Abstract
Thanks to developments in the field of nanotechnology over the past decades, more and more biosafe nanoscale materials have become available for use as pharmaceutical adjuvants in medical research. Nanomaterials possess unique properties which could be employed to develop drug carriers with longer circulation time, higher loading capacity, better stability in physiological conditions, controlled drug release, and targeted drug delivery. In this review article, we will review recent progress in the application of representative organic, inorganic and hybrid biosafe nanoscale materials in pharmaceutical research, especially focusing on nanomaterial-based novel drug delivery systems. In addition, we briefly discuss the advantages and notable functions that make these nanomaterials suitable for the design of new medicines; the biosafety of each material discussed in this article is also highlighted to provide a comprehensive understanding of their adjuvant attributes.
Keywords: Drug Delivery, Adjuvant Nanomaterials, Biosafe, Multifunction, Nanoparticle, Controlled Release
INTRODUCTION
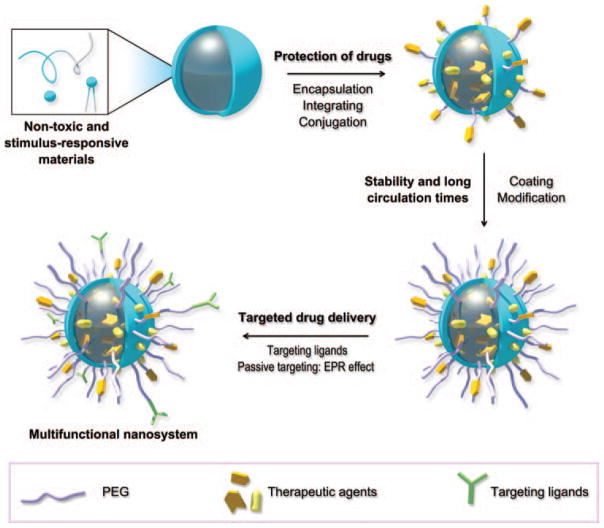
Nanoscale materials, as a new class of pharmaceutical adjuvants, have provided many strategies for designing and fabricating novel medicines with a variety of advantages over conventional medicines.1–4 In the past decades, numerous adjuvant materials were developed for drug delivery but few of them went further to clinical use. There are several reasons for this uncomfortable fact and we summarize these challenges in designing biosafe and efficient drug delivery systems as: (1) Inherent toxicity of adjuvant materials; (2) Drug leakage takes place before reaching the lesion; (3) The unfavorable pharmacokinetics, such as rapid elimination and poor stability in blood environment; (4) The non-targeted drug delivery in vivo. Therefore, the desired characteristics of pharmaceutical adjuvants are as shown in Figure 1. First, the adjuvant materials should be non-toxic as they are selected with an aim toward human health care. Meanwhile, stimulus-responsive materials that can provide controllable drug release are preferred. Drugs could be protected by the adjuvants through encapsulation, integration, or conjugation before they reach the disease site. After administration, adjuvants and drugs should be stable in physiological environment, avoiding from aggregation and reticuloendothelial system (RES) clearance. Surface coating and modification of drug-loaded nanoparticles, such as PEGylation, can improve their stability, resulting in a longer circulation time.5,6 Finally, targeted drug delivery, both active targeting by targeting ligands and passive targeting by taking advantage of the enhanced permeability and retention (EPR) effect (an effect that nano-sized particles and macromolecule drugs tend to accumulate in tumor site rather than in normal tissue due to hypervasculature and little recovery of tumor blood/lymphatic vessels), are desired in pharmaceutical research.7,8 Although nanomaterial’s biosafety remains controversial and bioeffect mechanism needs further study, their unique attributes, such as controllable size and shape,9–11 alternative surface modification,12–14 high surface area to volume ratio and environmentally-responsive structural deformation,15–17 still attract great interest from researchers and have enabled them to design new medicines with more functions, including controlled release of drugs in specific physiological environments, targeted delivery of drugs to lesions, longer circulation time, and better loading efficiency of insoluble drugs.18–21
Figure 1.
The desired characteristics of a multifunctional nanosystem composed of nanoscale pharmaceutical adjuvant materials. (A) The materials should be non-toxic to satisfy biosafety criteria and responsive to stimuli for triggered release of drugs. (B) Drugs should be carefully protected before they reach the disease site. (C) The drug vehicle should be stable in the physiological environment and resistant to clearance. (D) By conjugating with targeting ligands, active targeting can be achieved. Meanwhile, passive targeting can be realized by controlling the size of the multifunctional nanosystem.
Higher accumulation of small anticancer drug molecules in the tumor site can be achieved by encapsulating the drug into nanocarriers, because nanoparticles show preferred retention in tumor as a result of EPR effect.22,23 Targeted drug delivery can be achieved by modifying the surface shell of drug-loaded nanocarriers with ligands which specifically bind to disease markers. Figure 2 illustrates the EPR effect and targeted delivery of nanoparticles, in this case anticancer drug doxorubicin (DOX)-encapsulated micelles, in vitro and in vivo.24 The non-functionalized micelles (M-Dox) showed accumulation in tumor tissue as a result of EPR effect. This nanoparticle was then functionalized with tumor-penetrating peptide CRGDK, the receptor of which are known as neuropilin-1 (NRP-1) protein and are expressed by tumor vessel and by various human carcinoma cells.25–28 The tumor-penetrating peptide functionalized micelles (TPFM-Dox) are internalized more efficiently and exhibit better accumulation and penetration in tumor. By grafting small drug molecules into nanocarriers (nanoparticles, nanorods, nanocages etc.), it is possible to realize a higher loading capacity, and also to deliver insoluble drugs.29,30 Moreover, nanomaterials can be designed to release payloads triggered by environmental changes. In cancer treatment, the slightly altered acidic tumor microenvironment can be used to trigger drug release.31,32 These strategies can help to optimize the biodistribution of anticancer drugs, so that more drug accumulates in the tumor while less is located in the normal organs and the anticancer efficacy is improved with less unwanted side effects. If all these features can be combined in one nanoparticle system, a delivery system could be generated which acts as a multifunctional drug delivery system.
Figure 2.
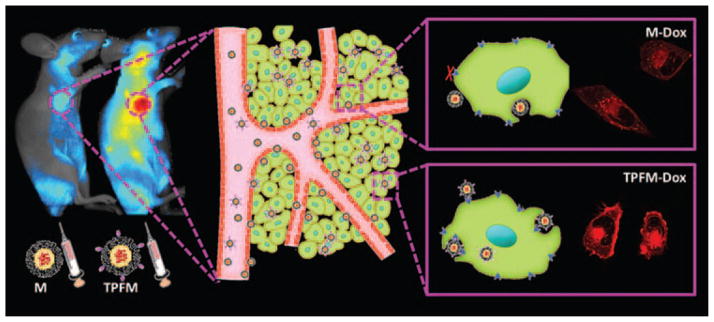
Passive and active targeting of nanoparticles. M-Dox: non-functionalized Dox-encapsulated micelle; TPFM-Dox: tumor-penetrating peptide functionalized Dox-encapsulated micelle. The shade of color in the images stands for the amount of nanoparticles in tumors and in cells. Reprinted with permission from [24], T. Wei, et al., Functionalized nanoscale micelles improve drug delivery for cancer therapy in vitro and in vivo. Nano Lett. 13, 2528 (2013). © 2013, American Chemical Society.
Traditional medical research has focused on finding and screening new chemical entities (NCEs), with the aim of curing the disease with one new drug. However, this method is expensive, time consuming, and full of unknown risks; in addition, the efficiency is very low. It usually takes more than 10 years, and thousands of candidates were tested. It is possible that only one chemical entity will gain access to the market. Some of the eliminated compounds may have had better efficacy than the final winner, but severe side effect or poor biodistribution prevented them from completing the screening process. Even the winner may have fatal drawbacks, like Paclitaxel (PTX). Due to its poor solubility in water, it was first sold in a formulation employing Cremophor EL and ethanol as solvents, which caused serious side effects in patients.33 It was later discovered that PTX and albumin could form nanoparticles in solution, and the efficacy was greatly improved without significant side-effects.34,35 Thus, nanomaterial-based formulation can be used to increase the efficacy of chemotherapeutic agents and reduce the side-effects.
Biosafety is a major issue in the design of novel drugs and researchers must give careful consideration to this when developing nanoscale materials for medical research. The physicochemical properties of nanoparticles, such as size, shape, charge, colloidal stability, and their interaction with environmental compounds, are related to their potential toxicity.36–39 When nanotechnology was first emerging in the 1980s, the biosafety of nanomaterials, including genotoxic and cytotoxic effects, toxicity to the immune system, in vivo biodistribution and clearance, and the long-term effects of treatment, was unclear and more studies were needed to prove that nanomaterials are biocompatible and biosafe.40,41 Biodegradable and biogenic nanoscale materials, such as proteins, nucleic acids, and phospholipids, are commonly used as pharmaceutical adjuvants. The two Food and Drug Administration (FDA) approved nanomaterial-based drug formulations, Doxil® and Abraxane®, employed organic nanomaterials (lipid in Doxil® and protein in Abraxane®).42,43 However, some of them may cause immune response (especially extrinsic proteins) and should be tested before clinical use. Inorganic nanomaterials, like silica and gold, are also employed as pharmaceutical adjuvants in research because there is evidence that they are biosafe.44 However, inorganic materials undergo biodegradation issue and clearance problem, and much research focusing on these two aspects has been done.45–47 How does inert materials, such as gold and carbon, are degraded and cleared from body is the major concern before their clinical application. Some research work also implied that some inorganic nanomaterials would cause genomic instability, inflammatory response, and protein phosphorylation.48–50 In conclusion, both organic and inorganic nanomaterials have their advantages and drawbacks when considering biosafety issue. Neither of them should be neglected when talking about biosafe nanomaterials. The concept “biosafe” in this review does not mean “no harm at all,” but indicates that the nanomaterials we talked about are with low toxicity and immunogenicity, would not cause severe damage in vivo at their applicable dose, and have acquired recognition as potential pharmaceutical adjuvants from most researchers.
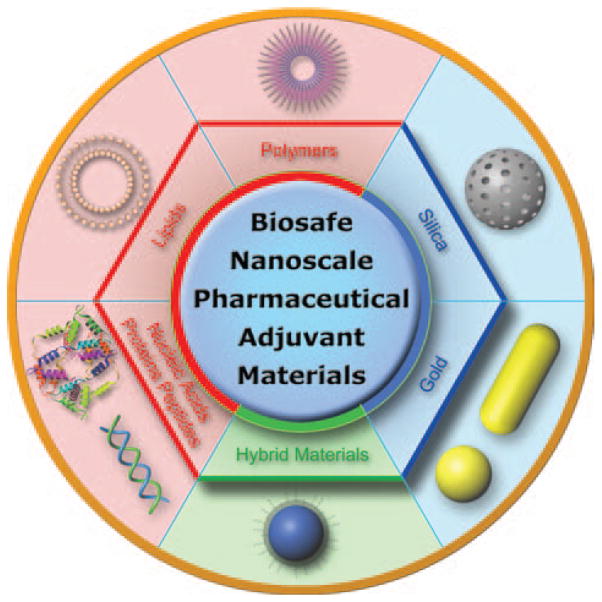
In this article, we will review recent progress in biosafe nanomaterial-based pharmaceutical research, using representative organic, inorganic and hybrid materials as examples (Fig. 3). We also highlight the advantages and remarkable functions that make these nanomaterials ideal for use in medicine design.
Figure 3.
Representative biosafe nanoscale pharmaceutical adjuvant materials.
ORGANIC NANOSCALE MATERIALS EMPLOYED FOR PHARMACEUTICAL DEVELOPMENT
Polymers
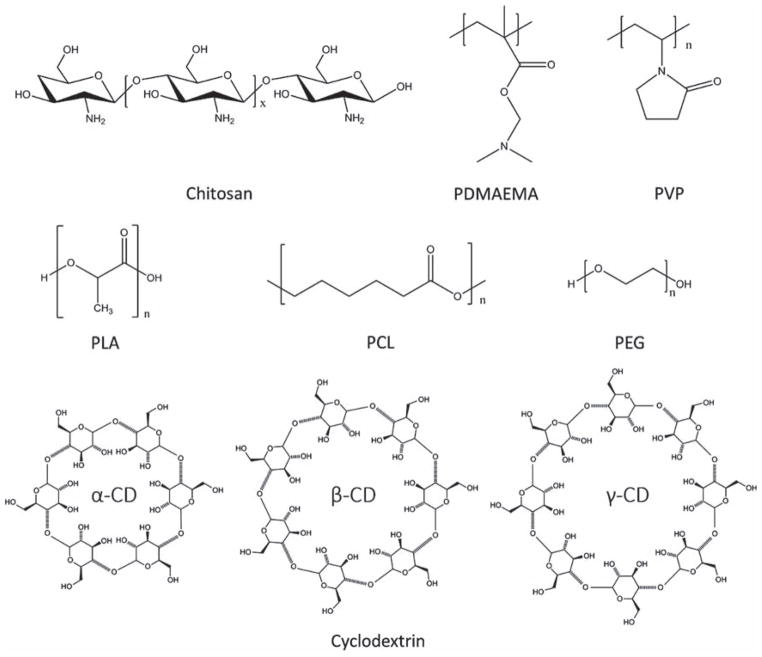
Polymers are an important class of nanomaterial in the nanomedicine field due to their ability to encapsulate and protect cargoes and to respond to specific extrinsic stimuli.51–53 They have been widely used in biomedical research, in applications such as drug delivery,54,55 gene therapy,56–58 cell imaging,59,60 and cancer diagnosis.61 Polymers can be classified into two categories, natural polymer and synthetic polymer, both of which are important pharmaceutical adjuvant materials. Chemical structures of frequently used polymers in nanomedicine design are listed in Figure 4.
Figure 4.
Chemical structures of representative polymers and cyclodextrins as pharmaceutical adjuvant materials.
