Abstract
Oculocerebrorenal syndrome of Lowe (OCRL) is a multisystem disorder characterized by congenital cataracts, hypotonia, and cognitive developmental delay with renal complications developing in the first few months of life. Clinical and laboratory findings of Lowe syndrome are well documented. Though a small number of case reports describe the neuroimaging features and the renal ultrasound manifestations of this disease, a comprehensive review of all the imaging manifestations has not been reported. The authors present a case of OCRL and review the neuroimaging and renal ultrasound manifestations of this multisystem disease.
Keywords: Lowe Syndrome, oculocerebrorenal syndrome, renal nephrocalcinosis, MRI, ultrasound
CASE REPORT
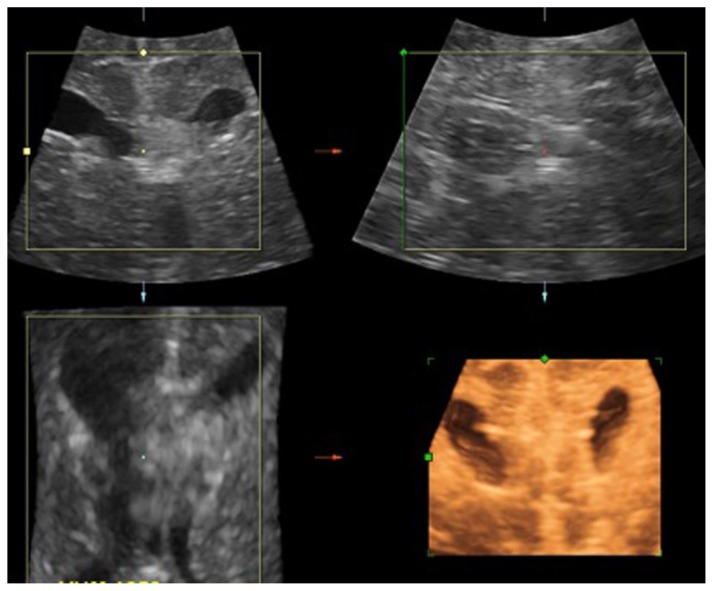
The patient is a 6-day-old infant with hypotonia, bilateral congenital cataracts, macrocephaly and neutropenia. This newborn male infant was born at 39-week gestation to a 42- year-old gravida 1, para 0 mother. Mother reports an uncomplicated pregnancy. The infant was delivered by scheduled C-section due to a maternal history of myomectomy. APGARS were 7 and 8 at 1 and 5 minutes respectively, but poor fetal tone was noted on examination. The infant was noted to have a large head with a head circumference greater than the 90th percentile. Initial cranial ultrasound (fig. 1) was performed to evaluate for hypotonia demonstrating macrocephaly and mild ventriculomegaly, but was otherwise normal. In the newborn period the patient was found to have bilateral cataracts. A complete blood count (CBC) performed to rule out infection showed neutropenia. Abdominal and renal ultrasounds performed were within normal limits (fig. 2).
Figure 1.
6-day-old male infant with hypotonia, bilateral congenital cataracts and macrocephaly, who was eventually diagnosed with Oculocerebrorenal syndrome of Lowe. FINDINGS: Initial cranial ultrasound in multiple imaging planes including coronal, sagittal, axial and 3D coronal (left-to-right, top-to-bottom) was performed to evaluate for hypotonia and showing macrocephaly and mild ventriculomegaly. TECHNIQUE: Renal ultrasound, grey-scale and 3 dimensional (3D) performed with an 7.0 MHz convex transducer.
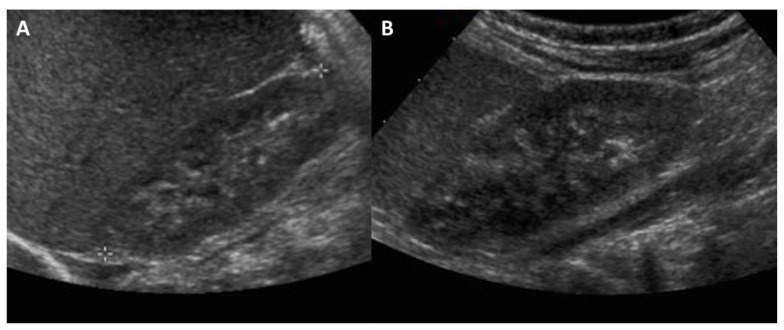
Figure 2.
