows. There is preservation of the peripontine cisterns as seen on the image on the right, indicating minimal local mass effect.
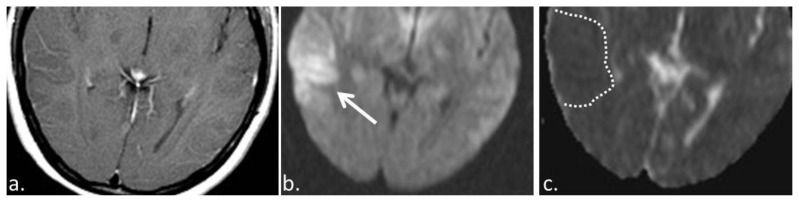
Figure 3.
19 year old female with anti-NMDA encephalitis. (a) T1 +C (3T MRI, TR 450, TE 30, post intravenous administration of 15 ml Optimark) axial image demonstrates no abnormal enhancement within the region of T2/FLAIR hyperintensity and gyral enlargement. (b) Diffusion weighted axial image (3 T MRI, TR 4000, TE 137) showing hyperintense signal in the right temporal lobe (solid arrow). (c) This region of DWI hyperintensity is associated with hypointensity on the ADC images (3 T MRI, TR 4000, TE 137) (dashed line) which is compatible with diffusion restriction.
MANAGEMENT
Further CSF tests for rickettsia, borrelia, Cryptococcus antigen, anti-cardiolipin antibody, anti-neutrophil cytoplasmic antibody, lupus, enterovirus, eastern equine encephalitis, California encephalitis, St. Louis encephalitis, western equine encephalitis, HIV, CMV, HSV were performed and all were negative. At this time the patient was given the presumptive diagnosis of anti-NMDA encephalitis. CSF was sent to an outside lab for indirect fluorescent antibody testing for anti-NMDA antibodies.
Due to the fact that anti-NMDA encephalitis is frequently associated with an underlying teratoma, a pelvic ultrasound and subsequently a CT scan of the abdomen was performed which were normal. Subsequently the outside laboratory confirmed the diagnosis of anti-NMDA encephalitis with the patient having a serum anti-NMDA antibody level of 1:160 (Normal <1:10) and CSF level of 1:10 (Normal <1:1).
FOLLOW-UP
The patient was started on intravenous immunoglobulin (IVIG) therapy in addition to high dose steroids and demonstrated significant improvement. She was discharged home with only a mild deficit in short term memory.
DISCUSSION
ETIOLOGY & DEMOGRAPHICS
Anti-NMDA encephalitis is an autoimmune disorder first reported in 1997 in a febrile patient who rapidly progressed to seizures, psychosis, twitching of the upper extremities and coma [1]. As with the current case, the patient described by Nokura et al. had no improvement with acyclovir and high dose steroids which are standard therapy for herpes encephalitis. Incidentally, an ovarian tumor was identified and removed, which was followed by a drastic recovery in cognition. Pathology revealed this tumor to be an immature teratoma. Since that time there have been several scattered reports of autoimmune encephalitis associated with teratoma in the literature [2, 3, 4].
Subsequently an antibody was discovered in patients diagnosed with anti-NMDA encephalitis that was not present in control groups [5]. Following a large survey of antigens it was found that this antibody bound to the extracellular portion of the NMDA receptor [5]. Tüzün et al. demonstrated that the presence of neuronal components within teratomas lead to immunologic sensitization against NMDA receptors [6].
Since the identification of the anti-NMDA antibody, hundreds of cases have been identified. An English study of all cases of encephalitis identified in 24 hospitals over 13 months demonstrated that 4% of encephalitis was etiologically attributable to anti-NMDA antibodies [7]. The largest case series of 100 patients demonstrated a mean age of 23 years old (range 5–76) and 91% female predisposition (Table 1). Of the 98 patients who underwent oncologic assessment, 58% were found to have associated tumors, the majority of which were ovarian teratomas [8].
Table 1.
