Abstract
Cloacal exstrophy is the most severe and rare form of the exstrophy-epispadias complex, presenting with exposed bladder halves extruding through an abdominal wall defect and variable genitourinary, gastrointestinal, musculoskeletal, and neurological defects. The authors report magnetic resonance imaging findings of a neurologically-intact, 24-month-old female with cloacal exstrophy who presented with anterior spinal dysraphism and diastematomyelia and duplicate pelvic floor musculature. The constellation of defects suggests a common genetic, biochemical, and embryological origin for duplication of the bladder, spinal cord, and pelvic floor muscles occurring in the fourth week of gestation.
Keywords: Diastematomyelia, Spinal Dysraphism, Duplicated Pelvic Floor, Cloacal Exstrophy, Exstrophy-Epispadias Complex, Etiology
CASE REPORT
A neurologically-intact, 24-month-old female presented for consultation following failed cloacal exstrophy (CE) closure complicated by rectal prolapse. This patient was recently adopted from China. Her family history was unknown. Prior surgical history was unclear, but appeared to include an attempted bladder closure and a rectal/hindgut pull-through.
The patient was brought to the operating room for an exam under anesthesia. The bladder halves were still outside the abdomen, but were closed in the center presumably from the original repair (Fig. 1). The anal opening was noticeable with prolapsing mucosa almost contiguous with the bottom of the bladder plate. Her buttocks were also abnormal as there was a central buttock flanked by two clefts with their own lateral buttock.
Figure 1.
24-month-old female with cloacal exstrophy. Pre-operative view of patient’s abdomen. The patient’s bladder halves are still outside the abdomen, but are closed in the center, presumably from the original repair.
Peña stimulation was used to stimulate anal sphincter contraction. During the perineal stimulation exam, an apparently external sphincter complex contracted 2 cm to the left of the central opening of the anus. Additional stimulation of her buttocks produced another anal wink on the right buttocks, well away from her central anal opening.
Imaging Findings
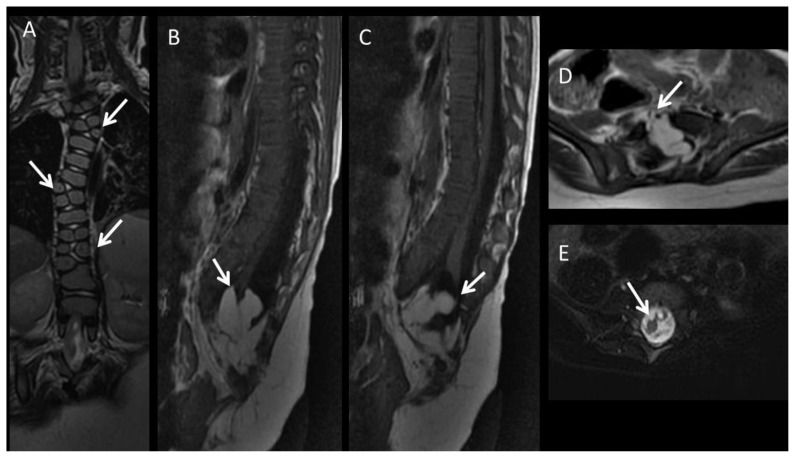
To elucidate the common pelvic bony malformations of our CE patient, a plain radiograph of the pelvis was taken which demonstrated an 8.8 cm pubic diastasis and an open-book configuration of the pelvis resulting from both the pubic diastasis and widened iliac wing angles (Fig. 2). Further evaluation of the entire spine and abdomen/pelvis was untaken with magnetic resonance imaging (MRI) (Optima MR450w, GE Medical Systems, Milwaukee, WI, USA) to fully map out the severity of multisystem involvement. Entire spine MRI demonstrated multi-level formation/segmentation anomalies of the vertebrae in the thoracic and lumbar spine including hemivertebrae and butterfly vertebrae (Fig. 3). Multilevel vertebral anomalies resulted in only minimal congenital dextroscoliosis centered in the midthoracic spine since the right and left-sided hemivertebrae were relatively balanced. Mild focal kyphosis was seen in the distal lumbar spine. Furthermore, there was partial sacral agenesis and a large bony defect in the anterior vertebral bodies at the lumbosacral junction representing anterior spinal dysraphism. In addition, the spinal cord was tethered and there was diastematomyelia with the ventral hemicord tethered and terminating into intradural fat (Fig. 3). This large intradural fat communicated with the peritoneal fat through the large anterior spinal dysraphic defect. The posterior hemicord was tethered and demonstrated evidence of syringomyelia (Fig. 3).