One month old male infant with splenomegaly, who was eventually diagnosed with Oculocerebrorenal syndrome of Lowe. FINDINGS: Initial renal ultrasound imaging of the right (a) and left (b) kidneys showing normal echotexture and echogenicity. TECHNIQUE: Renal ultrasound, grey scale performed with an 8.0 MHz convex transducer.
On neurological examination, the patient had moderate hypotonia and weakness, and absent deep tendon reflexes; therefore, there was concern for a neurologic disorder with both central and peripheral components such as a peroxisomal or lysosomal disorder, particularly given his cataracts. Labs including very long chain fatty acids, phytanic acid, pipecolic acid, lysosomal enzyme screen and creatine kinase (CK) were unrevealing. Further workup with EMG was performed which showed normal motor responses.
Urinalysis showed 2+ protein suggesting possible renal involvement. It was then questioned if OCRL might be a unifying diagnosis given his hypotonia, cataracts and proteinuria. Oculocerebrorenal-Lowe protein (OCRL1) gene testing was performed and came back positive, thus confirming the diagnosis of OCRL.
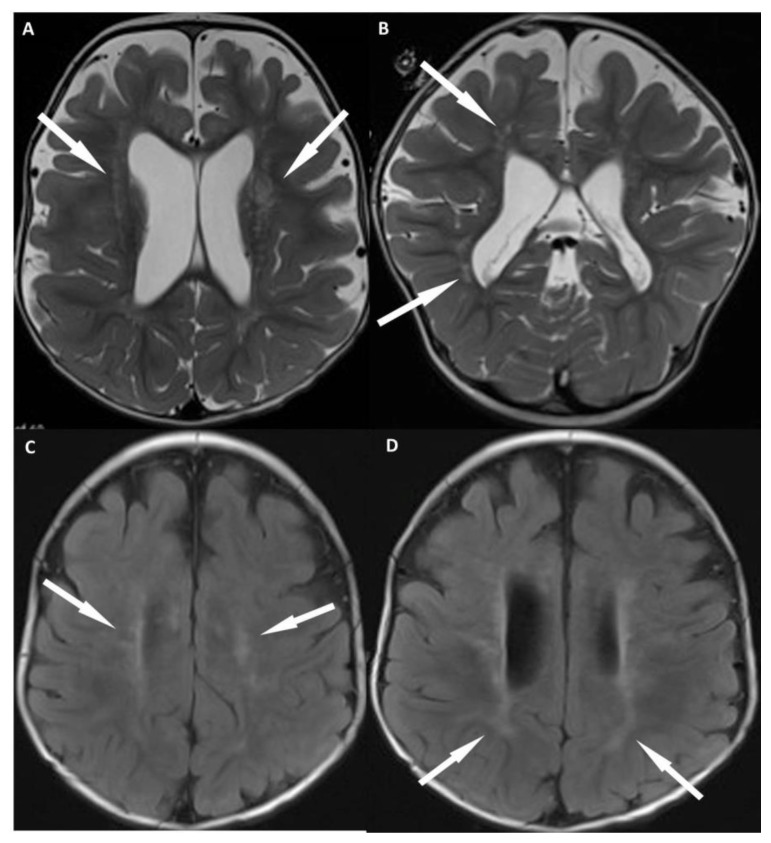
Over the course of the next 12 months, the patient made neurologic progress, however, continued to have difficulties with failure to thrive with persistent severe hypercalcinuria and renal tubular proteinuria. Follow-up renal ultrasound (fig. 3) was performed which showed extensive renal nephrocalcinosis. MR imaging (fig. 4) of the brain was also performed showing characteristic imaging features of OCRL. T2 weighted images show multiple prominent perivascular spaces within the periventricular and deep white matter (fig. 4A, B). T2/FLAIR (Fluid attenuated inversion recovery) imaging show confluent regions of T2 prolongation within the periventricular white matter with sparing of the subcortical white matter (fig. 4C, D), which is typical early in the course of the disease. T2 imaging also demonstrates absence of the lens of the eyes as the patient has undergone cataract surgery (fig. 5).