Natural Polymers
Biocompatible natural polymers have attracted great attentions of researchers, since they have many advantages such as low toxicity and biodegradability.62,63 The most widely studied polymers among them are chitosan and its derivatives. And in order to achieve various functions, many modifications have been applied on the natural polymers.64
Chitosan
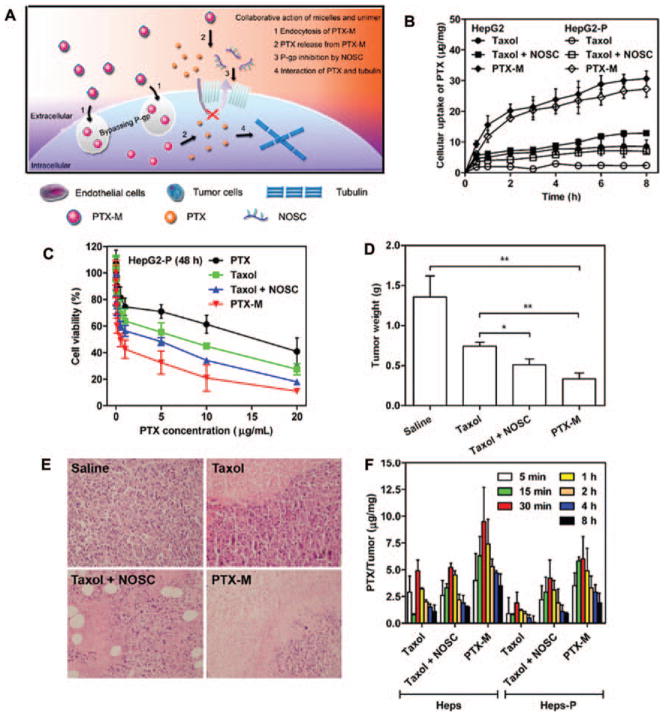
Chitosan, a natural linear polysaccharide, has been used to synthesize nanoparticles for drug delivery and tissue engineering due to its superior biocompatibility and versatile chemical properties.65–68 Chitosan nanoparticles will degrade into non-toxic compounds under the effect of lysozyme. These naturally synthesized biomaterials are biocompatible and biodegradable and are relatively easy to obtain at low cost.69 Chitosan nanoparticles can also be designed to load hydrophobic agents by introducing hydrophobic molecules to chitosan. Therefore, chitosan-based nanoparticles have been employed for delivery of several protein drugs and anticancer chemical drugs, including insulin,70 DOX,71 cisplatin,72 and docetaxel.73 However, chitosan is only soluble in acidic solvents, resulting in the instability of nanoparticles in neutral or alkaline conditions.74,75 Fortunately, chitosan provides chemical functional groups which make chemical modification on chitosan possible. A chemical modified chitosan, N-imidazolyl-O-carboxymethyl chitosan (IOCMCS), was introduced by Bi and co-workers to improve the solubility of chitosan in water and make the IOCMCS-formed nanoparticle positively charged.76 Under the N/P ratio of 5, IOCMCS could efficiently bound with plasmid DNA. It also showed higher transfection efficiency and lower cytotoxicity compared to polyethyleneimine (PEI) and Lipofectamine™ 2000. This work is one of the various successful attempts to make chemical modification of chitosan to break the obstacle of solubility and made it a promising material in the field of medicine research. Another interesting work reported by Zhang et al. recently suggested a new application of chitosan.77 A modified chitosan, N-octyl-O-sulfate chitosan (NOSC), was applied to assemble into micelle for PTX loading. According to previous report, NOSC was possible to inhibit the key drug resistance-related protein P-glycoprotein (P-gp, also known as MDR-1).78–80 Thus, PTX-encapsulated NOSC micelle (PTX-M) could deliver PTX into cells bypassing P-gp. After internalization, NOSC micelle disassembled and inhibited P-gp function, improving PTX retention in cells (Fig. 5(A)). Anticancer efficacy towards HepG2 cell and drug resistance cell HepG2-P of different PTX formulations, including taxol (PTX dissolved in Cremophor EL and ethanol), taxol + NOSC (physical mixture of taxol and NOSC), and PTX-M, was assessed in vitro and in vivo. Cytotoxicity evaluation result indicated that PTX-M possessed the best cell killing efficacy in HepG2-P cells with the lowest half maximal inhibitory concentration (IC 50) (0.55 μg/mL). Meanwhile, IC 50 of PTX was 16.39 μg/mL (Fig. 5(C)). PTX-M also exhibited the best tumor growth inhibition efficacy in vivo as tumor growth was significantly controlled after PTX-M injection (Figs. 5(D), (E)). Further study of PTX accumulation in cells and tumors of different formulations revealed that PTX-M could increase the amount of PTX in cells and tumor tissues which may be a result of the dysfunction of P-gp (Figs. 5(B), (F)). Therefore, drug resistance of HepG2-P cell was successfully overcome in vitro and in vivo by encapsulating PTX into P-gp inhibiting chitosan derivative NOSC. NOSC in this work is a P-gp inhibitor as well as a drug carrier. More interesting work could be developed basing on this material.
Figure 5.
A chitosan-based micelle that can overcome drug resistance of HepG2 cells. (A) Scheme of P-gp inhibition of NOSC and P-gp bypassing of PTX. (B) Cellular uptake of different PTX formulations in HepG2 and HepG2-P cells. (C) Cytotoxicity evaluation of different PTX formulations toward HepG2-P cells after 48 h treatment. (D) Tumor weights of HepG2-P xenograft mice model after different PTX formulation treatment at day 21. (E) H&E stained tumor sections after the treatment at day 21. (F) PTX accumulation in tumor tissue after intravenous injection of different PTX formulations into Heps and Heps-P tumor-bearing mice. Reprinted with permission from [77], X. Jin, et al., Paclitaxel-loaded N-octyl-O-sulfate chitosan micelles for superior cancer therapeutic efficacy and overcoming drug resistance. Mol. Pharm. 11, 145 (2014). © 2014, American Chemical Society.
Synthetic Polymers
Compared to natural ones, synthetic polymers are more designable, and can also be biocompatible by using appropriate compounds while synthesizing.81,82 The method of synthesizing can be various, such as polymerization, covalently binding, and physical assembly. Optimizing polymers to be more functional and biocompatible is now the most important research field of polymer science and attracts many scientists’ attentions.83–85
PEG and Its Derivatives
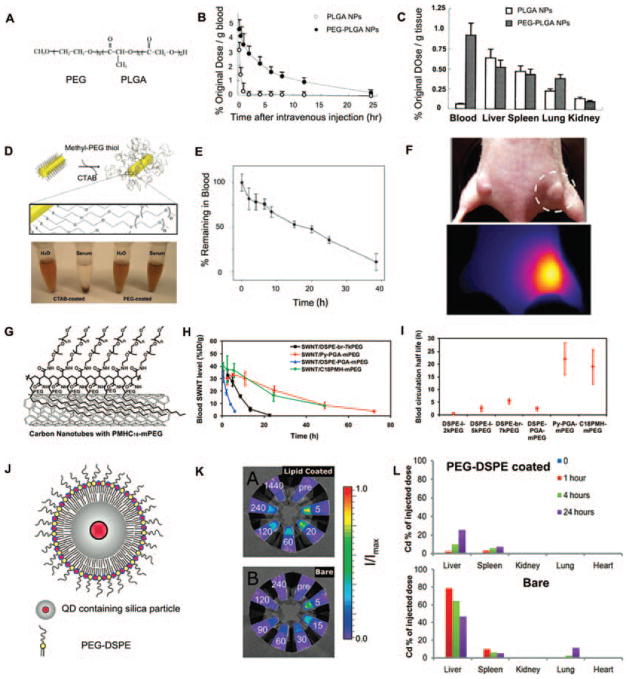
Polyethylene glycol (PEG) is another important pharmaceutical adjuvant in the nanomedicine research field. Most unmodified nanostructures, such as liposomes, micelles, and nanocrystals, usually have a high rate of uptake by the RES, leading to significant accumulation of nanomaterials in the liver and spleen.86 PEGylation, the process of covalently attachment of PEG to another molecule, can increase the hydrodynamic size of nanoparticles and hinder the interaction between nanoparticles and plasma proteins, resulting in reduced immunogenicity. Nanoparticles will then show improved biocompatibility, with ultra-long circulation times and high water solubility after PEGylation.87 Nowadays, many PEGylated medicines, especially PEG-liposomes and PEG-proteins, have got their FDA approval because of prolonged circulation time and reduced immunogenicity.88 Figure 6 summarized several PEG-protected nanoparticles with improved pharmacokinetics. PEG was conjugated with another FDA-approved adjuvant materials, poly(lactic-co-glycolic acid) (PLGA), and reported by Pei and co-workers.89 I125-labeled bovine serum albumin (BSA) was loaded in PEG-PLGA and PLGA nanoparticles to trace circulation and biodistribution of two nanoparticles in vivo. The PEG-PLGA nanoparticle exhibited longer blood circulation time than PLGA nanoparticle. Moreover, PEG-PLGA nanoparticles remained a much higher concentration in blood than PLGA nanoparticle 12 h after intravenous injection (Figs. 6(A)–(C)). PEG is also important surface coating agent of inorganic nanomaterials. PEG-protected gold nanorods (Au NRs) obtained by replacing CTAB with methyl-PEG thiol were stable in serum, while CTAB-protected Au NRs aggregated.90 Blood clearance test showed that PEG-protected Au NRs has a very high half-life of ~ 17 h. 72 h after intravenous injection, PEG-Au NRs were still visible in tumor site by thermal imaging (Figs. 6(D)–(F)). In another work, Dai et al. functionalized single-walled carbon nanotubes (SWNTs) with a branched PEG polymer, named as PMHC18-mPEG.91 The blood circulation time of PEG polymer-protected SWNTs in mice upon intravenous injection was greatly prolonged (t1/2 = 22.1 h) compared to the previous record (t1/2 = 5.4 h). The PMHC18-mPEG was also compared with DSPE-PEG in SWNT protection in vivo and PMHC18-mPEG-coated SWNT showed longer half-life in blood (Figs. 6(G)–(I)). PEG holds great potential in imaging probe protection in vivo. Mulder et al. investigated the biocompatibility and pharmacokinetics of quantum dots (QDs, an important imaging probe) containing silica nanoparticles after PEG coating.92 Both bare and PEG-coated nanoparticles were injected into mice and blood sample were obtained several minutes after injection. Then fluorescence intensity, resulting from QDs, was determined and the results showed that bare nanoparticles were rapidly cleared from blood. Meanwhile, PEG-coated nanoparticles remained in blood and exhibited fluorescence signal even 240 min post injection. Biodistribution analysis demonstrated that less PEG-coated nanoparticles were uptake by RES organ than bare nanoparticles (Figs. 6(J)–(L)). Reduced adsorption of protein on PEG corona is the main mechanism of PEG protection. Griesser et al. provide some evidence to support this viewpoint.93 Nonspecific adsorption of proteins on naked and PEG-protected silica nanoparticles was determined by X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) analysis, and the results confirmed that protein was hardly adsorbed by PEG-protected nanoparticles (Figs. 6(M)–(O)).
Figure 6.
PEG-protected nanoparticles with improved pharmacokinetics. (A) Structure of the PEG–PLGA copolymer. (B) Blood clearance curves of I125-labeled BSA in PEG-PLGA and PLGA nanoparticles. (C) Tissue distribution of I125-labeled BSA at 12 h in PEG-PLGA and PLGA nanoparticles. Reprinted with permission from [89], Y.-P. Li, et al., PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control Release 71, 203 (2001). © 2001, Elsevier. (D) Molecular schematic of PEG coating on NR surface and stability of NRs in water and serum. (E) Blood clearance curves of PEG-protected Au NRs. (F) Thermal imaging of PEG-protected Au NRs in tumor tissue 72 h post intravenous injection. Reprinted with permission from [90], G. von Maltzahn, et al., Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 69, 3892 (2009). © 2009, American Association for Cancer Research. (G) SWNTs coated with PMHC18-mPEG. (H) Blood circulation curves of different PEG-coated SWNTs. (I) Blood circulation half-lives of different PEG-coated SWNTs. Reprinted with permission from [91], G. Prencipe, et al., PEG branched polymer for functionalization of nanomaterials with ultra-long blood circulation. J. Am. Chem. Soc. 131, 4783 (2009). © 2009, American Chemical Society. (J) Schematic representation of PEG-protected QD containing silica particle. (K) Fluorescence intensity of blood samples taken at different time points (indicated in minutes). (L) Biodistribution analysis of bare and PEG-protected particle by cadmium (Cd, a component of the QD) quantification. Reprinted with permission from [92], M. M. van Schooneveld, et al., Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: A multimodality investigation. Nano Lett. 8, 2517 (2008). © 2008, American Chemical Society. (M) Schematic structure of the PEGylated silica nanoparticle. (N) Nonspecific protein adsorption of protein on naked and PEG-protected silica nanoparticles after incubation with protein solution. (O) FTIR spectra of naked and PEG-protected silica nanoparticles after incubation with 5% serum solution. Reprinted with permission from [93], B. Thierry, et al., Electrostatic self-assembly of PEG copolymers onto porous silica nanoparticles. Langmuir 24, 8143 (2008). © 2008, American Chemical Society.
PLA and Its Derivatives
Poly(lactic acid) (PLA) and PLGA are the most widely used polymers as they have been approved by the FDA.94,95 PLA- and PLGA-based nanocarriers were developed to deliver various drugs;96,97 however, the rapid burst of cargo release without any specificity limited further in vivo application due to the low drug concentration at the desired site.98,99 Thus, subsequent work focused on improving the specific release of drugs at the disease site by linking drug release with a specific biological process. Scientists have now successfully synthesized polymeric nanoparticles which are responsive to specific external stimuli, such as light,100 temperature,101 and ultrasound,102 especially to inherently occurred low pH in tumor microenvironment and endosome.103 Compared to the neutral pH in healthy tissues, the lower pH in the tumor microenvironment and in endosomes can be exploited so that nanoparticles release their drug payloads in the right place.104 The most widely used strategy to design pH-responsive polymeric nanoparticles is to introduce a protonation group, such as carboxylic acids and tertiary amines, into the polymer structure which can be ionized at different pH values. Another strategy to achieve pH-responsive polymeric structures is to use acid-labile functional groups which can be hydrolyzed to reveal a new hydrophilic group; the polymer then becomes water-soluble and the nanoparticle structure decomposes, thus releasing the loaded agent. For example, Fréchet et al. synthesized acetal-derivatized dextran-based particles which degraded in a pH-dependent manner (Fig. 7(A)).105 At pH 7.4, the nanoparticles possessed a half-life of 360 h, as judged by measuring free dextran, but when the pH was adjusted to 5, the nanoparticles became unstable and the half-life decreased to 10 h. The ultra-long half-life at neutral pH and significantly decreased stability at acidic pH are ideal for pH-sensitive drug delivery. Nanoparticles would be extremely stable in normal tissue and response sensitively once they get to the disease site. Another merit of this approach is that both hydrophobic and hydrophilic agents were successfully encapsulated within the nanoparticles and, as dextran is biocompatible, these particles are non-toxic to cells in vitro.
Figure 7.
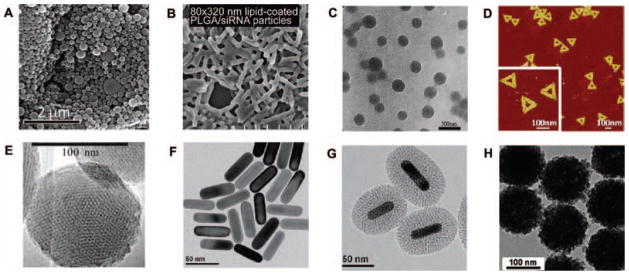
Microscope images of (A) acetal-derivatized dextran nanoparticles. Reprinted with permission from [105], E. M. Bachelder, et al., Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 130, 10494 (2008). © 2008, American Chemical Society. (B) lipid-coated PLGA/siRNA particles. Reprinted with permission from [130], W. Hasan, et al., Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 12, 287 (2011). © 2011, American Chemical Society. (C) cisplatin-loaded milk protein nanoparticles. Reprinted with permission from [148], X. Zhen, et al., Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles. Biomaterials 34, 1372 (2012). © 2012, Elsevier. (D) DNA origami nanostructures. Reprinted with permission from [173], Q. Jiang, et al., DNA origami as a carrier for circumvention of drug resistance. J. Am. Chem. Soc. 134, 13396 (2012). © 2012, American Chemical Society. (E) MSNs. Reprinted with permission from [191], I. Slowing, et al., Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 128, 14792 (2006). © 2006, American Chemical Society. (F) Au NRs; (G) Au@SiO2 nanoparticles. Reprinted with permission from [212], Z. Zhang, et al., Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 24, 1418 (2012). © 2012, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (H) PNiPAM/AA@SiO2 nanoparticles. Reprinted with permission from [227], X. Hu, et al., Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J. Mater. Chem. B 1, 1109 (2013). © 2013, Royal Society of Chemistry.
PVP and Its Derivatives
Poly(N-vinylpyrrolidone) (PVP) has a lot of properties which make it an outstanding candidate as pharmaceutical adjuvant material, such as solubility in water, very low toxicity, good biocompatibility, and high complexation ability.106 PVP was initially used as a blood plasma substitute and then be widely used in medicine and pharmacy.107 The highly hydrated structure of PVP suppressed its interaction with immune system and made PVP a good choice as coating agent to reduce immunogenicity of nanomaterials.108 The slow degradation of PVP under ultraviolet (UV) or ultrasound irradiation is another promising aspect.109 It has also been reported that PVP can cause intracellular vesicular swelling and diminish the fusion of endosomes to lysosomes.110 These unique features of PVP make it a good drug and gene carrier. However, the non-biodegradability of macromolecular PVP will lead to accumulation in vivo. To deal with this problem, PVP with molecular weight below the kidney threshold was synthetized through various methods. Saxena et al. reported a cross-linked PVP nanoparticle of size less than 100 nm which can encapsulate plasmid DNA.111 The result showed these particles have high transfection efficiency both in vitro and in vivo. This synthesis method provided a low molecular weight PVP nanoparticle which was a promising vehicle for drug and gene delivery.