Figure 2.
24-month-old female with cloacal exstrophy. Findings: Anteroposterior radiograph of the pelvis demonstrates the profound pubic diastasis as annotated by the measurement on the image. Note the lack of normal conical shape and the “open book” or “square-like” appearance of the pelvic bones due to pubic diastasis and increased iliac wing angles. Note the partial sacral agenesis. Technique: Anteroposterior radiograph.
Figure 3.
24-month-old female with cloacal exstrophy. Findings: A: Coronal T2-w image (TR: 4000 TE: 106, slice thickness: 3 mm) demonstrates multilevel butterfly vertebra and additional hemivertebrae adjacent to them (arrows). B: Sagittal T1-w FLAIR image (TR: 2250 TE: 13, slice thickness: 3 mm) demonstrates the partial agenesis of the sacrum. Ventral part of the cord terminates at the intradural lipoma (arrow), whereas the dorsal part attaches posteriorly (C). D: Axial T1-w image (TR: 492 TE: 12, slice thickness: 4 mm) shows the anterior spinal dysraphism (arrow), with intradural lipoma extending into the peritoneal cavity. E: Axial T2-w image (TR:3232 TE: 105, slice thickness: 4 mm) with fat saturation demonstrates diastematomyelia (arrow), and syringomyelia. Technique: MRI performed at a 1.5T MR scanner (Optima MR450w, GE Medical Systems, Milwaukee, WI, USA).
MRI of the pelvis demonstrated a midline defect holding the two extruded bladder plates with bowel situated posteriorly (Fig. 4). Interestingly, the widened pelvic floor resembled a “double V” appearance representing two adjacent puborectalis muscles (Fig. 4), instead of a normal “single V” pattern.
Figure 4.
24-month-old female with cloacal exstrophy. Findings: A: Coronal T2-w image (TR: 4437 TE: 80, slice thickness: 5 mm) demonstrates the double V shape of the puborectalis muscle (arrows). B: Axial T1-w image (TR: 681 TE: 7.7, slice thickness: 5 mm) confirms the double V shape of the puborectalis muscle (arrows). Note the open exposed two bladder halves (arrow heads). Immediately posterior to the bladder halves bowel loops are noted. Technique: MRI performed at a 1.5T MR scanner (Optima MR450w, GE Medical Systems, Milwaukee, WI, USA).
Excess amount of subcutaneous fat was noted extending toward the pubic diastasis and underlying the pelvic floor musculature.
Bilateral kidneys were present in their respective renal fossae. They were of normal size, but the left kidney was observed to be minimally malrotated. The liver, spleen, pancreas, and gallbladder were all normal.
Management & Follow-Up
Two months after the MRI, the patient underwent bilateral innominate and vertical iliac osteotomies with external fixation to close her pubic diastasis to 2 cm. During this procedure, painless stimulation continued to demonstrate contraction in half of the left-sided anus along with the right gluteal tissue. These findings supported the MRI depiction of the divided musculature to the right and left with the off-centered anus.
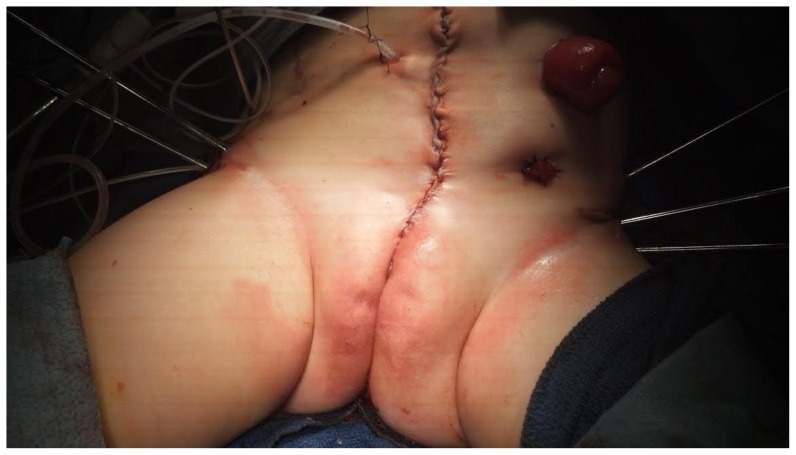
On post-op day 15, the patient was brought back to the operating room for her exstrophy re-closure and colostomy. Neurosurgery to relieve the tethered cord and syringomyelia was scheduled for a later date since the patient did not display any overt neurologic defects. Following the procedure (Fig. 5), the patient remained in Buck’s modified traction along with external fixation for six weeks. After fixation and pin removal, she was in a mermaid wrapped for two weeks until discharge. Unfortunately, the patient was lost to follow-up after the procedure since the parents gave her back up to adoption.