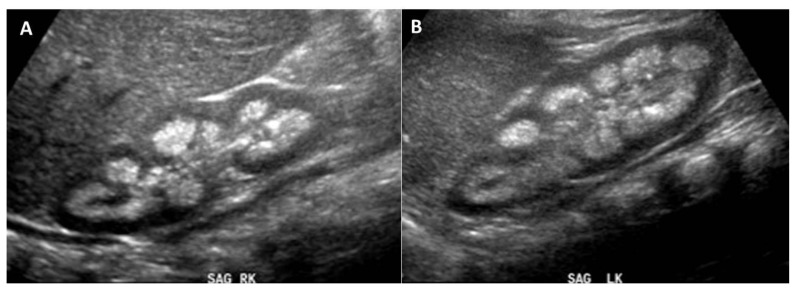
Figure 3.
12 month old with male failure to thrive with persistent severe hypercalcinuria and renal tubular proteinuria. The patient was eventually diagnosed with Oculocerebrorenal syndrome of Lowe. FINDINGS: Follow-up renal ultrasound imaging of the right (a) and left (b) kidneys showing extensive renal medullary nephrocalcinosis. TECHNIQUE: Renal ultrasound, grey scale performed with an 8.0 MHz convex transducer.
Figure 4.
12 month old with male failure to thrive and diagnosis of OCRL. FINDINGS: MR imaging of the brain shows characteristic imaging features of OCRL. Axial T2 (a) and coronal T2 (b) weighted images show prominent perivascular spaces within the periventricular and deep white matter (arrows). Axial FLAIR (c, d) show confluent regions of T2 prolongation within the periventricular white matter with sparing of the subcortical white matter (arrows), which is typical early in the course of the disease. Also, note the lack of small cystic structures in the periventricular and deep white matter, which typically don’t develop until later in the disease course. TECHNIQUE: (4a, b) Siemens 3T SKYRA MR scanner, axial T2-weighted images, TR= 3312, TE = 89, 4 mm slice thickness, no contrast. (4c, d) Siemens 3T SKYRA MR scanner, axial FLAIR images, TR= 7602 TE =132, 4 mm slice thickness, no contrast.
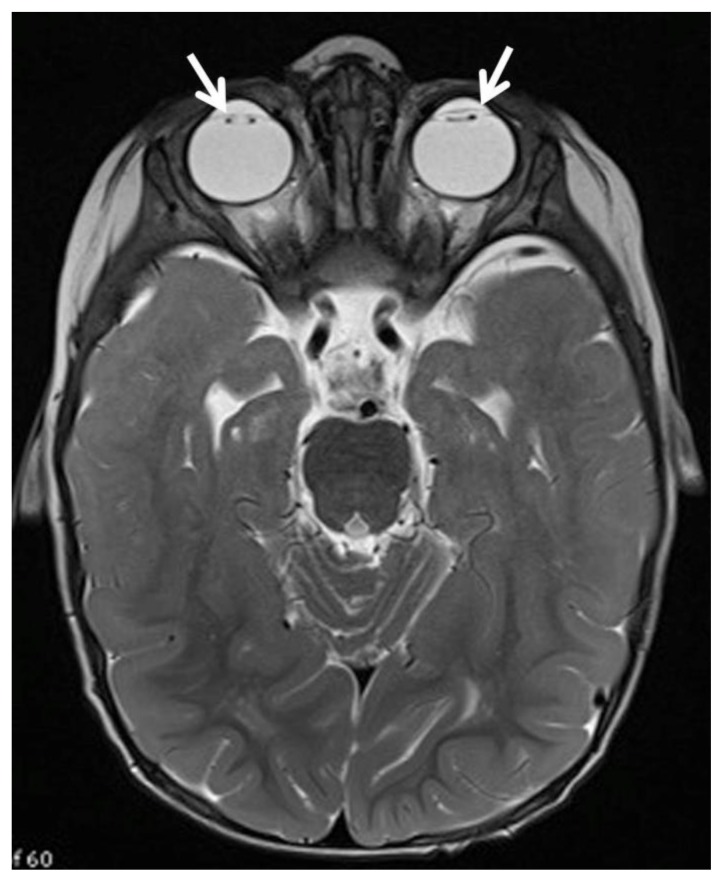
Figure 5.