Amphiphilic Copolymers
Copolymers, especially amphiphilic copolymers, are the most common polymers in nanotechnology.112–114 Amphiphilic copolymers can self-assemble into micelles or nanoparticles in aqueous solution, with a hydrophobic core inside and a hydrophilic shell outside. Each chain of the copolymer can also provide different functions, to make the particle multifunctional. For example, Guo et al. synthesized a copolymer composed of three polymers, methoxy polyethylene glycol-block-(polycaprolactone-graft-poly(2-(dimethylamino)ethyl methacrylate)) (PEG-b-(PCL-g-PDMAEMA)).115 The polycaprolactone (PCL) backbone can (1) form a hydrophobic core to carry hydrophobic drugs such as PTX and DOX; (2) improve drug/gene delivery efficiency by enhancing the cellular uptake through effective interaction between the hydrophobic chains and cell membrane. The side chain poly(2-(dimethyl-amino)ethyl methacrylate (PDMAEMA) is positively charged which can condense plasmid DNA and provide endosome escaping capability. As described above, PEG, the biocompatible part of backbone, forms a hydrophilic shell to protect the particle and decrease the surface positive charge. This well designed amphiphilic copolymer can self-assemble into nanoparticle and shows high gene delivery efficiency in vitro.
Lipids
Lipid is a group of natural molecules including fats, sterols, fat-soluble vitamins, glycerides, phospholipids, and others. Among these lipids, phospholipids are widely employed as nanoscale pharmaceutical adjuvants in the form of liposomes and micelles and will be highlighted in this review.116,117 Phospholipids are amphiphilic compounds, with a hydrophilic polar group and a hydrophobic chain, which mimic the components of naturally occurring membrane bilayers.
The best-known lipid-based nanostructures are liposomes, in which an internal aqueous core is separated from the bulk aqueous phase by a closed lipid bilayer shell.118 Micelles, a nanostructure similar to liposomes, are usually composed of closed lipid monolayers with a fatty acid core and a polar surface, or a polar core with fatty acids on the surface (inverted micelle). Nowadays, many amphiphilic polymer-based micelles have been developed as a result of their more versatile structure and function compared to that of lipid.119,120 Liposomes are capable of carrying both hydrophobic and hydrophilic drugs at the same time. The hydrophobic drugs can be integrated into the fatty acid shell while the hydrophilic drugs can be loaded into the aqueous core. In contrast, either hydrophobic or hydrophilic drugs can be encapsulated into micelles, determined by the core’s polarity. Micelles are much smaller in size (1–20 nm) than liposomes (100–200 nm); however, the size of micelles is more uniform. The surface of liposomes can also be functionalized with a variety of ligands and reporters, such as antibodies,121 nucleic acids,122 and luminescent sensors.123 The methods for liposome preparation are well developed and the process is very easy to control. Under specific conditions, amphiphilic lipids will self-assemble into liposomes. By adjusting the formulation (lipid ratio, source) and changing the synthesis conditions (temperature, solvent, ion concentration, and sonication), the size, uniformity, and stability of liposomes can be manipulated. The first FDA-approved nanodrug, known as Doxil® in the market, is a liposome loaded with the anticancer drug DOX. DOX is commonly used in the treatment of a wide range of cancers; however, the adverse side effect is life-threatening heart damage.124 Using the Doxil® formulation, the drug circulation time is prolonged and more drug accumulates in the tumor site as a result of the passive targeting of liposomes to the leaky tumor vasculature.125
Lipids can also be grafted onto other nanomaterials as surface coatings for further modification, better stability, and improved biocompatibility.126,127 PLGA nanoparticles are great siRNA carriers due to their stability and low toxicity.128 However, the transfection efficiency of PLGA nanoparticles is low and not comparable to that of cationic lipid nanoparticles.129 Thus, DeSimone et al. combined them and developed lipid-coated PLGA nanoparticles that could deliver multiple siRNAs for the treatment of prostate cancer (Fig. 7(B)).130 The encapsulation efficiency was high (32–46%) and target gene expression was greatly down-regulated.
Lipids can be classified according to their surface charge: neutral lipids, anionic lipids, and cationic lipids. Cholesterol is separately introduced due to its special role in lipid-based nanomaterials. Table I shows the commonly used lipids in pharmacy research.
Table I.
Structures of commonly used lipids in pharmacy research.
| Lipid | Abbr. | Structure |
|---|---|---|
| L-α-phosphatidylcholine, hydrogenated (Soy) | HSPC |
|
| 1,2-dioctadecanoyl-sn-glycero-3-phosphoethanolamine | DSPE |
|
| 1,2-dioctanoyl-sn-glycerol | DG |
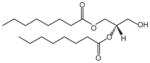
|
| L-α-phosphatidic acid | PA |
|
| L-α-phosphatidylglycerol | PG |
|
| 1,2-dilauroyl-sn-glycero-3-ethylphosphocholine | Ethyl PC |
|
| 1,2-di-O-octadecenyl 3-trimethylammonium propane | DOTMA |
|
| Cholesterol | Chol. |
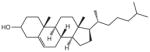
|
Neutral Lipids and Anionic Lipids
Neutral lipids and anionic lipids are similar in character and application. They are the main composition of conventional liposomes for pharmaceutical applications. Phosphatidylcholine (PC), phosphoethanolamine (PE), 1,2-dioctanoyl-sn-glycerol (DG), L-α-phosphatidic acid (PA), L-α-phosphatidylglycerol (PG) and their derivatives are commonly used in various liposomes. For instance, PC is a major constituent of cell membranes, which have been shown to stabilize membranes and can form a lamellar structure that is insensitive to the pH.131 Due to their membrane structure and neutral or negatively charged surface, neutral and anionic liposomes can decrease the non-specific protein adsorption and remain longer in blood circulation in vivo. Awasthi et al. prepared PEGylated neutral and anionic liposome-encapsulated hemoglobin (LEH).132 The neutral and anionic LEH showed long-lasting ability and the circulation half-life of LEH was improved after PEGylation. Besides, neutral and anionic lipids can be modified and cooperated with other functional groups. We have recently reported a novel pH-sensitive liposome which can circumvent the DOX-resistance of cancer cells.133 One way that cells develop multi-drug resistance (MDR) is by expressing high levels of certain membrane proteins, such as P-gp, which mediates efflux of intracellular drugs.79,80 We developed a system to create a sudden increase in the intracellular drug level, thus killing the cell before drug export can occur. This liposome is composed of neutral lipids hydrogenated soy phosphatidylcholine (HSPC) and PEGylated 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE). We loaded liposomes with ammonium bicarbonate and DOX and demonstrated that at pH 5.0, the bicarbonate ions decomposed and released CO2 gas, which raised the internal pressure and ruptured the liposomes. When these liposomes reached the endosomes (pH ~ 5.0) in MDR breast cancer cells, they released all their DOX at the same time and the intracellular drug concentration reached the effective concentration immediately. Thus, resistant cells were killed before their MDR mechanisms had time to work. This work illustrates a new strategy for designing and synthesizing pH-sensitive liposomes.
Cationic Lipids
Cationic lipids, for example ethyl phosphocholine (ethyl PC), 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA), and cationic cholesterol, have cationic hydrophilic head to attract negatively charged molecules, especially nucleic acids, and form complexes. In this way, cationic lipids can be used as nucleic acid carriers and are regarded to be the most promising non-viral gene vectors.134 Tenchov et al. synthesized various ethyl PCs with different hydrophobic moieties, and investigated their transfection efficiencies in human umbilical artery endothelial cells (HUAEC). The result showed that the compounds exhibited very high transfection efficiencies.135 Through the investigation of liposome phase-conversion during transfection process, they found that high transfection efficiency was resulted from enhanced liposome fusion with cellular membranes. This work could be useful to assess the effect of cationic lipid carriers and for further design of new and effective gene delivery system.
Cholesterol
Cholesterol is an essential structural constituent of animal cell membranes. The addition of cholesterol to lipid bilayers decreases its permeability to water and thus drug leakage is prevented.136 Cholesterol is also incorporated into the liposome layers to increase their ordered state as it has an additional stabilizing effect.137 It limits the mobility of acyl chains of phospholipids, rigidifies the membrane in the liquid-crystalline phase, and increases the mobility of these chains while in the gel phase. This character decreases the phase transition temperature of lipid membranes, a parameter that is important for the relative fluidity and mobility of lipid molecules in the bilayer. Thus, cholesterol is a regulator in lipid bilayer and plays an important role in lipid-based nanoparticles.
Proteins, Peptides and Nucleic Acids
Proteins, peptides, and nucleic acids have many different roles in the development of nanomedicines. They can act as drugs (e.g., interferon, insulin, tesamorelin, siRNA), target ligands (e.g., aptamer, antibody, nuclear localization signal peptide), and carriers (e.g., albumin, DNA origami). As they are the most common materials in the human body, their biodegradability is in no doubt. Much higher systemic concentrations of proteins, peptides, and nucleic acids can be administered than that of inorganic nanoscale materials. As pharmaceutical adjuvants, proteins, peptides, and nucleic acids are irreplaceable because of their targeting ability. Illustrative examples of targeting ligands, including proteins, peptides, and nucleic acids, are listed in Table II. Metabolism mechanism of them is also clearly studied, making them more advantageous than other materials. However, the disadvantage is that their structures are vulnerable and it is difficult to make modifications. Once the fine-designed structure is changed, they will lose functions and integrity. Another issue that should be addressed is the immunogenicity as immune system will be activated by exogenous proteins and DNA sequences.138
Table II.
Examples of targeting ligands.
| Category | Name | Target and indication | Refs. |
|---|---|---|---|
| Protein | |||
| Antibody | Trastuzumab (Herceptin®) | HER2/neu | [230, 231] |
| Bevacizumab (Avastin®) | VEGF | [232] | |
| Cetuximab (Erbitux®) | EGF receptor | [233, 234] | |
| Rituximab (Rituxan®) | CD20 | [235] | |
| Ibritumomab tiuxetan (Zevalin®) | CD20 | [236] | |
| Tositumomab and tositumomab (Bexxar®) | CD20 | [237] | |
| Gemtuzumab ozogamicin (Mylotarg®) | CD33 | [238] | |
| Alemtuzumab (Campath®) | CD52 | [239] | |
| Others | Transferrin | Transferrin receptor | [240, 241] |
| Low density lipoprotein | Folate receptor | [242] | |
| Heavy-chain ferritin | Transferrin receptor 1 | [243] | |
| Peptide | RGD | ανβ3-integrin | [244, 245] |
| CRGDK | Neuropilin-1 | [24] | |
| NGR | CD13 | [246] | |
| SP5-52 | Tumor neovasculature | [247] | |
| PIVO-8 | Tumor angiogenesis | [248] | |
| PIVO-24 | Tumor angiogenesis | [248] | |
| LyP-1 | Tumor hypoxia and lymphangiogenesis | [249] | |
| RVG | Acetylcholine receptor | [250] | |
| TAT | Cell nucleus | [166, 251] | |
| Nucleic acid: aptamer | Macugen | Vascular endothelial growth factor | [252] |
| AS1411 | Nucleolin | [253, 254] | |
| sgc8 | Protein tyrosine Kinase 7 | [255] | |
| S1.3/S2.2 | Mucin 1 | [256] | |
| TD05 | Immunoglobulin μ-heavy chains | [255] | |
| A10 | Prostate-specific membrane antigen | [257] | |
| TBA | α-thrombin | [258] | |
| Apt-αvβ3 | αvβ3 integrin | [259] | |
| B28 | HIV gp120 | [260] | |
| A30 | HER3 | [261] | |
Proteins
Self-assembled protein-based nanocages can be considered as natural drug carriers. In nature, some protein structures, such as microtubules, are assembled from multiple copies of protein subunits.139 Recently, albumin, gelatin, and silk protein-based protein nanoparticles have been developed as drug delivery systems following the successful model of Abraxane®.140–142 These particles are biosafe and non-antigenic; furthermore, they can realize active targeting as well as passive targeting. For example, albumin can penetrate the microvessel endothelial cells in angiogenic tumor vasculature with the assistance of the albumin-binding protein gp60 and combine with the target protein SPARC which is over-expressed in many tumors.143,144 Cao and co-workers used protein nanoparticles for active tumor targeting.145 They employed human ferritin H-chain protein (FTH1) as the framework and combined it with epidermal growth factor (EGF), the ligand of EGFR, a receptor that is over-expressed in many malignant tissues.146,147 The EGF-FTH1 nanoparticles were specifically internalized by two breast cancer cell lines, MCF-7 and MDA-MB-231, but the uptake by normal epithelial MCF-10A cells was negligible. In vivo experiments also demonstrated the accumulation of EGF-FTH1 nanoparticles in breast tumors in a mouse xenograft model. Jiang et al. cross-linked the milk protein casein with transglutaminase to form nanoparticles, which were then loaded with cisplatin, a model anti-cancer drug (Fig. 7(C)).148 In the murine xenograft model, cisplatin-loaded nanoparticles inhibited tumor growth 1.5-fold more effectively than free cisplatin and analysis of tumor sections demonstrated that the casein nanoparticles could penetrate through the tumor tissue and deliver cis-platin to cells far from the vasculature.
Collagen is another important biosafe nanomaterial that should be addressed. It is the main structural protein in animals and accounts for one third of total protein in human.149 28 types of collagen have been identified in vertebrate and 90% of the collagen in the body is type I.150,151 As collagen is the major component of the extracellular matrix (ECM), the biocompatibility of collagen is good.152–154 In a recent work reported by Cai and coworkers, collagen was linked to silica nanoparticles via disulfide bond as a protein cap to protect encapsulated fluorescence probe FITC.155 Silica nanoparticle is an excellent reservoir for drugs, and end-capping of them could protect encapsulated cargos, separate cargos from external environment. As a capping material, collagen has advantages in biosafety. In addition, collagen is full of sulfydryl, which are necessary to form disulfide bond that can be broke by various reducing agents, including cell-expressed glutathione.156 Then, specific cargo release from nanoparticles in cytoplasm can be realized. This work subtly utilized this property and conjugated collagen with nanoparticle through disulfide bond. Redox-responsive release of cargo FITC from nanoparticles was successfully identified after adding another reducing agent, dithiothreitol (DTT), to the nanoparticle solution. Targeting moiety, lactobionic acid (LA), was also introduced to the collagen-capped nanoparticle and was demonstrated to be effective in cell-specific targeting in vitro. Therefore, redox-responsive release and targeted delivery were achieved in this work.
Gelatin, a derivative of collagen, has similar potential as pharmaceutical adjuvant just like collagen. It is widely used in food and pharmaceuticals as gelling agent.157 Gelation would be easily triggered by increasing the temperature and suddenly cooling of gelatin solution. Nanoparticles protected by a gelatin cap could then be obtained by this method. He, Wang, and co-workers reported a work employing gelatin as a cap to protect DOX-loaded nanoparticles recently.158 The gelatin cap was successfully grafted to the nanoparticles by temperature-induced gelation and stabled by glutaraldehyde mediated cross-linking. Interestingly, gelatin is a natural pH-sensitive agent and the authors take this advantage and successfully made the gelatin-capped nanoparticles pH-sensitive. As a mixture of protein and peptide, gelatin contains many protonation groups like carboxyl and amine, making it negatively or positively charged determined by the pH value. Moreover, the isoelectric point of gelatin can be modified by removing or retaining the protonation group.159 Thus, the charge of gelatin can be precisely controlled. In this work, the loaded-DOX was well-protected upon neutral pH and released from the nanoparticles once the solution was adjusted to be acidic. This pH-sensitive behavior was also confirmed in vitro by co-localization analysis of DOX and endosome assisted by confocal laser scanning microscopy (CLSM). DOX was first trapped in endosome, exhibiting high co-localization ratio with endosome at 5 h. Then DOX escaped from endosome and entered into cell nucleus at 12 h, indicating that gelatin cap was eliminated from the nanoparticle surface.