Figure 5.
24-month-old female with cloacal exstrophy. View of patient’s abdomen and pelvis status post closure and application of external fixator.
DISCUSSION
Etiology & Demographics
Cloacal exstrophy (CE) is a severe, rare form of the exstrophy-epispadias complex (EEC) with variable clinical presentation. CE occurs in one of 200,000 to 400,000 live births with a male to female ratio ranging from 2:1 to 1:1 [1, 2]. There are no known risk factors for CE [3]. However, assisted-reproductive technologies, such as in vitro fertilization, may increase the risk of the exstrophy-epispadias complex [4].
CE is the most severe presentation of EEC and is commonly referred to as the omphalocele, exstrophy of bladder, imperforate anus and spinal dysmorphism (OEIS) complex. Typical CE spinal anomalies include skin-covered, posterior spinal deformities including sacral agenesis, meningocele, lipomyelomeningocele and myelocystocele. Female CE patients may also have Müllerian defects presenting as uterine or vaginal duplication. The assemblage of anomalies composing the OEIS complex has led to theories regarding a common embryological etiology [5].
CE is usually attributed to abnormal development of the cloacal membrane. The cloacal membrane consists of the ectodermal and endodermal layers that are normally separated during the 5th and 6th weeks as the mesoderm grows caudally toward the midline. At the same time, the urorectal septum extends toward the cloacal membrane dividing it into a primitive urogenital sinus and rectum. At the 8th week of gestation the rupture of the cloacal membrane yields the normal anatomy of abdomen and pelvis.
It is hypothesized that abnormal migration of the mesodermal layer between the 5th and 8th weeks of gestation results in premature rupture of cloacal membrane, and the timing of this abnormality determines whether EEC patients present with CE, classic bladder exstrophy, or epispadias. Early rupture during the 4th and 5th weeks affects the infraumbilical mesoderm, urorectal septum, and the lumbosacral somites, which may result in this patient’s presentation of a rudimentary hindgut with imperforate anus, omphalocele, failure of genital tubercle fusion, vertebral dysmorphism, and spinal cord dysraphism. While spinal dysraphism usually occurs posteriorly, our patient had an anterior spinal dysraphism at the lumbosacral junction. The early rupture of the cloacal membrane preventing the caudal migration of mesoderm and cranial migration of the urorectal septum results in shortened pubic rami, outward rotation of the innominate bones, and exposed bladder wall, which leads to a pubic diastasis and “open book like” appearance of the pelvic bones [6].
The hedgehog signaling pathway has been implicated to coordinate the embryological development of the bladder and dorsal genital tubercle in a mouse model, and may cross-talk with fibroblast growth factor, bone morphogenic protein, and Wnt signaling pathways [7]. If any one of the 100 genes that have been suggested to play a role in EEC also codes for a protein in the hedgehog signaling pathway, a patient may develop CE with the previously described constellation of defects resulting from the affected downstream signaling pathways [8]. In addition, because 12 of the 100 suggested genes lead to development of the nervous system, widespread genetic abnormalities may also cause the presentation seen here.
While the etiology is unknown, but most likely multifactorial, the authors presented two hypotheses of an explanation for this CE patient who presented with the first reported case of a duplicated pelvic floor and diastematomyelia.
Clinical & Imaging Findings
The common denominator of the complex multisystem anomalies in our patient was each system’s duplicated appearance: duplicated bladder halves, duplicated puborectalis muscle, and two separate hemicords (thus diastematomyelia). Interestingly, despite the severity of the spinal column and cord anomalies with multilevel formation and segmentation defects, our patient had no obvious neurologic defects in the lower extremities.
While many of the patient’s anomalies can be explained by previous embryological studies, the duplicated puborectalis presenting as a double V shape and two distinct anal stimulation response foci is not clear. It has been hypothesized that the levator ani muscles grow in a dorsal-ventral pattern during the first trimester of gestation and inserts medially into the pubic surface [9]. A disruption of the lumbosacral somites, which may have resulted in our patient’s anterior spinal dysraphism at the lumbosacral junction, could also be the reason the two puborectalis muscles grew independently creating the duplicated pattern. Both of these muscles seem to function independently as evidenced by the two distinct anal winks elicited by Peña stimulation. This embryological insult could also yield the observed duplicated appearance of the buttocks. The genetic abnormalities discussed in the previous section could lead to downstream developmental problems that led to these clinical findings.