Summary table for anti-NMDA encephalitis
| Etiology | Paraneoplastic syndrome with antibodies against NMDA receptors |
| Incidence | Approximately 4% of cases of encephalitis |
| Gender | 91% female |
| Age | Median age of 23 years |
| Risk Factors | 58% have teratomas |
| Treatment | Steroids, IVIG and resection of tumors if identified. |
| Prognosis | 75% recover with no or mild deficits. 25% of cases result in severe deficits or death. |
| Findings on Imaging | 50% have a normal MRI. The remaining 50% can have T2/FLAIR hyperintensity and restricted diffusion in the hippocampi, cerebral cortex, cerebellar cortex, insula, basal ganglia, brainstem, or infrequently the spinal cord. There is typically no enhancement or hemorrhage. |
CLINICAL & IMAGING FINDINGS
This patient presented with deficits in speech and auditory hallucinations. Medical attention was sought when her symptoms progressed with seizures. This is consistent with the descriptions in the literature, which describe psychiatric symptoms in all patients and seizures in 76% of cases [8]. The patient’s response to IVIG therapy is also typical of patients with anti-NMDA encephalitis who receive timely immunosuppression [11,12]. Delmau et al. reported memory deficits in all 100 patients in their case series and this was the only persisting deficit in our patient at the time of discharge [8]. Although not present in this patient, dyskinesias, autonomic instability and hypoventilation are also common manifestations.
The initial CT of the brain was unremarkable with the areas of abnormality seen on the later MRIs appearing isodense to normal adjacent cortex. MRI demonstrated T2/FLAIR hyperintensity in the temporal lobe with corresponding low signal on T1 images and diffusion restriction on DWI/ADC. The MRI of our patient did not demonstrate abnormal contrast enhancement which is consistent with imaging descriptions in the literature. Furthermore, no frank or petechial hemorrhage was identified.
TREATMENT & PROGNOSIS
Hughes et al. demonstrated that the pathomechanism of anti-NMDA encephalitis is a reversible decrease in NMDA receptor density via capping and internalization of receptors from the synaptic surface [9]. The symptomatology of this encephalitis is therefore due to diminished glutamatergic function and loss of synapses rather than to neuronal death. The severity of the clinical symptoms correlates with CSF antibody titer [8,10]. Seki et al showed that removal of the patient’s ovarian teratoma led to a decrease anti-NMDA antibody CSF titer associated with clinical improvement [10]. However, as mentioned previously, a teratoma is identified in only 58% of cases. In 2008, two reports were published describing marked improvement in symptomatology and cognition in patients in which a teratoma was not resected [11,12]. Instead, immunosuppression alone was successful in improving symptom severity by decreasing CSF antibody titers.
Our case was an example of a patient who underwent a significant workup without identifiable cause. Once anti-NMDA encephalitits was diagnosed, further work-up did not reveal the presence of a teratoma. Therefore, the patient was treated with high dose steroids and IVIG which lead to a marked improvement in symptom severity. Early treatment with immunotherapy has been shown to be associated with better outcomes in cognition, memory and mortality [8]. Of those patients with early treatment 75% of patients have been shown to have full recovery or only mild deficits, whereas the late or no treatment group progressed to severe deficits or death. Despite work up at three separate hospitals, our patient appears to have received timely therapy and at the time of this report only demonstrates mild residual short term memory loss.
DIFFERENTIAL DIAGNOSES
In a younger patient population, a rapid onset of cognitive and behavioral abnormalities is most concerning for herpes encephalitis, which accounts for approximately 19% of encephalitides [7]. The medial temporal and frontal lobes are most commonly involved as the herpes virus is typically reactivated from the trigeminal ganglion medial to the temporal lobes. In this patient the right lateral temporal lobe was predominantly involved with only minimal involvement of the medial temporal lobe. Furthermore, herpes encephalitis frequently demonstrates patchy enhancement and petechial hemorrhages which are absent in this case (Table 2).
Table 2.