12 month old with male failure to thrive and diagnosis of OCRL. Axial T2 MR imaging of the brain (Siemens 3 Tesla magnet, TR 11730, TE 91, 2.5 mm slice thickness) demonstrates absence of the lens of the eyes as the patient has undergone cataract surgery (white arrows).
DISCUSSION
Etiology and Demographics
Oculocerebrorenal syndrome of Lowe, or (OCRL) is a multisystem disorder first described in 1952 [1]. The disease is inherited in an X - Linked recessive manner, caused by a mutation in the gene encoding oculocerebrorenal-Lowe protein (OCRL1), localized at Xq26.1, coding for the enzyme phosphatidylinositol (4,5) bisphosphate 5 phosphatase [2]. OCRL is present in all races, with a predominance in Caucasian and Asian ancestries. OCRL is an extremely rare disease, with estimated prevalence in the general population of 1 in 500,000 [2]. Given the X - Linked recessive manner of inheritance, males are usually only affected, though rare cases of affected females with OCRL have been reported [2, 3].
Clinical and Imaging
Clinically, OCRL presents with congenital cataracts, hypotonia, and cognitive developmental delay, with renal complications developing in the first few months of life [2]. Cataracts are present at birth in all patients and glaucoma is often detected within the first year of life. Motor development is delayed and diminished, with hypotonia leading to respiratory issues early in the clinical course. Cognitively, mental retardation is moderate or severe in almost all cases, and obsessive-compulsive behavior is often present. Renal disease is characterized by renal tubular acidosis. Many children are asymptomatic at birth, however, salt and water wasting ultimately results in failure to thrive. A large number of patients will develop chronic renal failure later in life [2].
Neuroimaging may show a number of different nonspecific findings including delayed myelination, dilated perivascular spaces, confluent region of T2 prolongation and multiple small cystic lesions in the deep and periventricular white matter, which develop later in the course of the disease [4–9]. A tigroid pattern of confluent T2 prolongations with internal hypointense radially oriented stripes has also been described [10]. Limited radiology literature reports of the renal manifestations have shown diffuse hyperechogenicity throughout the renal pyramids on ultrasound [11].
Advanced MR imaging techniques have been performed in evaluation of OCRL [12, 13]. MR spectroscopy in OCRL shows an elevation at 3.56 ppm, likely corresponding to elevated levels of myo-inositol. This suggests the abnormal parenchymal signal is due to gliosis, given that myo-inositol is a glial marker [12]. The MR spectra also showed normal choline and N-acetyl aspartate (NAA) peaks, which also suggests gliosis, as demyelination typically produces an elevated choline and reduced NAA peak [12]. Reports of diffusion MR show an elevated diffusion pattern in OCRL [12]. Unfortunately, this is a non-specific finding as active demyelinating processes result in low diffusion patterns; however, there are elevated diffusion patterns when active demyelination is not occurring.
Treatment and Prognosis
Treatment for ocular issues related to OCRL includes cataract extraction and glaucoma control. Cataracts should be removed early in order to avoid amblyopia. Early use of eyeglasses or contact lenses improves visual function and consequently psychosocial skills. Early rehabilitation therapy is necessary to address hypotonia and its complications. Occupational and physical therapies are necessary to maintain articular mobility in order to avoid contractures. Osteopenia and pathological fractures can be prevented through treatment of rickets with phosphate and vitamin D supplementation [2].