Treatment & Prognosis
CE was previously considered a fatal congenital anomaly. Patients who survived gestation later died in the neonatal period or soon after from renal complications [10, 11]. Since the advancement of surgical techniques, survival rates have risen to nearly 100%. Like the classic bladder exstrophy repair, the surgical management of CE includes pelvic osteotomy and immobilization, bladder and abdominal wall closure, an anti-reflux procedure, and usually augmentation cystoplasty with continent urinary diversion. Due to its many associated anomalies, such as the neurospinal defects seen in this patient, CE requires more arduous pre and post-closure management and procedures. The single most important predictor of long-term bladder growth and continence is a successful primary bladder closure [12, 13]. Therefore, CE patients should be management by experienced reconstructive pediatric urologists and pediatric surgeons.
Differential Diagnosis
Due to the variable constellation of presentations of the exstrophy-epispadias complex, any combination of omphalocele and exstrophied bladder/intestine usually leads to the quick CE diagnosis. Furthermore, the plain film, computed tomography (CT), and MRI findings of two extruded bladder plates with posteriorly situated bowel, a wide pubic diastasis, a markedly increased iliac wing angle, gastrointestinal (GI) and genitourinary (GU) abnormalities, abnormal pelvic floor musculature, and multi-level neurospinal anomalies strengthen this diagnosis. Hydronephrosis may even present on ultrasound (US) due to the chronic vesicourethral reflux (VUR) [3]. CE variants such as a skin-covered or with an intravesical phallus may lead to the diagnosis of omphalocele or gastroschisis; however, these diagnoses do not present any radiographic findings besides extruded gut on CT. Some CE patients may even present without extruding gut making the differential include bladder exstrophy as well [14]. Bladder exstrophy presents similarly to CE on radiographic imaging, but with less wide pubic diastasis, less increased iliac wing angles, and usually no neurospinal defects on MRI. Still, if there is any suspicion of any presentation of the exstrophy-epispadias complex, the patient should be seen by a team of expert reconstructive surgeon and radiologists for final diagnosis.
TEACHING POINT
To the best of the authors’ knowledge, this is the first demonstration of this constellation of defects in a cloacal exstrophy patient. While the embryological pathology for this defect is uncertain, a genetic or biochemical insult may cause an embryological defect during the fourth week that eventually leads to the MRI findings of duplicated pelvic floor musculature, anterior spinal dysraphism, and diastematomyelia.
Table 1.
Summary table for cloacal exstrophy outlining the salient points of cloacal exstrophy and usual imaging findings
| Etiology | Unknown, but multifactorial |
| Incidence | 1:200,000 – 1:400,000 |
| Gender Ratio | M:F of 2:1 to 1:1 |
| Age Predilection | Neonate |
| Risk Factors | None known |
| Treatment |
|
| Prognosis | Survival nearly 100% Continence depends on successful primary bladder closure |
| Findings on Imaging | Two extruded bladder plates with bowel situated posteriorly Wide pubic diastasis Multi-level neurospinal anomalies (e.g. hemivertebrae, butterfly vertebrae, sacral agenesis, spinal dysraphism, diastematomyelia with tethered hemicord) |
Table 2.
Differential diagnosis table of neuroimaging features of Oculocerebrorenal syndrome of Lowe (OCRL).
| Cloacal Exstrophy | Bladder Exstrophy | Omphalocele | Gastroschisis | |
|---|---|---|---|---|
| X-ray | Markedly wide pubic diastasis. Markedly increased iliac wing angle. |
Wide pubic diastasis. Increased iliac wing angle. |
N/A | N/A |
| US | Possible hydronephrosis due to VUR. | Possible hydronephrosis due to VUR. | N/A | N/A |
| CT | Exposed bladder plate and gut. GU and GI abnormalities. Neurospinal defects. |
Exposed bladder plate. GU and GI abnormalities. |
Extruded gut | Extruded gut |
| MRI (T1) | GU and GI abnormalities. Neurospinal defects. Abnormal pelvic floor musculature. |
GU Abnormalities. Abnormal pelvic floor musculature. |
N/A | N/A |
| MRI (T2) | GU and GI abnormalities. Neurospinal defects. Abnormal pelvic floor musculature. |
GU Abnormalities. Abnormal pelvic floor musculature. |
N/A | N/A |
| Pattern of Contrast Enhancement | Contrast is not necessary in the evaluation of this congenital anomaly. | Contrast is not necessary in the evaluation of this congenital anomaly. | Contrast is not necessary. | Contrast is not necessary in the evaluation of this congenital anomaly. |
Abbreviations: (CBC) Complete Blood Count; (FLAIR) Fluid attenuated inversion recovery; (CK) creatine kinase; (NAA) N-acetyl aspartate; (VLCFA) very long chain fatty acid
ACKNOWLEDGEMENTS
We would like to thank Dr. Henry Lau for explaining Peña stimulation.