Peptides
Peptide, natural outcome from protein metabolism, played a crucial role in biological activity. Accompanied by the progress of peptide synthesis, peptides are more widely used as therapeutics, targeting ligands and drug vectors.160,161 Especially, peptide presents an advanced and rapid developing in the field of drug delivery. Compared to other drug carrier materials, peptides possess several advantages for drug delivery, including smallness, versatile structures, facile synthesis, biocompatibility, and biodegradability.162
Many studies have confirmed the potential and superiority of peptide applied as drug delivery system. For example, Divita and co-workers successfully developed a peptide-based novel method to delivery siRNA into mammalian cells.163 They designed an amphiphilic peptide (CADY) of 20 residues combining aromatic tryptophan (Trp, W) and cationic arginine (Arg, R) residues. The recombinant peptide forms stable complexes with siRNA, thereby increasing the stability of the complexes forming from peptide and siRNA and improving their transfection efficiency into a wide variety of mammalian cells, notably including primary cell lines. The siRNA delivery based on the CADY peptide significantly knockdown the target gene at subnanomolar concentration of siRNA. And more importantly, the cytotoxicity results showed that the CADY peptide was non-toxic and entered cells in an endosome-independent way, delivering therapeutics into cells without enzymolysis under lysosome enzymes. CADY peptide can then be used for the delivery of unstable drugs and those whose therapeutic efficacy are compromised due to the lysosome sequestration. Meanwhile, peptides possess a great potential in the chemotherapy drug delivery. Recently, a self-assembled peptide hydrogel formed by peptide and anti-cancer drug taxol was successfully designed by Yang and co-workers.161 The peptide hydrogels of taxol could markedly inhibit tumor growth and metastasis through local administration of taxol hydrogels. In addition, the peptide-based taxol hydrogels enhanced the dosage tolerance of mice to taxol by reducing the plasma concentration of taxol which is due to the local administration, and finally decreased the side effects of taxol. Peptide hydrogel can be applied for controlled and sustained release of drugs to skin tissues, which is important for wound healing, anti-inflammation, and dermatosis.
Except for drug carrier, peptide also plays an important role as functional agent in drug delivery system. Cell penetrating peptides (CPPs) and pH-sensitive peptides are two major categories of functional peptides. CPPs are a series of peptides which can facilitate cellular uptake, endosomal escape, and nuclear targeting.164,165 Tat peptide, a CPP derived from transactivator of transcription (tat) of type I human immunodeficiency virus (HIV-1), has been widely used in drug delivery and gene transfection.166,167 Polyhistidine, as a famous example of pH-sensitive peptide, has a pKa of ~ 6.5. When the pH is turned to be below 6.5, polyhistidine would shift from hydrophobic to hydrophilic.168,169 Caruso et al. recently successfully combined these two functional peptides in one, named H4R4, and reported its application in nanoparticle-based drug delivery.170 R4 stands for 4 arginines, and it is mimicking the functional region of tat peptide which is rich of arginine (GRKKRRQRRRPQ). H4 is 4 histidines, and is the pH-sensitive part of the combined peptide. R4, like many other CPPs, is highly hydrophilic and cannot be used alone without conjugation in nanoparticle functionalization. However, conjugation makes it hard to vary the amount of CPP used.171 Here H4 provides a hydrophobic part and makes H4R4 an amphiphilic peptide under neutral pH. It is simply mixed with a PEG-DOX conjugate and a poly(2-diisopropylaminoethyl methacrylate) (PDPA) homopolymer. PEG-DOX and PDPA can assemble into a nanoparticle with a hydrophilic corona and hydrophobic core, which provide space for the amphiphilic H4R4. In the acidic endosomal compartment, H4 become hydrophilic and H4R4 is released from the nanoparticle. Then, R4 could facilitate the release of PEG-DOX from endosome and its further entrance into cell nucleus. The PEG-DOX loaded in H4R4 mixed nanoparticle exhibits better endosome escape ability and higher accumulation in nucleus than that loaded in non-H4R4 mixed one, confirmed by co-localization analysis. More importantly, the cytotoxicity of the nanoparticle is tunable by adjusting the amount of H4R4 mixed. This work provides us a new sight in the application of peptides. Peptide is versatile in functions and combination of two functional peptides is easy. We may have more multifunctional drug delivery systems in peptide-involved nanoparticles.
Nucleic Acids
Nucleic acids are considered to be fragile due to their instability in physiological environments which contain DNase and RNase. It may seem impossible that nucleic acids could be used as pharmaceutical adjuvants to carry drugs, but the opposite has proved to be true. Compared to the common linear structure, engineered DNA structures can be very stable, and have been used to build nanoparticles for the past 30 years. DNA nanostructures have great potential in the areas of molecular and cellular biophysics, energy transfer and photonics, and diagnostics.172 Recently, Ding and co-workers successfully applied “origami” DNA nanostructures, made of folded nucleic acid strands, to the drug delivery field (Fig. 7(D)).173 The electrostatic attraction between DOX and DNA structure was subtly used in this work. The stability issue of DNA was also settled by converting linear strands into origami nanostructure. These self-assembled, spatially addressable, DNA origami nanostructures were very stable in cell lysates, and the DOX loading efficiency was high because the drug was non-covalently bound to the DNA through intercalation. The cellular uptake of DOX was increased by this DNA origami nanostructure, resulting in a better cell killing efficiency. Meanwhile, RNA nanotechnology has achieved rapid development in biomedicine applications in recent years. A packing RNA (pRNA) of bacteriophage phi29 DNA-packaging motor is developed by Guo and coworkers to work as a highly efficient nanovector to carry siRNA (MT-IIA) for cancer therapy.174 The pRNA/siRNA can protect the siRNA from RNase and keep stable in serum. The messenger RNA (mRNA) levels of MT-IIA and its downstream gene survivin were down-regulated by this pRNA/siRNA complex, resulting in suppressed cell proliferation. Furthermore, the dimmers that were obtained by tagging the pRNA/siRNA complex with folate can be delivered cell–specifically to ovarian cancer cells which express folate receptor. From both examples we can find that stability is the major problem for nucleic acids as pharmaceutical adjuvants. If this can be settled properly, DNA and RNA have a promising future as a potent tool for drug delivery.
Other Organic Materials
Besides polymer, lipid, protein, peptide, and nucleic acid, saccharide is another important nanoscale pharmaceutical adjuvant materials. Cyclodextrins (CDs), a family of compounds made of saccharide molecules forming a ring structure, will be introduced as an outstanding saccharide. They are produced from starch by enzymatic conversion.175 CDs are composed of 5 or more α-D-glucopyranoside units and typical CDs are formed by 6–8 monomers in a cone shape, known as α-CD (6 monomers), β-CD (7 monomers), and γ-CD (8 monomers) (Fig. 4).176 Compared to chitosan and other polymers, CDs are commonly used as one of the most versatile aids in pharmaceutical technology to enhance the aqueous solubility and chemical stability of biologically active compounds.177 Especially by using guest-host chemistry, CDs can introduce the guest functional groups to the host compounds. Ma et al. conjugated β-CDs to a branched PEI as a host unit.178 Then a guest hydrophobic polymer poly(β-benzyl L-aspartate) (PBLA) was introduced to the host scaffold to mediate the assembly process. The hydrophobic core formed by PBLA serves as a nanocarrier to load hydrophobic drugs, while the positively charged hydrophilic PEI shell is capable of condensing plasmid DNA and achieve its transfection in vitro. In this way CDs produce a new way of assembly, and can be used as a new generation of multifunctional nanocarriers.
INORGANIC NANOSCALE MATERIALS AS POTENTIAL ADJUVANTS IN PHARMACEUTICS
Silica
In the past decade, silica-based nanoparticles have attracted more and more attention as pharmaceutical adjuvants.179 The biocompatibility of silica nanoparticles has also been thoroughly studied in different cell lines, and there is strong evidence that they are only toxic at a high dose.180 Some recent results indicated that silica nanoparticles are biocompatible in mice after serological, hematological, and histopathological examinations of blood samples and tissues.181
Silica Nanoparticles
The most well-developed of these silica-based nanoparticles are mesoporous silica nanoparticles (MSNs) because of their tailored mesoporous structure and high surface area.182–184 Mesopores can be modified with different chemical groups to be hydrophobic or hydrophilic, enabling MSNs to encapsulate a variety of drugs. Meanwhile, high surface area allows a much higher drug loading capacity compared to that of solid nanoparticles.
By preferentially manipulating the biodistribution of encapsulated pharmaceutical agents in the target organ/tissue, MSN-based systems have the potential to deliver an effective concentration of drug to the lesion at a relatively low systemic drug dose. The adverse side-effects can therefore be minimized without compromising treatment efficacy. To accomplish this goal, the surface of MSNs can be modified with a variety of functional agents, such as polymers, proteins, and stimulus-responsive groups. These surface coatings work as gatekeepers to protect the pharmaceuticals inside the pores and achieve a controlled release.185,186 β-CD, a saccharide which is responsive to the acidic conditions within endosomes, was employed by Zink and co-workers to work as a nanovalve in MSNs for pH-sensitive drug release.187 This nanovalve is tightly closed at pH 7.4 and acidic environment is the key to open the valve and release cargos. The leak and rapid burst of drug from nanoparticles are crucial problems for drug delivery and nanovalve is a good strategy to overcome these challenges. Mesopores can be sealed under physiological condition and release drug slowly under certain stimuli. Figure 8 shows three typical nanovalve-based controlled release mechanisms. Gatekeeper molecules are covalently attached to MSNs while plug molecules are then plugged into the gatekeepers through host–guest interaction. In some work only gatekeeper molecules are employed. Initially, cargo molecules are loaded in the channels of MSNs. Drug release can be initiated in three ways:
Figure 8.
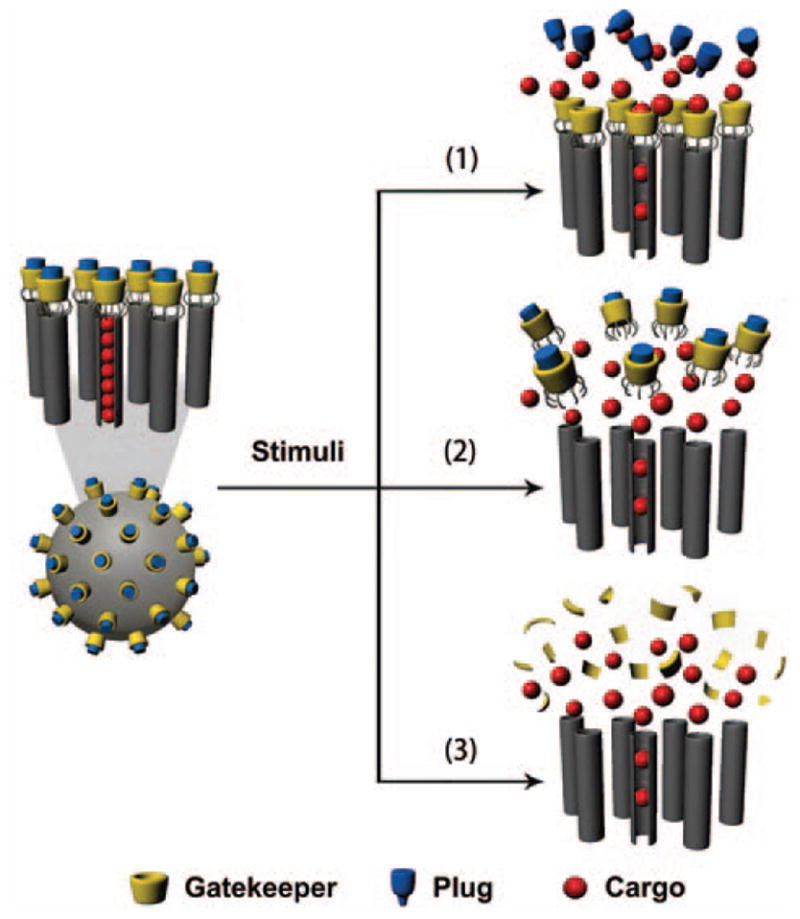
Schematic illustration of three different nanovalve-based stimuli-responsive controlled drug release mechanisms in MSNs.
cutting off the covalent bonds between gatekeeper molecules and MSNs;
pulling out the plug molecules by interfering host-guest interaction;
destroying gatekeeper molecules.
These mechanisms can be designed to be responsive to various stimuli, including pH, light, thermal, ultrasound, oxygen stress, and several enzymes. It is a hot pursued area to develop smart drug delivery systems with nanovalves. Reversible and precisely controlled nanovalves will be the future trend.
MSNs are also good candidates for multifunctional nanoparticles. Nanoparticle-based drug delivery systems are confronting many challenges, including uptake by the RES, interaction with serum proteins, and poor accumulation at the disease site. Thus, researchers have concentrated much effort on integrating several functions into one particle and, as a result, many MSN-based multifunctional nanoparticles have recently been developed.188–190 Lin et al. functionalized MSNs with folate groups, the receptors of which are highly expressed in human cancer cells (Fig. 7(E)).191 They demonstrated that the endocytosis of the modified nanoparticles is a receptor-mediated process and can be inhibited by free folic acid. Hyeon and co-workers reported functionalized MSNs that could be used for magnetic resonance imaging (MRI), fluorescence imaging, and drug delivery, thus combining therapeutic and diagnostic functions.192 DOX was loaded into the pores and the particles were stabilized by coating with PEG to increase the circulation time. The anticancer effect of DOX-loaded nanoparticles was also observed in vitro and in vivo. In this work, both MRI and PEG coating are employed for integrated multifunction. MRI is superior than fluorescence imaging in clinical use because the latter will photobleach and the imaging depth is only a few millimeters. PEG is the best stealth delivery cover so far as introduced in the polymers section. MSN is a well-established integration platform on which multiple functions could be combined thanks to the versatility and compatibility of MSNs.
Compared to other adjuvant materials, MSNs are superior in various aspects: higher available surface area allows for more functionalization; tailored mesoporous structure provides sealed compartment for hydrophilic and hydrophobic cargos; strong adsorptive capacity results in higher drug loading; toxicity study and previous application as food additive stands for their biosafety. However, we should also pay attention to the hydrolysis of MSNs’ backbone (Si–O–Si) caused by acidic pH environment. In addition, unmodified silanol groups on MSNs will interact with red blood cell membrane and cause hemolysis.193 Further modification of silanol groups and hemolysis test are needed before MSNs’ intravenous injection in the pre-clinical development.
Silica Nanotubes
Silica nanotube (SNT), a novel nanomaterial, has been developed for drug delivery,194 biocatalysis,195 and protein immobilization.196 SNT has several advantages including large internal volume, large surface area, favorable biocompatibility, and the ability of being chemical modified.197 More importantly, the size of SNT can be controlled easily.198 Because of their unique physicochemical properties, SNT used as drug delivery system drew considerable attention from pharmaceutics researchers in recently. SNT possesses the potential as effective drug delivery vehicle owing to
the large internal space of SNT can improve the drug loading capacity compared with other vectors. And the drug molecules incorporated in the inner of material cannot interact with the environment, so that the drug leakage was prevented until they reach the target destination;
SNT possesses a plentiful of hydroxide radical on the surface which lead to the high hydrophilicity of nanotubes. The drug delivered by SNT can effectively avoid the capture by the RES, and improve the bioavailability of drug molecules;
SNT can be further modified to acquire more functions through the active hydroxide radicals on the surface. Bhattacharyya and coworker reported the sol–gel template synthesized SNTs that can be applied for controlled drug delivery in which ultrasound acts as the trigger.194
In order to control drug delivery, SNTs were coated by dihydroxynaphthalene (DN) and ibuprofen was chose as a model drug. The ibuprofen loading of SNTs and DN-SNTs analyzed by thermogravimetric analysis was 46% and 25%, respectively. The release kinetics of ibuprofen from SNTs demonstrated that the drug release is heavily dependent on the ultrasound impulse in vitro. Fluorescent silica nanotubes prepared via sol–gel reaction were also successfully applied for plasmid DNA delivery.199 This work further demonstrated that SNTs have enormous potential in biomolecule drug delivery.