Renal tubular acidosis must be recognized early and treated promptly with alkali supplements including sodium and/or potassium citrate and sodium bicarbonate in variable doses and combinations. Seizures require treatment with antiepileptic drugs specific to the symptoms. Behavioral problems and obsessive-compulsive disorder occurring during adolescence are often difficult to treat, requiring extensive psychologic counseling and psychiatric involvement. Drugs such clomipramine, paroxetine and risperidone appear promising in treating these behavioral problems [2]. Life span for those affected with OCRL rarely exceeds 40 years.
Differential Diagnosis
White matter lesions of T2 prolongation are nonspecific and several pediatric white matter diseases may appear similar. These include Alexander disease, Canavan disease, Krabbe disease, metachromatic leukodystrophy and X - Linked Adrenoleukodystrophy. Dilated perivascular spaces are commonly seen in mucopolysaccharidosis, though periventricular white matter lesions are less common. Medullary nephrocalcinosis on renal ultrasound is also nonspecific and is seen in numerous conditions including hyperparathyroidism, medullary sponge kidney, hypercalcemia, sickle cell disease and renal tubular acidosis, the later which results in the imaging findings seen in OCRL. Thus, neuroimaging findings and renal ultrasound findings are not specific for Lowe syndrome when taken into account independent of one another. Therefore, a combination of the clinical, laboratory, neuroimaging and renal ultrasound findings are all needed to establish the correct diagnosis.
TEACHING POINT
Radiologists and clinicians should be aware that delayed myelination, dilated perivascular spaces, confluent white matter T2 prolongation and multiple small cystic lesions on neuroimaging, and medullary nephrocalcinosis on renal ultrasound are nonspecific if not taken in the appropriate context. The combination of these imaging manifestations is particularly useful in aiding in the diagnosis, as both renal and cranial imaging manifestations are typically not seen in peroxisomal, lysosomal and other pediatric white matter diseases, which clinically appear similar to OCRL.
Table 1.
Summary table for Oculocerebrorenal syndrome of Lowe (OCRL)
| Etiology | Inherited in an X – Linked recessive manner. Caused by a mutation in the gene encoding oculocerebrorenal-Lowe protein (OCRL1), localized at Xq26.1, coding for the enzyme phosphatidylinositol (4,5) bisphosphate 5 phosphatase. |
| Incidence | 1 in 500,000. |
| Gender Ratio | Exclusively males with rare reports of female involvement. |
| Age Predilection | Ocular and Neurologic manifestations at birth. Renal manifestations present later, but typically within the first year of life. |
| Risk Factors | X – Linked recessive inheritance. |
| Treatment | Early cataract surgery, rehabilitation therapy for hypotonia, correction of renal tubular acidosis and oral phosphates/vitamin D to treat rickets. |
| Prognosis | Death usually occurs between the end of the second decade and the beginning of the fourth decade of life. |
| Imaging Findings | Delayed myelination, dilated perivascular spaces and confluent periventricular and deep white matter regions of T2 prolongation. Small cystic lesions in the deep and periventricular white matter develop latter in the course of the disease. Renal findings may include medullary nephrocalcinosis. |
Table 2.
Differential diagnosis table of neuroimaging features of Oculocerebrorenal syndrome of Lowe (OCRL).