Differential diagnosis table for anti-NMDA encephalitis
| Anti-NMDA encephalitis | Posterior reversible encephalopathy syndrome | Rabies encephalitis | Herpes encephalitis | Stroke | Infiltrating Glioma | |
|---|---|---|---|---|---|---|
| Age | Early twenties | Middle aged adults | None | Two peaks: younger than 20 years old and older than 50 years old | > 65 | 40’s |
| Gender Predilection | 91% women | None | None | None | Slightly male | None |
| Distribution of findings | No specific distribution | Most commonly parieto-occipital, but can have a holohemispheric watershed pattern, superior frontal sulcus pattern, or a combination of the three. | Basal ganglia and thalamus along with the deep white matter and brainstem | Medial temporal and frontal lobes | Vascular territory, most commonly in the distribution of the middle cerebral artery | No specific distribution |
| CT | Normal | Vasogenic edema | Decreased attenuation | Decreased attenuation | Decreased attenuation | Decreased attenuation |
| MRI T1 | Normal | Normal | Hyperintense signal in areas of involvement | Normal | Normal | Low to normal signal intensity |
| MRI T2 | Hyperintense signal in areas of involvement | Hyperintense signal in areas of involvement | Hyperintense signal in areas of involvement | Hyperintense signal in areas of involvement | Normal signal intensity early in the disease course, hyperintense late | Hyperintense signal in areas of involvement |
| MRI DWI/ADC | Restricted diffusion possible | Typically normal | No characteristic findings | Can demonstrate restricted diffusion | Restricted diffusion in cases of acute or subacute stroke | None |
| Pattern of contrast enhancement | No enhancement | No enhancement | Minimal to no enhancement | Patchy parenchymal or gyral enhancement | Late enhancement | Variable |
Posterior reversible encephalopathy syndrome predominantly involves the parieto-occipital regions, unlike in this case. The distribution of rabies encephalitis within the basal ganglia, deep gray matter and brainstem is also incompatible with the findings of this case. Stroke and primary infiltrating glioma do frequently demonstrate T2/FLAIR hyperintensity and diffusion restriction; however, these entities occur in an older population. Indeed, young patients with predisposing conditions such as coagulopathy or vasculitis can have strokes, but these usually occur either in a vascular territory or as punctate infarcts at the gray white border. Neither of these findings are present in the current case and can be excluded confidently particularly with a clinical picture of diffuse cognitive and behavioral abnormalities rather than a focal deficit.
TEACHING POINT
Anti-NMDA is a relatively new type of encephalitis predominantly effecting women with ovarian teratomas. This diagnosis should be considered in young women in whom herpes encephalitis is ruled out since decreasing CSF titers of anti-NMDA antibodies via immunosuppression is associated with rapid improvement in symptomatology and mortality risk.
ABBREVIATIONS
- ADC
Apparent diffusion coefficient
- CMV
Cytomegalovirus
- CSF
Cerebral Spinal Fluid
- CT
Computerized Tomography
- DWI
Diffusion weighted images
- FLAIR
Fluid attenuated inversion recovery
- HIV
Human immunodeficiency virus
- HSV
Herpes simplex virus
- IVIG
Intravenous immunoglobulin
- MRI
Magnetic resonance imaging
- NMDA
N-methyl-D-aspartate
REFERENCES
- 1.Nokura K, Yamamoto H, Okawara Y, Koga H, Osawa H, Sakai K. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol Scand. 1997;95(6):367–373. doi: 10.1111/j.1600-0404.1997.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 2.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(7):1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 3.Okamura H, Oomori N, Uchitomi Y. An acutely confused 15-year-old girl. Lancet 1997. 1997;350(9076):488. doi: 10.1016/S0140-6736(97)06208-9. [DOI] [PubMed] [Google Scholar]
- 4.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Delmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58(4):594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007 Jan;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009 Dec;118(6):737–43. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010 Dec;10(12):835–44. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 8.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008 Dec;7(12):1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balic-Gordon RJ. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. The Journal of Neuroscience. 2010 Apr;30(17):5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki M, Suzuki S, Iizuka T, Shimizu T, Nihei Y, Suzuki N, Dalmau J. Neurological response to early removal of ovarian teratoma in antiNMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324–326. doi: 10.1136/jnnp.2007.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, Dalmau J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishiura H, Matsuda S, Higashihara M, Hasegawa M, Hida A, Hanajima R, Yamamoto T, Shimizu J, Dalmau J, Tsuji S. Response of antiNMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71:1921–1923. doi: 10.1212/01.wnl.0000336648.43562.59. [DOI] [PubMed] [Google Scholar]