Gold
Among metal nanomaterials, those made of gold have been widely utilized in biomedical research. Gold nanomaterials have a variety of potential applications, including gene and drug delivery,200 cell imaging,201 thermotherapy,202 and colorimetric detection of biomolecules and ions.203–205 Nowadays, the synthesis and modification of gold nanomaterials can be effectively manipulated, and spheroids, rods, dodecahedra, octahedra, and cubes can be designed with a high level of precision.206 The surface can also be modified with chemical functional groups, including amines, lipids, antibodies, peptides, oligonucleotides, and small molecules. In addition, the inherent non-toxicity and unique properties of gold nanomaterials, such as size- and shape-dependent cellular uptake and high surface area to volume ratio, make them excellent drug carriers.207 Thus, considerable effort has been put in to develop gold nanomaterials as pharmaceutical adjuvants.
Gold Nanoparticles
Gold nanoparticles (Au NPs) have many attributes which meet the needs of nanomedicine research. Many works have focused on the modification of Au NPs with therapeutic agents and targeting ligands. Liang and co-workers recently synthesized novel small (2 nm) Au NPs that were functionalized with a therapeutic peptide, PMI (p12), and a targeting peptide, CRGDK, which selectively binds with cancer cells through the over-expressed receptor NRP-1.208 The results demonstrated that these targeting nanocarriers improve the delivery of the therapeutic p12 peptide, which is thought to activate p53, into cancer cells. When employing Au NPs as drug carriers, conjugation is commonly used for drug loading and ligand modification. The problem is that available chemical groups on the surface are limited, so it is difficult to functionalize Au NPs with both therapeutics and targeting ligands. This work employed 2 nm Au NPs, the surface area to volume ratio of which is much higher than that of commonly used Au NPs, to provide more space for drugs and functional groups.
Gold Nanorods
The localized surface plasmon resonance (LSPR) effect is a widely utilized property of some special shaped gold nanomaterials, including nanorods, nanocages, nanoshells, and nanostars. Plasmon resonance occurs when gold nanomaterials are irradiated by light with specific wavelengths, and the luminous energy is converted into heat.209 Direct killing of surrounding cells can be achieved by taking advantage of the heat released. This is called photothermal therapy. The heat released can also be further utilized to control the behavior of nanomaterials by introducing thermosensitive materials into them.210 Therefore, many gold nanomaterial-based light-controlled drug release systems have been developed based on the LSPR effect. Moreover, the absorption of light with specific wavelengths can be utilized for in vivo imaging (Fig. 9).
Figure 9.
Applications of LSPR effect of gold nanostructures in pharmaceutical research.
Among these gold nanostructures with LSPR effect, Au NRs are an outstanding one due to two reasons: (1) the uniformity and size of Au NRs are easier to control than that of other materials; (2) by manipulating the aspect ratio, the LSPR maximum of Au NRs can be tuned to the near-infrared (NIR) window (700–900 nm), which is the wavelength range in which lasers can penetrate deeply through tissues.211 Wu and co-workers reported promising results in this area (Figs. 7(F), (G)).212 They coated light-responsive Au NRs with mesoporous silica to overcome two major disadvantages of Au NRs, namely low specific surface area and poor stability. DOX was encapsulated into the mesoporous silica shell of the Au@SiO2 nanoparticles. Low intensity laser irradiation triggered drug release, while high intensity laser irradiation also triggered hyperthermia. Furthermore, the Au@SiO2 nanoparticles can be visualized by two-photon microscopy, so that imaging is also successfully integrated into this platform. The advantage of Au NRs is that they can be used as drug vehicle, photothermal agent, and two-photon microscopy probe. Thus, Au NRs are an inherent multifunctional drug delivery system. However, low loading capacity and template residues caused toxicity impede their application as pharmaceutical adjuvant. This work successfully solved both challenges at the same time by mesoporous silica coating, the unique properties of Au NRs were also maintained and utilized.
Photothermal therapy is a unique application of gold nanomaterials as they are not just drug vehicles, also therapy agents. The irradiation area, time, and intensity are tunable and the killing effect is direct. Future work should focus on the targeting ability of photothermal therapy agents as the therapy efficacy is determined by the biodistribution of heat source. Moreover, gold is inert in most conditions and its degradation and clearance remains unclear. As a result, the accumulation and excretion mechanism of gold nanomaterials in cells, organs, and bodies needs further study.
Other Gold Nanostructures
Other gold nanostructures, such as gold nanocages, gold nanoshells, and gold nanostars, are developed recently as photothermal therapy agent. Many work successfully combined photothermal therapy with imaging and drug delivery by employing these novel gold nanostructures.213,214 For, example, a NIR laser-controlled drug release system which was based on smart polymers covered gold nanocages was successfully developed by Xia et al. The smart polymer undergoes a phase transition from a hydrophilic state to a hydrophobic state when the temperature is raised above its low critical solution temperature (LCST). Once covalently anchored to the surface of gold nanocages, the smart polymer would undergo the phase transition and aggregate under the heat converted by gold nanocages after NIR laser irradiation. The pre-loaded drug, lysozyme, can therefore be released in a controllable fashion using photothermal conversion of gold nanocages. Furthermore, gold nanocages have a potential for multi-functional drug delivery system after functionalized with targeting ligands. Hollow gold nanostructures are superior in drug loading as it would be easier than that of Au NRs and Au NPs as drug will diffuse into the hollow structure when incubating with these gold nanostructures and no chemical reaction is needed. Moreover, hollow structure can provide more space compared to surface area. The biggest challenge is that the synthesis of these novel gold nanostructures is much more complicated than that of Au NRs and Au NPs. Thus, more efforts should be done to optimize the synthesis method and we believe that these gold nanostructures would become important members of gold-based nanoscale pharmaceutical adjuvant materials.
Other Inorganic Materials
Apart from silica and gold, other inorganic nanomaterials, such as carbon,215 iron,216–218 and calcium,219 also have been used as pharmaceutical adjuvants. Some of these have special applications in pharmaceutical adjuvant research. For example, calcium phosphate nanoparticles can work as vaccine adjuvants, inducing immunity to viruses.220 Results indicated that calcium phosphate nanoparticles were more potent as vaccine adjuvant than traditional aluminium adjuvant because less inflammation was caused and high titer of antibody was induced. Graphene oxide, a novel carbon nanomaterial with monolayer structure, is promising in aromatic drug delivery via non-covalently π–π stacking. SN38, a water-insoluble aromatic anticancer drug derived from camptothecin, is attached to graphene oxide by Dai and co-workers.221 The obtained composite exhibited excellent water solubility while the anticancer efficacy was not compromised. Graphene oxide also showed potential in photothermal therapy, making it popular in novel medicine design.222 Our previous work demonstrated that metallofullerene nanoparticles Gd@C82(OH)22, another promising carbon-based nanomaterial, can circumvent tumor resistance to cisplatin by reactivating endocytosis, which is a new strategy to fight with tumor drug resistance.223 Further studies demonstrated that Gd@C82(OH)22 nanoparticles are inherently anticancer drug.224,225 They can inhibit pancreatic tumor metastasis by affecting matrix metalloproteinase-9 (MMP-9) function. Another potential pharmaceutical adjuvant material is superparamagnetic iron oxide nanoparticles (SPIONs) which are well-known as contrast agents.226 Thus, SPIONs are appropriate for design of traceable drug delivery system. Tumor site accumulated SPIONs will enable to monitor the growth, metastasis, and location of tumor tissue. Following these established examples, inorganic nanomaterials can be used selectively to design drug vehicles with more functions.
HYBRID ORGANIC AND INORGANIC NANOMATERIALS FOR PHARMACEUTICAL APPLICATION
Multifunctional nanoparticles, which are desired for advanced drug delivery, can be created by incorporating more than one nanomaterial into a nanostructure. These combined nanomaterials are known as hybrid nanomaterials, and they combine the advantages of each component. In another way, fatal drawbacks of one component are made up by the other one. Recently, thermal and pH dual responsive hybrid nanoparticle based on this strategy was successfully developed (Fig. 7(H)).227 The hybrid nanoparticles had a SiO2 shell and a polymer core made of poly(N-isopropylacrylamide) (PNiPAM)-co–acrylic acid (AA) hydrogel, which is responsive to both heat and pH. By lowering the temperature, DOX was loaded into the nanoparticles and by manipulating the pH, DOX was rapidly released. Compared with traditional MSNs, these hybrid nanoparticles had a better uniformity and stability. Moreover, the in vitro results showed that these novel nanoparticles were more bio-compatible and less toxic than conventional MSNs, while the anticancer efficiency was not compromised. In this work, SiO2 is employed as the drug container, but pH-responsive drug release could not be realized in pure silica system. As a result, pH-sensitive polymer is introduced as the core of the nanoparticle. This core–shell drug delivery system works like a tank: SiO2 shell is the armor, DOX is the cannonball, and PNiPAM/AA hydrogel is the fire control system. It cruises everywhere, resists attack from clearance system, and fires in the battle-field (slightly altered acidic tumor microenvironment and endosomes).
Theranostic, a new concept combins therapy and diagnostic, is emerging with the development of hybrid adjuvants.228,229 It is difficult to realize theranostic with only one adjuvant material. Therefore, hybrid adjuvants composed of more than one material are employed. For instance, after the drug-loaded nanoparticles are administered for therapeutic treatment, biodistribution of these nanoparticles is expected to be monitored in organs and disease site. In this case, fluorescence probes or MRI contrast agents can be integrated into the drug vehicle. The labeled hybrid nanoparticles are then monitored non-invasively throughout the treatment and researchers can get more information from a single injection.
Actually, most examples we mentioned in this article are hybrid materials but only one component is discussed in detail in individual context. It is a trend to combine more functions in one nanoparticle, resulting in the widely application of hybrid nanomaterials. Although it is fascinating to design multifunctional drug delivery system with hybrid nanomaterials, there are two disadvantages under consider in application. First, the preparation will become complicated compared to that of single-component nanoparticles, resulting in the reduction of reproducibility. Second, the drug release pathway and metabolism mechanism will be unclear after the addition of another material. Both of them are of great importance for pharmaceutical adjuvants. Therefore, every aspect of hybrid materials should be thoroughly studied.
CONCLUSIONS
Recent progress reveals the great potential of nanoscale materials as novel pharmaceutical adjuvants. Nanomaterials are capable of transporting insoluble drugs in aqueous solution, reducing adverse side-effects, delivering dual therapeutic agents simultaneously, targeting drugs to disease lesions and controlling drug release in the desired environment. However, despite the enormous potential of nanoscale materials, considerable challenges must be addressed before they can be translated into clinical use. Pharmaceutical adjuvants must fulfill specific criteria, like biocompatibility, stability in physicochemical conditions, protection of active drugs, and low toxicity, in order to meet the requirements of the FDA. In addition, these materials should be reproducible in a scalable manner with high purity and low cost. Most reported nanomaterials are synthesized and modified in small batches. Standard protocols must be provided for industrial-scale production of nanoscale pharmaceutical adjuvant materials. Comprehensive assessment of the biosafety issues of nanomaterials, such as biocompatibility, biodegradation and biodistribution, should also be provided. Drug delivery mechanisms should be further studied for a better understanding of pharmacodynamics and pharmacokinetics of different nanocarriers. As far as we know, size, shape, charge, functional groups, and other propeties of nanomaterials all have an impact on their interaction with biosystems. We need to understand the role played by each property, so that the pharmacological attributes of nanomaterials can be optimized. Nowadays, many scientists are working hard to overcome these challenges and we are sure that nanoscale materials have a bright future as pharmaceutical adjuvants.
Acknowledgments
This work was supported by the Chinese Natural Science Foundation project (Nos. 30970784, 81171455), a National Distinguished Young Scholars grant (31225009) from the National Natural Science Foundation of China, the National Key Basic Research Program of China (2009CB930200), the Chinese Academy of Sciences (CAS) “Hundred Talents Program” (07165111ZX), the CAS Knowledge Innovation Program and the State High-Tech Development Plan (2012AA020804). The authors also appreciate the support by the “Strategic Priority Research Program” of the Chinese Academy of Sciences, Grant No. XDA09030301. This work was also supported in part by NIH/NIMHD 8 G12 MD007597, and USAMRMC W81XWH-10-1-0767 grants.
Biographies
 Shubin Jin is a graduate student and he is now pursuing his Ph.D. in Nanoscience and Technology at National Center for Nanoscience and Technology, China (NCNST). He received his B.E. of Biotechnology at Beijing University of Technology in 2011. Currently he is working on design and fabrication of visible drug delivery and release nanosystem.
Shubin Jin is a graduate student and he is now pursuing his Ph.D. in Nanoscience and Technology at National Center for Nanoscience and Technology, China (NCNST). He received his B.E. of Biotechnology at Beijing University of Technology in 2011. Currently he is working on design and fabrication of visible drug delivery and release nanosystem.
 Shengliang Li received his B.E. of Pharmacy at Henan University in 2009. Currently he is pursuing his medical doctor’s degree in Zhongshan School of Medicine at Sun Yat-Sen University, China. And he is a joint training M.D. candidate under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). His research interests focuses on functionalization and engineering of nanomaterials for nanomedicine.
Shengliang Li received his B.E. of Pharmacy at Henan University in 2009. Currently he is pursuing his medical doctor’s degree in Zhongshan School of Medicine at Sun Yat-Sen University, China. And he is a joint training M.D. candidate under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). His research interests focuses on functionalization and engineering of nanomaterials for nanomedicine.
 Chongxi Wang received his B.S. of Chemical Engineering and Technology at Tianjin University in 2011. Currently he is pursuing his master’s degree in Chemical Engineering at Tianjin University. And he is a joint student under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). His main research interest focuses on polycations-based gene delivery system.
Chongxi Wang received his B.S. of Chemical Engineering and Technology at Tianjin University in 2011. Currently he is pursuing his master’s degree in Chemical Engineering at Tianjin University. And he is a joint student under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). His main research interest focuses on polycations-based gene delivery system.
 Juan Liu received her B.S. of Biopharmaceutics at Huazhong University of Science and Technology in 2011. Currently she is pursuing her Ph.D. in Physical Chemistry under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). Her main research interest focuses on nanomaterial-based drug delivery systems.
Juan Liu received her B.S. of Biopharmaceutics at Huazhong University of Science and Technology in 2011. Currently she is pursuing her Ph.D. in Physical Chemistry under the guidance of Professor Xing-Jie Liang at National Center for Nanoscience and Technology, China (NCNST). Her main research interest focuses on nanomaterial-based drug delivery systems.
 Xiaolong Yang received his B.E. of Biotechnology at Tianjin University of Science and Technology in 2012. Currently he is pursuing his master’s degree in Pharmacology at Tianjin University of Science and Technology. He is now a joint student working on nanomaterials-induced stem cell differentiation at National Center for Nanoscience and Technology, China (NCNST).
Xiaolong Yang received his B.E. of Biotechnology at Tianjin University of Science and Technology in 2012. Currently he is pursuing his master’s degree in Pharmacology at Tianjin University of Science and Technology. He is now a joint student working on nanomaterials-induced stem cell differentiation at National Center for Nanoscience and Technology, China (NCNST).
 Paul Wang is the Director of the Molecular Imaging Laboratory and a Professor in the Department of Radiology, Howard University. Dr. Wang graduated from Fu Jen University in 1974 with a B.S. in physics. He received a Ph.D. in applied radiation physics from MIT’s Nuclear Engineering Department in 1982. Dr. Wang established the Molecular Imaging Laboratory as a core facility at Howard University to promote multi-disciplinary research using imaging techniques for biomedical applications. His research interests include MRI and NMR spectroscopy studies of cancer, cardiovascular and neurodegenerative diseases, multidrug interactions, and development of nanoparticles as drug delivery vehicles for targeted chemotherapy and diagnostic imaging. Dr. Wang has been a Visiting Professor at the Chinese National Center for Nanoscience and Technology since 2007. He received Howard University College of Medicine’s Outstanding Faculty Researcher Award in 2008, and the Dr. M. Wharton Young Research Award in 2010.