| Diagnosis | Distribution of WM lesions | Dilated perivascular spaces | MR T2/FLAIR | Contrast Enhanced pattern | Diffusion | MR Spectra |
|---|---|---|---|---|---|---|
| Mucopolysaccharidoses | Gliosis around dilated perivascular spaces | Corpus Callosum, Peritrigonal | Prolongation | None | N/A | Reduced NAA, Elevated Choline |
| X-linked adrenoleukodystrophy | Parietal & Occipital lobes | None | Prolongation | Leading edge enhancement | Reduced | Reduced NAA, Peaks 0.9 – 2.4 ppm (VLCFA) |
| Metachromatic leukodystrophy | Diffuse | None | Prolongation | None | Reduced | Elevated Choline |
| Alexander disease | Frontal lobes | None | Prolongation | Intense early | Elevated | Reduced NAA, +/− Lactate |
| Canavan disease | Diffuse | None | Prolongation | None | Reduced early | Markedly elevated NAA |
| Krabbe disease | Periventricular and deep white matter, cerebellum | None | Prolongation | +/− leading edge enhancement | Reduced early | Reduced NAA, Elevated Choline |
| Oculocerebrorenal syndrome of Lowe | Periventricular | Periventricular and deep white matter | Prolongation | None | Elevated | + myo-inositol, Normal NAA and Choline |
Abbreviations: (CBC) Complete Blood Count; (FLAIR) Fluid attenuated inversion recovery; (CK) creatine kinase; (NAA) N-acetyl aspartate; (VLCFA) very long chain fatty acid
ABBREVIATIONS
- MRI
Magnetic Resonance Imaging
REFERENCES
- 1.Lowe CU, Terrey M, MacLachlan EA. Organic aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation. Am J Dis Child. 1952;83:164–184. doi: 10.1001/archpedi.1952.02040060030004. [DOI] [PubMed] [Google Scholar]
- 2.Loi M. Lowe syndrome. Orphanet J Rare Dis. 2006;1:16. doi: 10.1186/1750-1172-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knaap MS, Valk J. Magnetic resonance of myelination and myelin disorders. Third edition. New York: Springer; 2005. Lowe syndrome; pp. 387–391. [Google Scholar]
- 4.Carvalho-Neto Ad, Ono SE, de Cardoso GM, et al. Oculocerebrorenal syndrome of Lowe: magnetic resonance imaging findings in the first six years of life. Arq Neuropsiquiatr. 2009;67(2A):305–7. doi: 10.1590/s0004-282x2009000200027. [DOI] [PubMed] [Google Scholar]
- 5.Carroll WJ, Woodruff WW, Cadman TE. MR findings in oculocerebrorenal syndrome. AJNR Am J Neuroradiol. 1993;14(2):449–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Ono J, Harada K, Mano T, Yamamoto T, Okada S. MR findings and neurologic manifestations in Lowe oculocerebrorenal syndrome. Pediatr Neurol. 1996;14(2):162–4. doi: 10.1016/0887-8994(96)83274-7. [DOI] [PubMed] [Google Scholar]
- 7.Demmer LA, Wippold FJ, II, Dowton SB. Periventricular white matter cystic lesions in Lowe (Oculocerebrorenal) syndrome. Pediatric Radiology. 1992;22:76–77. doi: 10.1007/BF02011619. [DOI] [PubMed] [Google Scholar]
- 8.Pueschel SM, Brem AS, Nittoli P. Central nervous system and renal investigations in patients with Lowe syndrome. Child’s Nerv System. 1992;8:45–48. doi: 10.1007/BF00316562. [DOI] [PubMed] [Google Scholar]
- 9.Savolaine ER, Bielke DJ. Cranial magnetic resonance imaging in Lowe’s syndrome. Clin Imaging. 1993;17(2):133–6. doi: 10.1016/0899-7071(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 10.Onur MR, Senol U, Mihçi E, Lüleci E. Tigroid pattern on magnetic resonance imaging in Lowe syndrome. J Clin Neurosci. 2009;16(1):112–4. doi: 10.1016/j.jocn.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Kraus RA, Gaisie G, Young LW. Increased renal parenchymal echogenicity: causes in pediatric patients. Radiographics. 1990;10(6):1009–18. doi: 10.1148/radiographics.10.6.2259758. [DOI] [PubMed] [Google Scholar]
- 12.Sener RN. Lowe syndrome: proton MR spectroscopy, and diffusion MR imaging. J Neuroradiol. 31:238–240. doi: 10.1016/s0150-9861(04)97001-0. [DOI] [PubMed] [Google Scholar]