ABBREVIATIONS
- CE
Cloacal exstrophy
- CT
Computed tomography
- EEC
Exstrophy-epispadias complex
- GI
Gastrointestinal
- GU
Genitourinary
- MRI
Magnetic resonance imaging
- OEIS
Omphalocele, exstrophy of bladder, imperforate anus, and spinal dysmorphism
- US
Ultrasound
- VUR
Vesicoureteral reflux
REFERENCES
- 1.Gearhart JP, Matthews R. Exstrophy-Epispadias Complex. In: Kavoussi L, Wein A, Novick A, Partin A, Peters C, editors. Campbell-Walsh Urology. 10 ed. Vol. 4. Elsevier Saunders; Philadelphia: 2012. pp. 3325–3378.e5. [Google Scholar]
- 2.Stec AA. Embryology and bony and pelvic floor anatomy in the bladder exstrophy-epispadias complex. Semin Pediatr Surg. 2011;20(2):66–70. doi: 10.1053/j.sempedsurg.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Inouye BM, Tourchi A, Di Carlo HN, Young EE, Gearhart JP. Modern Management of the Exstrophy-Epispadias Complex. Surgery Research and Practice. 2014 doi: 10.1155/2014/587064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye BM, Massanyi EZ, Di Carlo H, Shah BB, Gearhart JP. Modern management of bladder exstrophy repair. Curr Urol Rep. 2013;14(4):359–65. doi: 10.1007/s11934-013-0332-y. [DOI] [PubMed] [Google Scholar]
- 5.Stec AA, Pannu HK, Tadros YE, Sponseller PD, Fishman EK, Gearhart JP. Pelvic floor anatomy in classic bladder exstrophy using 3-dimensional computerized tomography: initial insights. J Urol. 2001;166(4):1444–9. [PubMed] [Google Scholar]
- 6.Martinez-Frias ML, Bermejo E, Rodriguez-Pinilla E, Frias JL. Exstrophy of the cloaca and exstrophy of the bladder: two different expressions of a primary developmental field defect. Am J Med Genet. 2001;99(4):261–9. doi: 10.1002/ajmg.1210. [DOI] [PubMed] [Google Scholar]
- 7.Haraguchi R, Motoyama J, Sasaki H, et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134(3):525–33. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 8.Qi L, Chen K, Hur DJ, et al. Genome-wide expression profiling of urinary bladder implicates desmosomal and cytoskeletal dysregulation in the bladder exstrophy-epispadias complex. Int J Mol Med. 2011;27(6):755–65. doi: 10.3892/ijmm.2011.654. [DOI] [PubMed] [Google Scholar]
- 9.Copin HD, Bourdelat D, Barbet JP. Dorso-ventral gradient of maturation for puborectalis muscle. Morphologie. 1999;83(262):19–21. [PubMed] [Google Scholar]
- 10.Howell C, Caldamone A, Snyder H, Ziegler M, Duckett J. Optimal management of cloacal exstrophy. J Pediatr Surg. 1983;18(4):365–9. doi: 10.1016/s0022-3468(83)80182-1. [DOI] [PubMed] [Google Scholar]
- 11.Lund DP, Hendren WH. Cloacal exstrophy: experience with 20 cases. J Pediatr Surg. 1993;28(10):1360–8. doi: 10.1016/s0022-3468(05)80328-8. discussion 1368–9. [DOI] [PubMed] [Google Scholar]
- 12.Novak TE, Costello JP, Orosco R, Sponseller PD, Mack E, Gearhart JP. Failed exstrophy closure: management and outcome. J Pediatr Urol. 2010;6(4):381–4. doi: 10.1016/j.jpurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Baradaran N, Cervellione RM, Orosco R, Trock BJ, Mathew RI, Gearhart JP. Effect of failed initial closure on bladder growth in children with bladder exstrophy. J Urol. 2011;186(4):1450–4. doi: 10.1016/j.juro.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 14.Ciftci AO, Soyer T, Tanyel FC. A previously unreported variant of exstrophy cloaca. Turk J Pediatr. 2008;50(6):609–12. [PubMed] [Google Scholar]