Paul Wang is the Director of the Molecular Imaging Laboratory and a Professor in the Department of Radiology, Howard University. Dr. Wang graduated from Fu Jen University in 1974 with a B.S. in physics. He received a Ph.D. in applied radiation physics from MIT’s Nuclear Engineering Department in 1982. Dr. Wang established the Molecular Imaging Laboratory as a core facility at Howard University to promote multi-disciplinary research using imaging techniques for biomedical applications. His research interests include MRI and NMR spectroscopy studies of cancer, cardiovascular and neurodegenerative diseases, multidrug interactions, and development of nanoparticles as drug delivery vehicles for targeted chemotherapy and diagnostic imaging. Dr. Wang has been a Visiting Professor at the Chinese National Center for Nanoscience and Technology since 2007. He received Howard University College of Medicine’s Outstanding Faculty Researcher Award in 2008, and the Dr. M. Wharton Young Research Award in 2010.
 Xin Zhang is Professor of Institute of Process Engineering (IPE), Chinese Academy of Sciences (CAS). She received her Ph.D. degree from University of Strasbourg. At the same year, she worked as an assistant professor at The French National Institute of Health and Medical Research. Her primary research focus is on the gene therapy, including design, synthesis and application of gene delivery vectors. The works has been published in peer-reviewed scientific journal, such as Journal of Materials Chemistry, Biomaterials and Nano Letters, which has been cited overall more than 400 times.
Xin Zhang is Professor of Institute of Process Engineering (IPE), Chinese Academy of Sciences (CAS). She received her Ph.D. degree from University of Strasbourg. At the same year, she worked as an assistant professor at The French National Institute of Health and Medical Research. Her primary research focus is on the gene therapy, including design, synthesis and application of gene delivery vectors. The works has been published in peer-reviewed scientific journal, such as Journal of Materials Chemistry, Biomaterials and Nano Letters, which has been cited overall more than 400 times.
 Xing-Jie Liang got Ph.D. at National Key Laboratory of Biomacromolecules, Institute of Biophysics at CAS. He finished his postdoc at Center for Cancer Research, NCI, NIH, and worked as a Research Fellow at Surgical Neurology Branch, NINDS. Dr. Liang worked on Molecular imaging at School of Medicine, Howard University before he became deputy director of CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology of China. Dr. Liang is current editorial board member of Advances in Nano Research, Theranostics, Journal of Nanomaterials, Biomaterials Research, and Current Nanoscience, associated editor of Biophysics Report and guest editor of Biotechnology Advances. Dr. Liang was honored with 2004, 2005, 2006 Fellows Award for Research Excellence in NIH; Special Government Allowances by Department of State, 2011; National Distinguished Young Scholars by NSFC; and Young Pharmaceutics Scholar by CPA, 2012. Developing drug delivery strategies for prevention/treatment of AIDS and cancers are current program ongoing in Dr. Liang’s lab based on understanding of basic physical-chemical and biological processes of nanomedicine.
Xing-Jie Liang got Ph.D. at National Key Laboratory of Biomacromolecules, Institute of Biophysics at CAS. He finished his postdoc at Center for Cancer Research, NCI, NIH, and worked as a Research Fellow at Surgical Neurology Branch, NINDS. Dr. Liang worked on Molecular imaging at School of Medicine, Howard University before he became deputy director of CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology of China. Dr. Liang is current editorial board member of Advances in Nano Research, Theranostics, Journal of Nanomaterials, Biomaterials Research, and Current Nanoscience, associated editor of Biophysics Report and guest editor of Biotechnology Advances. Dr. Liang was honored with 2004, 2005, 2006 Fellows Award for Research Excellence in NIH; Special Government Allowances by Department of State, 2011; National Distinguished Young Scholars by NSFC; and Young Pharmaceutics Scholar by CPA, 2012. Developing drug delivery strategies for prevention/treatment of AIDS and cancers are current program ongoing in Dr. Liang’s lab based on understanding of basic physical-chemical and biological processes of nanomedicine.
References
- 1.Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2009;9:172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 2.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 4.Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Delivery Rev. 2008;60:1278. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Cole AJ, David AE, Wang J, Galbán CJ, Yang VC. Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials. 2011;32:6291. doi: 10.1016/j.biomaterials.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Delivery Rev. 2012;64:246. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 8.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 9.Pileni M. Control of the size and shape of inorganic nanocrystals at various scales from nano to macrodomains. J Phys Chem C. 2007;111:9019. [Google Scholar]
- 10.Qiao ZA, Guo B, Binder AJ, Chen J, Veith GM, Dai S. Controlled synthesis of mesoporous carbon nanostructures via a “silica-assisted” strategy. Nano Lett. 2012;13:207. doi: 10.1021/nl303889h. [DOI] [PubMed] [Google Scholar]
- 11.Nalwa HS, editor. Encyclopedia of Nanoscience and Nanotechnology. American Scientific Publishers; Los Angeles, CA: 2004/2011. pp. 1–25. [Google Scholar]
- 12.Bagwe RP, Hilliard LR, Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kohler N, Zhang M. Surface modification of super-paramagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 14.Nalwa HS, editor. Handbook of Nanostructured Materials and Nanotechnology. Academic Press; San Diego, CA: 2000. pp. 1–5. [Google Scholar]
- 15.Na K, Lee KH, Lee DH, Bae YH. Biodegradable thermosensitive nanoparticles from poly(L-lactic acid)/poly (ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci. 2006;27:115. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Yan B, Boyer JC, Branda NR, Zhao Y. Near-infrared light-triggered dissociation of block copolymer micelles using upconverting nanoparticles. J Am Chem Soc. 2011;133:19714. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- 17.Sonia T, Sharma CP. pH sensitive thiolated cationic hydrogel for oral insulin delivery. J Biomed Nanotechnol. 2014;10:642. doi: 10.1166/jbn.2014.1740. [DOI] [PubMed] [Google Scholar]
- 18.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125:4451. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 19.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles. Proc Natl Acad Sci USA. 2008;105:17356. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra S, Gaur U, Ghosh P, Maitra A. Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74:317. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 21.Sandiford L, Phinikaridou A, Protti A, Meszaros LK, Cui X, Yan Y, Frodsham G, Williamson PA, Gaddum N, Botnar RM. Bisphosphonate-anchored PEGylation and radiolabeling of superparamagnetic iron oxide: Long-circulating nanoparticles for in vivo multimodal (T 1 MRI-SPECT) imaging. ACS Nano. 2012;7:500. doi: 10.1021/nn3046055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Delivery Rev. 2012;64:24. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 23.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Delivery Rev. 2011;63:170. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Wei T, Liu J, Ma H, Cheng Q, Huang Y, Zhao J, Huo S, Xue X, Liang Z, Liang XJ. Functionalized nanoscale micelles improve drug delivery for cancer therapy in vitro and in vivo. Nano Lett. 2013;13:2528. doi: 10.1021/nl400586t. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson JM, Banerjee S, Saxena NK, Cherian R, Banerjee SK. Neuropilin-1 is differentially expressed in myoepithelial cells and vascular smooth muscle cells in preneoplastic and neoplastic human breast: A possible marker for the progression of breast cancer. Int J Cancer. 2002;101:409. doi: 10.1002/ijc.10611. [DOI] [PubMed] [Google Scholar]
- 26.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 27.Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol. 2012;226:50. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 28.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westesen K, Bunjes H, Koch M. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J Control Release. 1997;48:223. [Google Scholar]
- 30.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 31.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using 1 H MRSI. Magn Reson Med. 1999;41:743. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Engin K, Leeper D, Cater J, Thistlethwaite A, Tupchong L, McFarlane J. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 33.Weiss RB, Donehower R, Wiernik P, Ohnuma T, Gralla R, Trump D, Baker J, Jr, Van Echo D, Von Hoff D, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 34.Green M, Manikhas G, Orlov S, Afanasyev B, Makhson A, Bhar P, Hawkins M. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 35.Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV., III Abraxane in the treatment of ovarian cancer: The absence of hypersensitivity reactions. Gynecol Oncol. 2006;100:437. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Rivera-Gil P, De Aberasturi DJ, Wulf V, Pelaz B, Del Pino P, Zhao Y, De La Fuente JM, De Larramendi IR, Rojo T, Liang XJ. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc Chem Res. 2012 doi: 10.1021/ar300039j. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Gondikas AP, Marinakos SM, Auffan M, Liu J, Hsu-Kim H, Meyer JN. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ Sci Technol. 2011;46:1119. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 39.Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5:846. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Nalwa HS. Nanotechnology and health safety toxicity and risk assessments of nanostructured materials on human health. J Nanosci Nanotechnol. 2007;7:3048. doi: 10.1166/jnn.2007.922. [DOI] [PubMed] [Google Scholar]
- 41.Zhao YL, Nalwa HS, editors. Nanotoxicology: Interactions of Nanomterials with Biological Systems. American Scientific Publishers; Los Angeles, CA: 2007. [Google Scholar]
- 42.Lyass O, Uziely B, Ben-Yosef R, Tzemach D, Heshing NI, Lotem M, Brufman G, Gabizon A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4:99. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angew Chem Int Ed. 2011;123:3226. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8:331. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5:316. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 48.Li JJ, Lo SL, Ng CT, Gurung RL, Hartono D, Hande MP, Ong CN, Bay BH, Yung LYL. Genomic instability of gold nanoparticle treated human lung fibroblast cells. Biomaterials. 2011;32:5515. doi: 10.1016/j.biomaterials.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Ganguly K, Upadhyay S, Irmler M, Takenaka S, Pukelsheim K, Beckers J, De Angelis MH, Hamelmann E, Stoeger T, Schulz H. Impaired resolution of inflammatory response in the lungs of JF1/Msf mice following carbon nanoparticle instillation. Respir Res. 2011;12:1. doi: 10.1186/1465-9921-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge Y, Bruno M, Wallace K, Winnik W, Prasad RY. Proteome profiling reveals potential toxicity and detoxification pathways following exposure of BEAS-2B cells to engineered nanoparticle titanium dioxide. Proteomics. 2011;11:2406. doi: 10.1002/pmic.201000741. [DOI] [PubMed] [Google Scholar]
- 51.Sershen S, Westcott S, Halas N, West J. Temperature-sensitive polymer–nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res. 2000;51:293. doi: 10.1002/1097-4636(20000905)51:3<293::aid-jbm1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 52.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed. 2003;42:4640. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 53.Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and near-IR triggered release from polymeric nanoparticles. J Am Chem Soc. 2010;132:9540. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 55.Qiu LY, Bae YH. Polymer architecture and drug delivery. Pharm Res. 2006;23:1. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 56.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A polymer library approach to suicide gene therapy for cancer. Proc Natl Acad Sci USA. 2004;101:16028. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benns JM, Choi JS, Mahato RI, Park JS, Kim SW. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly (L-histidine)-graft-poly (L-lysine) comb shaped polymer. Bioconjugate Chem. 2000;11:637. doi: 10.1021/bc0000177. [DOI] [PubMed] [Google Scholar]
- 58.Tiram G, Scomparin A, Ofek P, Satchi-Fainaro R. Interfering cancer with polymeric siRNA nanomedicines. J Biomed Nanotechnol. 2014;10:50. doi: 10.1166/jbn.2014.1715. [DOI] [PubMed] [Google Scholar]
- 59.Xiong HM, Xu Y, Ren QG, Xia YY. Stable aqueous ZnO@polymer core–shell nanoparticles with tunable photoluminescence and their application in cell imaging. J Am Chem Soc. 2008;130:7522. doi: 10.1021/ja800999u. [DOI] [PubMed] [Google Scholar]
- 60.Li K, Pan J, Feng SS, Wu AW, Pu KY, Liu Y, Liu B. Generic strategy of preparing fluorescent conjugated-polymer-loaded poly(DL-lactide-co-glycolide) nanoparticles for targeted cell imaging. Adv Funct Mater. 2009;19:3535. [Google Scholar]
- 61.Wadajkar AS, Menon JU, Nguyen KT. Polymer-coated magnetic nanoparticles for cancer diagnosis and therapy. Rev Nanosci Nanotechnol. 2012;1:284. [Google Scholar]
- 62.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Delivery Rev. 2006;58:487. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762. [Google Scholar]
- 64.Zhang J, Xia W, Liu P, Cheng Q, Tahi T, Gu W, Li B. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8:1962. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Delivery Rev. 2010;62:28. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 66.VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 67.Kumar JP, Lakshmi L, Jyothsna V, Balaji D, Saravanan S, Moorthi A, Selvamurugan N. Synthesis and characterization of diopside particles and their suitability along with chitosan matrix for bone tissue engineering in vitro and in vivo. J Biomed Nanotechnol. 2014;10:970. doi: 10.1166/jbn.2014.1808. [DOI] [PubMed] [Google Scholar]
- 68.Victor SP, Paul W, Sharma CP. Chitosan self-aggregates and micelles in drug delivery. J Nanopharm Drug Deliv. 2013;1:193. [Google Scholar]
- 69.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Delivery Rev. 2010;62:3. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Ma Z, Lim TM, Lim LY. Pharmacological activity of peroral chitosan–insulin nanoparticles in diabetic rats. Int J Pharm. 2005;293:271. doi: 10.1016/j.ijpharm.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 71.Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MAJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73:255. doi: 10.1016/s0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]
- 72.Kim JH, Kim YS, Park K, Lee S, Nam HY, Min KH, Jo HG, Park JH, Choi K, Jeong SY. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release. 2008;127:41. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128:23. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Kim TH, Park IK, Nah JW, Choi YJ, Cho CS. Galactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrier. Biomaterials. 2004;25:3783. doi: 10.1016/j.biomaterials.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 75.Qin C, Du Y, Xiao L, Li Z, Gao X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int J Biol Macromol. 2002;31:111. doi: 10.1016/s0141-8130(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 76.Shi B, Shen Z, Zhang H, Bi J, Dai S. Exploring N-imidazolyl-O-carboxymethyl chitosan for high performance gene delivery. Biomacromolecules. 2011;13:146. doi: 10.1021/bm201380e. [DOI] [PubMed] [Google Scholar]
- 77.Jin X, Mo R, Ding Y, Zheng W, Zhang C. Paclitaxel-loaded N-octyl-O-sulfate chitosan micelles for superior cancer therapeutic efficacy and overcoming drug resistance. Mol Pharm. 2014;11:145. doi: 10.1021/mp400340k. [DOI] [PubMed] [Google Scholar]
- 78.Zhang C, Qu G, Sun Y, Wu X, Yao Z, Guo Q, Ding Q, Yuan S, Shen Z, Ping Q. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials. 2008;29:1233. doi: 10.1016/j.biomaterials.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 79.Persidis A. Cancer multidrug resistance. Nat Biotechnol. 1999;17:94. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 80.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 81.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cells Mater. 2003;5:1. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Liu C, Li M, Liu F, Feng L, Zhang L, Zhang N. Polymer–polymer conjugation to fabricate multi-block polymer as novel drug carriers: Poly(lactic acid)–poly(ethylene glycol)–poly(L-lysine) to enhance paclitaxel target delivery. J Biomed Nanotechnol. 2014;10:948. doi: 10.1166/jbn.2014.1796. [DOI] [PubMed] [Google Scholar]
- 83.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 84.Bertin PA, Gibbs JM, Shen CKF, Thaxton CS, Russin WA, Mirkin CA, Nguyen ST. Multifunctional polymeric nanoparticles from diverse bioactive agents. J Am Chem Soc. 2006;128:4168. doi: 10.1021/ja056378k. [DOI] [PubMed] [Google Scholar]
- 85.Kim JH, Burnett RD, Gabriel A. Stimuli-responsive hollow polymer nanoparticles for use as novel delivery systems. J Biomed Nanotechnol. 2012;8:432. doi: 10.1166/jbn.2012.1397. [DOI] [PubMed] [Google Scholar]
- 86.Löbenberg R, Araujo L, von Briesen H, Rodgers E, Kreuter J. Body distribution of azidothymidine bound to hexyl-cyanoacrylate nanoparticles after iv injection to rats. J Control Release. 1998;50:21. doi: 10.1016/s0168-3659(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 87.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alconcel SN, Baas AS, Maynard HD. FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polym Chem. 2011;2:1442. [Google Scholar]
- 89.Li YP, Pei YY, Zhang XY, Gu ZH, Zhou ZH, Yuan WF, Zhou JJ, Zhu JH, Gao XJ. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J Control Release. 2001;71:203. doi: 10.1016/s0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 90.von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai H. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J Am Chem Soc. 2009;131:4783. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, Tang CY, Gordon RE, Nicolay K, Meijerink A. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: A multimodality investigation. Nano Lett. 2008;8:2517. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 93.Thierry B, Zimmer L, McNiven S, Finnie K, Barbeì C, Griesser HJ. Electrostatic self-assembly of PEG copolymers onto porous silica nanoparticles. Langmuir. 2008;24:8143. doi: 10.1021/la8007206. [DOI] [PubMed] [Google Scholar]
- 94.Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, Farokhzad OC. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 95.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 96.Naha PC, Byrne HJ, Panda AK. Role of polymeric excipients on controlled release profile of Glipizide from PLGA and Eudragit RS 100 Nanoparticles. J Nanopharm Drug Deliv. 2013;1:74. [Google Scholar]
- 97.Durán-Lobato M, Muñoz-Rubio I, Holgado MÁ, Álvarez-Fuentes J, Fernández-Arévalo M, Martín-Banderas L. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly(lactic-co-glycolic acid) nanoparticles. J Biomed Nanotechnol. 2014;10:1068. doi: 10.1166/jbn.2014.1806. [DOI] [PubMed] [Google Scholar]
- 98.Xu P, Gullotti E, Tong L, Highley CB, Errabelli DR, Hasan T, Cheng JX, Kohane DS, Yeo Y. Intracellular drug delivery by poly (lactic-co-glycolic acid) nanoparticles, revisited. Mol Pharm. 2008;6:190. doi: 10.1021/mp800137z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamaguchi Y, Takenaga M, Kitagawa A, Ogawa Y, Mizushima Y, Igarashi R. Insulin-loaded biodegradable PLGA microcapsules: Initial burst release controlled by hydrophilic additives. J Control Release. 2002;81:235. doi: 10.1016/s0168-3659(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 100.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei H, Cheng SX, Zhang XZ, Zhuo RX. Thermosensitive polymeric micelles based on poly (N-isopropyl-acrylamide) as drug carriers. Prog Polym Sci. 2009;34:893. [Google Scholar]
- 102.Kim HJ, Matsuda H, Zhou H, Honma I. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)–mesoporous silica composite. Adv Mater. 2006;18:3083. [Google Scholar]
- 103.Quesada M, Muniesa C, Botella P. Hybrid PLGA-organosilica nanoparticles with redox-sensitive molecular gates. Chem Mater. 2013;25:2597. [Google Scholar]
- 104.Geisow MJ, Evans WH. pH in the endosome: Measurements during pinocytosis and receptor-mediated endocytosis. Exp Cell Res. 1984;150:36. doi: 10.1016/0014-4827(84)90699-2. [DOI] [PubMed] [Google Scholar]
- 105.Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Freìchet JMJ. Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J Am Chem Soc. 2008;130:10494. doi: 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu X, Ji Y, Wang M, Miao F, Ma H, Shen H, Jia N. Water-soluble and biocompatible MnO@PVP nanoparticles for MR imaging in vitro and in vivo. J Biomed Nanotechnol. 2013;9:976. doi: 10.1166/jbn.2013.1602. [DOI] [PubMed] [Google Scholar]
- 107.Fischer F, Bauer S. Polyvinylpyrrolidon. Ein tausendsassa in der chemie. Chem unserer Zeit. 2009;43:376. [Google Scholar]
- 108.Zhi X, Fang H, Bao C, Shen G, Zhang J, Wang K, Guo S, Wan T, Cui D. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34:5254. doi: 10.1016/j.biomaterials.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 109.Aarthi T, Shaama M, Madras G. Degradation of water soluble polymers under combined ultrasonic and ultraviolet radiation. Ind Eng Chem Res. 2007;46:6204. [Google Scholar]
- 110.Ciftci K, Levy RJ. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int J Pharm. 2001;218:81. doi: 10.1016/s0378-5173(01)00623-8. [DOI] [PubMed] [Google Scholar]
- 111.Saxena A, Mozumdar S, Johri AK. Ultra-low sized cross-linked polyvinylpyrrolidone nanoparticles as non-viral vectors for in vivo gene delivery. Biomaterials. 2006;27:5596. doi: 10.1016/j.biomaterials.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 112.Hickey RJ, Haynes AS, Kikkawa JM, Park SJ. Controlling the self-assembly structure of magnetic nanoparticles and amphiphilic block-copolymers: From micelles to vesicles. J Am Chem Soc. 2011;133:1517. doi: 10.1021/ja1090113. [DOI] [PubMed] [Google Scholar]
- 113.Wang H, Zhao Y, Wu Y, Hu Y-l, Nan K, Nie G, Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 114.Tyrrell ZL, Shen Y, Radosz M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog Polym Sci. 2010;35:1128. [Google Scholar]
- 115.Guo S, Huang Y, Wei T, Zhang W, Wang W, Lin D, Zhang X, Kumar A, Du Q, Liang XJ. Amphiphilic and biodegradable methoxy polyethylene glycol-block-(polycaprolactone-graft-poly(2-(dimethylamino) ethyl methacrylate)) as an effective gene carrier. Biomaterials. 2011;32:879. doi: 10.1016/j.biomaterials.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li S, Goins B, Zhang L, Bao A. Novel multifunctional ther-anostic liposome drug delivery system: Construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjugate Chem. 2012;23:1322. doi: 10.1021/bc300175d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu R, Law WC, Lin G, Ye L, Liu J, Liu J, Reynolds JL, Yong KT. PEGylated phospholipid micelle-encapsulated near-infrared PbS quantum dots for in vitro and in vivo bioimaging. Theranostics. 2012;2:723. doi: 10.7150/thno.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Jamal WT, Kostarelos K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for ther-anostic nanomedicine. Acc Chem Res. 2011;44:1094. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 119.Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, Liu S. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials. 2011;32:6595. doi: 10.1016/j.biomaterials.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 120.Bigot J, Charleux B, Cooke G, Delattre F, Fournier D, Lyskawa J, Sambe L, Stoffelbach F, Woisel P. Tetrathiafulvalene end-functionalized poly(N-isopropylacrylamide): A new class of amphiphilic polymer for the creation of multistimuli responsive micelles. J Am Chem Soc. 2010;132:10796. doi: 10.1021/ja1027452. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad I, Longenecker M, Samuel J, Allen TM. Antibody-targeted delivery of doxorubicin entrapped in sterically stabilized liposomes can eradicate lung cancer in mice. Cancer Res. 1993;53:1484. [PubMed] [Google Scholar]
- 122.Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, Lu Y. Reversible Cell-specific drug delivery with Aptamer-functionalized liposomes. Angew Chem Int Ed. 2009;48:6494. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 123.Al-Jamal WT, Al-Jamal KT, Bomans PH, Frederik PM, Kostarelos K. Functionalized-quantum-dot–liposome hybrids as multimodal nanoparticles for cancer. Small. 2008;4:1406. doi: 10.1002/smll.200701043. [DOI] [PubMed] [Google Scholar]
- 124.Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:156. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 125.Barenholz YC. Doxil®—The first FDA-approved nano-drug: Lessons learned. J Control Release. 2012;160:117. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 126.Koole R, van Schooneveld MM, Hilhorst J, Castermans K, Cormode DP, Strijkers GJ, de Mello Donegá C, Vanmaekelbergh D, Griffioen AW, Nicolay K. Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: A new contrast agent platform for multimodality imaging. Bioconjugate Chem. 2008;19:2471. doi: 10.1021/bc800368x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Messerschmidt SK, Musyanovych A, Altvater M, Scheurich P, Pfizenmaier K, Landfester K, Kontermann RE. Targeted lipid-coated nanoparticles: Delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009;137:69. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Koby G, Ofra B, Dganit D, Marcelle M. Poly(D,L-lactide-co-glycolide acid) nanoparticles for DNA delivery: Waiving preparation complexity and increasing efficiency. Biopolymers. 2007;85:379. doi: 10.1002/bip.20697. [DOI] [PubMed] [Google Scholar]
- 129.RaviKumar M, Mohapatra S, Kong X, Jena P, Bakowsky U, Lehrd C. Cationic poly(lactide-co-glycolide) nanoparticles as efficient in vivo gene transfection agents. J Nanosci Nanotechnol. 2004;4:990. doi: 10.1166/jnn.2004.130. [DOI] [PubMed] [Google Scholar]
- 130.Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, DeSimone JM. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2011;12:287. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Felnerova D, Viret JF, Glück R, Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518. doi: 10.1016/j.copbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Awasthi V, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: Effect of postinserted poly(ethylene glycol)-distearoylphosphati-dylethanolamine on distribution and circulation kinetics. J Pharmacol Exp Ther. 2004;309:241. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- 133.Liu J, Ma H, Wei T, Liang XJ. CO2 gas induced drug release from pH-sensitive liposome to circumvent doxorubicin resistant cells. Chem Commun. 2012;48:4869. doi: 10.1039/c2cc31697h. [DOI] [PubMed] [Google Scholar]
- 134.Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med Res Rev. 2007;27:696. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 135.Tenchov BG, Wang L, Koynova R, MacDonald RC. Modulation of a membrane lipid lamellar–nonlamellar phase transition by cationic lipids: A measure for transfection efficiency. BBA-Biomembranes. 2008;1778:2405. doi: 10.1016/j.bbamem.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 136.Lee SC, Lee KE, Kim JJ, Lim SH. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J Liposome Res. 2005;15:157. doi: 10.1080/08982100500364131. [DOI] [PubMed] [Google Scholar]
- 137.Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980;186:591. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schellekens H. Immunogenicity of therapeutic proteins: Clinical implications and future prospects. Clin Ther. 2002;24:1720. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- 139.Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH. Microtubule structure at 8 Å resolution. Structure. 2002;10:1317. doi: 10.1016/s0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 140.Ding D, Zhu Z, Liu Q, Wang J, Hu Y, Jiang X, Liu B. Cisplatin-loaded gelatin-poly(acrylic acid) nanoparticles: Synthesis, antitumor efficiency 〈i〉 in vivo 〈i〉 and penetration in tumors. Eur J Pharm Biopharm. 2011;79:142. doi: 10.1016/j.ejpb.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 141.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 142.Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials. 2010;31:4583. doi: 10.1016/j.biomaterials.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schnitzer J, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol Heart Circ Physiol. 1992;263:H1872. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- 144.John TA, Vogel SM, Minshall RD, Ridge K, Tiruppathi C, Malik AB. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J Physiol. 2001;533:547. doi: 10.1111/j.1469-7793.2001.0547a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li X, Qiu L, Zhu P, Tao X, Imanaka T, Zhao J, Huang Y, Tu Y, Cao X. Epidermal growth factor–ferritin H-chain protein nanoparticles for tumor active targeting. Small. 2012;8:2505. doi: 10.1002/smll.201200066. [DOI] [PubMed] [Google Scholar]
- 146.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Destro A, Ceresoli G, Falleni M, Zucali P, Morenghi E, Bianchi P, Pellegrini C, Cordani N, Vaira V, Alloisio M. EGFR overexpression in malignant pleural mesothelioma: An immunohistochemical and molecular study with clinicopathological correlations. Lung Cancer. 2006;51:207. doi: 10.1016/j.lungcan.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 148.Zhen X, Wang X, Xie C, Wu W, Jiang X. Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles. Biomaterials. 2012;34:1372. doi: 10.1016/j.biomaterials.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 149.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 151.Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J Biol Chem. 2006;281:3494. doi: 10.1074/jbc.M509333200. [DOI] [PubMed] [Google Scholar]
- 152.Wang Z, Zhang C, Xie L. Biosafety evaluation of collagen-based bone repairing material. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30:105. [PubMed] [Google Scholar]
- 153.Takeda U, Odaki M, Yokota M, Sasaki H, Niizato T, Kawaoto H, Watanabe H, Ito T, Ishiwatari N, Hayasaka H. Acute and subacute toxicity studies on collagen wound dressing (CAS) in mice and rats. J Toxicol Sci. 1982;7:63. doi: 10.2131/jts.7.supplementii_63. [DOI] [PubMed] [Google Scholar]
- 154.Peng C, Zhu ZA, Li GL, Sun DC, Li JG, Qiu ZY, Hu CL, Liu ZH, Sheng HB, Cui FZ. Enhancement of bone healing in rabbit ulnar critical bone defect by injectable nanohydroxyapatite/collagen/(calcium sulfate hemihydrate) mixed with autogenous bone marrow. J Biomater Tissue Eng. 2013;3:534. [Google Scholar]
- 155.Luo Z, Cai K, Hu Y, Zhao L, Liu P, Duan L, Yang W. Mesoporous Silica nanoparticles end-capped with collagen: Redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Ed. 2011;50:640. doi: 10.1002/anie.201005061. [DOI] [PubMed] [Google Scholar]
- 156.Kim H, Kim S, Park C, Lee H, Park HJ, Kim C. Glutathione-induced intracellular release of guests from mesoporous silica nanocontainers with cyclodextrin gatekeepers. Adv Mater. 2010;22:4280. doi: 10.1002/adma.201001417. [DOI] [PubMed] [Google Scholar]
- 157.Djagny KB, Wang Z, Xu S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit Rev Food Sci Nutr. 2001;41:481. doi: 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- 158.Zou Z, He D, He X, Wang K, Yang X, Qing Z, Zhou Q. Natural gelatin capped mesoporous silica nanoparticles for intracellular Acid-triggered drug delivery. Langmuir. 2013;29:12804. doi: 10.1021/la4022646. [DOI] [PubMed] [Google Scholar]
- 159.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 160.Larché M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 161.Wang H, Wei J, Yang C, Zhao H, Li D, Yin Z, Yang Z. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials. 2012;33:5848. doi: 10.1016/j.biomaterials.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 162.Zhang XX, Eden HS, Chen X. Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. J Control Release. 2012;159:2. doi: 10.1016/j.jconrel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crombez L, Aldrian-Herrada G, Konate K, Nguyen QN, McMaster GK, Brasseur R, Heitz F, Divita G. A new potent secondary amphipathic cell–penetrating peptide for siRNA delivery into mammalian cells. Mol Ther. 2008;17:95. doi: 10.1038/mt.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakase I, Konishi Y, Ueda M, Saji H, Futaki S. Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J Control Release. 2012;159:181. doi: 10.1016/j.jconrel.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 165.Melikov K, Chernomordik L. Arginine-rich cell penetrating peptides: From endosomal uptake to nuclear delivery. Cell Mol Life Sci. 2005;62:2739. doi: 10.1007/s00018-005-5293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA. 2001;98:8786. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome–DNA complexes. Proc Natl Acad Sci USA. 2003;100:1972. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Putnam D, Gentry CA, Pack DW, Langer R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc Natl Acad Sci USA. 2001;98:1200. doi: 10.1073/pnas.031577698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Du J, Armes SP. pH-responsive vesicles based on a hydrolytically self-cross-linkable copolymer. J Am Chem Soc. 2005;127:12800. doi: 10.1021/ja054755n. [DOI] [PubMed] [Google Scholar]
- 170.Liang K, Richardson JJ, Ejima H, Such GK, Cui J, Caruso F. Peptide-tunable drug cytotoxicity via onestep assembled polymer nanoparticles. Adv Mater. 2013 doi: 10.1002/adma.201305002. [DOI] [PubMed] [Google Scholar]
- 171.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release. 2007;118:216. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pinheiro AV, Han D, Shih WM, Yan H. Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol. 2011;6:763. doi: 10.1038/nnano.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, Wang ZG, Zou G, Liang X, Yan H, Ding B. DNA origami as a carrier for circumvention of drug resistance. J Am Chem Soc. 2012;134:13396. doi: 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- 174.Tarapore P, Shu Y, Guo P, Ho SM. Application of Phi29 motor pRNA for targeted therapeutic delivery of siRNA silencing metallothionein-IIA and survivin in ovarian cancers. Mol Ther. 2010;19:386. doi: 10.1038/mt.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Biwer A, Antranikian G, Heinzle E. Enzymatic production of cyclodextrins. Appl Microbiol Biotechnol. 2002;59:609. doi: 10.1007/s00253-002-1057-x. [DOI] [PubMed] [Google Scholar]
- 176.Villalonga R, Cao R, Fragoso A. Supramolecular chemistry of cyclodextrins in enzyme technology. Chem Rev. 2007;107:3088. doi: 10.1021/cr050253g. [DOI] [PubMed] [Google Scholar]
- 177.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Delivery Rev. 2007;59:645. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Sun H, Ma PX. Host-guest interaction mediated polymeric assemblies: Multifunctional nanoparticles for drug and gene delivery. ACS Nano. 2010;4:1049. doi: 10.1021/nn901213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao Y, Trewyn BG, II, Slowing V, Lin S-Y. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 180.He Q, Zhang Z, Gao Y, Shi J, Li Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small. 2009;5:2722. doi: 10.1002/smll.200900923. [DOI] [PubMed] [Google Scholar]
- 181.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6:1794. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou Y, Antonietti M. A novel tailored bimodal porous silica with well-defined inverse opal microstructure and supermicroporous lamellar nanostructure. Chem Commun. 2003:2564. doi: 10.1039/b307444g. [DOI] [PubMed] [Google Scholar]
- 183.Takahashi R, Sato S, Sodesawa T, Kawakita M, Ogura K. High surface-area silica with controlled pore size prepared from nanocomposite of silica and citric acid. J Phys Chem B. 2000;104:12184. [Google Scholar]
- 184.Liang W, He S, Wang Y, Fang J. Morphology and shape control of porous silica nanostructures with dual-templating approaches. J Nanosci Nanotechnol. 2014;14:4424. doi: 10.1166/jnn.2014.8243. [DOI] [PubMed] [Google Scholar]
- 185.Liu J, Detrembleur C, De Pauw-Gillet MC, Mornet S, Elst LV, Laurent S, Jérôme C, Duguet E. Heat-triggered drug release systems based on mesoporous silica nanoparticles filled with a maghemite core and phase-change molecules as gatekeepers. J Mater Chem B. 2014;2:59. doi: 10.1039/c3tb21229g. [DOI] [PubMed] [Google Scholar]
- 186.Muhammad F, Guo M, Qi W, Sun F, Wang A, Guo Y, Zhu G. pH-triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J Am Chem Soc. 2011;133:8778. doi: 10.1021/ja200328s. [DOI] [PubMed] [Google Scholar]
- 187.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, Zink JI, Nel AE. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc. 2010;132:12690. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed. 2008;47:8438. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 189.Tsai CP, Hung Y, Chou YH, Huang DM, Hsiao JK, Chang C, Chen YC, Mou CY. High-contrast paramagnetic fluorescent mesoporous silica nanorods as a multifunctional cell-imaging probe. Small. 2008;4:186. doi: 10.1002/smll.200700457. [DOI] [PubMed] [Google Scholar]
- 190.Wu SH, Lin YS, Hung Y, Chou YH, Hsu YH, Chang C, Mou CY. Multifunctional mesoporous silica nanoparticles for intracellular labeling and animal magnetic resonance imaging studies. ChemBioChem. 2008;9:53. doi: 10.1002/cbic.200700509. [DOI] [PubMed] [Google Scholar]
- 191.Slowing I, Trewyn BG, Victor SYL. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J Am Chem Soc. 2006;128:14792. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- 192.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc. 2009;132:552. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 193.Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VSY. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano. 2011;5:1366. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 194.Kapoor S, Bhattacharyya AJ. Ultrasound-triggered controlled drug delivery and biosensing using silica nanotubes. J Phys Chem C. 2009;113:7155. [Google Scholar]
- 195.Mitchell DT, Lee SB, Trofin L, Li N, Nevanen TK, Söderlund H, Martin CR. Smart nanotubes for bioseparations and biocatalysis. J Am Chem Soc. 2002;124:11864. doi: 10.1021/ja027247b. [DOI] [PubMed] [Google Scholar]
- 196.Ding HM, Shao L, Liu RJ, Xiao QG, Chen JF. Silica nanotubes for lysozyme immobilization. J Colloid Interface Sci. 2005;290:102. doi: 10.1016/j.jcis.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 197.Nan A, Bai X, Son SJ, Lee SB, Ghandehari H. Cellular uptake and cytotoxicity of silica nanotubes. Nano Lett. 2008;8:2150. doi: 10.1021/nl0802741. [DOI] [PubMed] [Google Scholar]
- 198.Jang J, Yoon H. Novel fabrication of size-tunable silica nanotubes using a reverse-microemulsion-mediated sol–gel method. Adv Mater. 2004;16:799. [Google Scholar]
- 199.Chen CC, Liu YC, Wu CH, Yeh CC, Su MT, Wu YC. Preparation of fluorescent silica nanotubes and their application in gene delivery. Adv Mater. 2005;17:404. [Google Scholar]
- 200.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc. 2008;130:10643. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.He H, Xie C, Ren J. Nonbleaching fluorescence of gold nanoparticles and its applications in cancer cell imaging. Anal Chem. 2008;80:5951. doi: 10.1021/ac8005796. [DOI] [PubMed] [Google Scholar]
- 202.Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther. 2008;7:1730. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Storhoff JJ, Lucas AD, Garimella V, Bao YP, Müller UR. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat Biotechnol. 2004;22:883. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sliem M, Ebeid E, Harith M. Selective colorimetric detection of viral DNA sequence of hepatitis C virus genotype-4 genetic material using gold nanoparticles. J Biomed Nanotechnol. 2007;3:360. [Google Scholar]
- 205.Bindhani B, Parida U, Biswal S, Panigrahi A, Nayak P. Gold nanoparticles and their biomedical applications. Rev Nanosci Nanotechnol. 2013;2:247. [Google Scholar]
- 206.Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008;37:1783. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 207.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 208.Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, Liang XJ. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2011;33:1180. doi: 10.1016/j.biomaterials.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 209.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjugate Chem. 2009;20:209. doi: 10.1021/bc800480k. [DOI] [PubMed] [Google Scholar]
- 211.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J Phys Chem B. 2006;110:7238. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 212.Zhang Z, Wang L, Wang J, Jiang X, Li X, Hu Z, Ji Y, Wu X, Chen C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater. 2012;24:1418. doi: 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- 213.Schütz M, Steinigeweg D, Salehi M, Kömpe K, Schlücker S. Hydrophilically stabilized gold nanostars as SERS labels for tissue imaging of the tumor suppressor p63 by immuno-SERS microscopy. Chem Commun. 2011;47:4216. doi: 10.1039/c0cc05229a. [DOI] [PubMed] [Google Scholar]
- 214.Cobley CM, Au L, Chen J, Xia Y. Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Expert Opin Drug Deliv. 2010;7:577. doi: 10.1517/17425240903571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 217.Rozanova N, Zhang JZ. Metal and magnetic nanostructures for cancer diagnosis and therapy. Rev Nanosci Nanotechnol. 2013;2:29. [Google Scholar]
- 218.Kleinauskas A, Kim JK, Choi GH, Kim HT, Roe K, Juzenas P. Superparamagnetic magnetite nanoparticles for cancer theranostics. Rev Nanosci Nanotechnol. 2012;1:271. [Google Scholar]
- 219.Roy I, Mitra S, Maitra A, Mozumdar S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int J Pharm. 2003;250:25. doi: 10.1016/s0378-5173(02)00452-0. [DOI] [PubMed] [Google Scholar]
- 220.He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol. 2000;7:899. doi: 10.1128/cdli.7.6.899-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Robinson JT, Tabakman SM, Liang Y, Wang H, Casalongue HS, Vinh D, Dai H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc. 2011;133:6825. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 223.Liang XJ, Meng H, Wang Y, He H, Meng J, Lu J, Wang PC, Zhao Y, Gao X, Sun B. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc Natl Acad Sci USA. 2010;107:7449. doi: 10.1073/pnas.0909707107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kang SG, Zhou G, Yang P, Liu Y, Sun B, Huynh T, Meng H, Zhao L, Xing G, Chen C. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82 (OH) 22 and its implication for de novo design of nanomedicine. Proc Natl Acad Sci USA. 2012;109:15431. doi: 10.1073/pnas.1204600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Song Y, Jin J, Li J, He R, Zhang M, Chang Y, Chen K, Wang Y, Sun B, Xing G. Gd@C82(OH)22 nanoparticles constrain macrophages migration into tumor tissue to prevent metastasis. J Nanosci Nanotechnol. 2014;14:4022. doi: 10.1166/jnn.2014.8277. [DOI] [PubMed] [Google Scholar]
- 226.Thorek DL, Chen AK, Czupryna J, Tsourkas A. Super-paramagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 227.Hu X, Hao X, Wu Y, Zhang J, Zhang X, Wang PC, Zou G, Liang XJ. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J Mater Chem B. 2013;1:1109. doi: 10.1039/C2TB00223J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3:137. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 229.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 230.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D. Anti-HER2 immunoliposomes enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172. [PubMed] [Google Scholar]
- 231.Chiu SJ, Ueno NT, Lee RJ. Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin®) conjugated polyethylenimine. J Control Release. 2004;97:357. doi: 10.1016/j.jconrel.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 232.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 233.Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 234.Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjugate Chem. 2004;15:185. doi: 10.1021/bc0341674. [DOI] [PubMed] [Google Scholar]
- 235.Borisch B, Semac I, Soltermann A, Palomba C, Hoessli D. Anti-CD20 treatments and the lymphocyte membrane: Pathology for therapy. Verh Dtsch Ges Pathol. 2001;85:161. [PubMed] [Google Scholar]
- 236.Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 237.Pagel JM, Pantelias A, Hedin N, Wilbur S, Saganic L, Lin Y, Axworthy D, Hamlin DK, Wilbur DS, Gopal AK. Evaluation of CD20, CD22, and HLA-DR targeting for radioimmunotherapy of B-cell lymphomas. Cancer Res. 2007;67:5921. doi: 10.1158/0008-5472.CAN-07-0080. [DOI] [PubMed] [Google Scholar]
- 238.Jager E, van der Velden VH, te Marvelde JG, Walter RB, Agur Z, Vainstein V. Targeted drug delivery by gemtuzumab ozogamicin: Mechanism-based mathematical model for treatment strategy improvement and therapy individualization. PloS one. 2011;6:e24265. doi: 10.1371/journal.pone.0024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lapalombella R, Zhao X, Triantafillou G, Yu B, Jin Y, Lozanski G, Cheney C, Heerema N, Jarjoura D, Lehman A. A novel Raji-Burkitt’s lymphoma model for preclinical and mechanistic evaluation of CD52-targeted immunotherapeutic agents. Clin Cancer Res. 2008;14:569. doi: 10.1158/1078-0432.CCR-07-1006. [DOI] [PubMed] [Google Scholar]
- 240.Kim TH, Jo YG, Jiang HH, Lim SM, Youn YS, Lee S, Chen X, Lee KC. PEG-transferrin conjugated TRAIL (TNF-related apoptosis-inducing ligand) for therapeutic tumor targeting. J Control Release. 2012;162:422. doi: 10.1016/j.jconrel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Han L, Ren Y, Long L, Zhong Y, Shen C, Pu P, Yuan X, Kang C. Inhibition of C6 glioma in vivo by combination chemotherapy of implantation of polymer wafer and intrac-arotid perfusion of transferrin-decorated nanoparticles. Oncol Rep. 2012;27:121. doi: 10.3892/or.2011.1459. [DOI] [PubMed] [Google Scholar]
- 242.Zhou P, Hatziieremia S, Elliott MA, Scobie L, Crossan C, Michie AM, Holyoake TL, Halbert GW, Jørgensen HG. Uptake of synthetic low density lipoprotein by leukemic stem cells—A potential stem cell targeted drug delivery strategy. J Control Release. 2010;148:380. doi: 10.1016/j.jconrel.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 243.Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J, Song L, Liang M, Yan X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nanotechnol. 2012;7:459. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 244.Lee MH, Kim JY, Han JH, Bhuniya S, Sessler JL, Kang C, Kim JS. Direct fluorescence monitoring of the delivery and cellular uptake of a cancer-targeted RGD peptide-appended naphthalimide theragnostic prodrug. J Am Chem Soc. 2012;134:12668. doi: 10.1021/ja303998y. [DOI] [PubMed] [Google Scholar]
- 245.Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, Ino Y, Nomoto T, Matsumoto Y, Koyama H. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anti-cancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano. 2013;7:8583. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- 246.Bieker R, Kessler T, Schwöppe C, Padró T, Persigehl T, Bremer C, Dreischalück J, Kolkmeyer A, Heindel W, Mesters RM. Infarction of tumor vessels by NGR-peptide–directed targeting of tissue factor: Experimental results and first-in-man experience. Blood. 2009;113:5019. doi: 10.1182/blood-2008-04-150318. [DOI] [PubMed] [Google Scholar]
- 247.Lee TY, Lin CT, Kuo SY, Chang DK, Wu HC. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007;67:10958. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
- 248.Chang DK, Chiu CY, Kuo SY, Lin WC, Lo A, Wang YP, Li PC, Wu HC. Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J Biol Chem. 2009;284:12905. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yan Z, Wang F, Wen Z, Zhan C, Feng L, Liu Y, Wei X, Xie C, Lu W. LyP-1-conjugated PEGylated liposomes: A carrier system for targeted therapy of lymphatic metastatic tumor. J Control Release. 2012;157:118. doi: 10.1016/j.jconrel.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 250.Kim JY, Choi WI, Kim YH, Tae G. Brain-targeted delivery of protein using chitosan-and RVG peptide-conjugated, pluronic-based nanocarrier. Biomaterials. 2012;34:1170. doi: 10.1016/j.biomaterials.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 251.Pan L, He Q, Liu J, Chen Y, Ma M, Zhang L, Shi J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J Am Chem Soc. 2012;134:5722. doi: 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- 252.Ng EW, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 253.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 254.Shieh YA, Yang SJ, Wei MF, Shieh MJ. Aptamer-based tumor-targeted drug delivery for photodynamic therapy. ACS Nano. 2010;4:1433. doi: 10.1021/nn901374b. [DOI] [PubMed] [Google Scholar]
- 255.Wu Y, Sefah K, Liu H, Wang R, Tan W. DNA aptamer–micelle as an efficient detection/delivery vehicle toward cancer cells. Proc Natl Acad Sci USA. 2010;107:5. doi: 10.1073/pnas.0909611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 257.Kim D, Jeong YY, Jon S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano. 2010;4:3689. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 258.Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355:564. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- 259.Hussain AF, Tur MK, Barth S. An Aptamer–siRNA Chimera Silences the Eukaryotic Elongation Factor 2 Gene and Induces Apoptosis in Cancers Expressing αvβ3 Integrin. Nucleic Acid Ther. 2013;23:203. doi: 10.1089/nat.2012.0408. [DOI] [PubMed] [Google Scholar]
- 260.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci Transl Med. 2011;3:66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chi-Hong BC, Chernis GA, Hoang VQ, Landgraf R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc Natl Acad Sci USA. 2003;100:9226. doi: 10.1073/pnas.1332660100. [DOI] [PMC free article] [PubMed] [Google Scholar]