Abstract
• Background and Aims Changes in number of trichomes and in composition and concentrations of their exudates throughout leaf development may have important consequences for plant adaptation to abiotic and biotic factors. In the present study, seasonal changes in leaf trichomes and epicuticular flavonoid aglycones in three Finnish birch taxa (Betula pendula, B. pubescens ssp. pubescens, and B. pubescens ssp. czerepanovii) were followed.
• Methods Trichome number and ultrastructure were studied by means of light, scanning and transmission electron microscopy, while flavonoid aglycones in ethanolic leaf surface extracts were analysed by high-pressure liquid chromatography.
• Key Results Density of both glandular and non-glandular trichomes decreased drastically with leaf expansion while the total number of trichomes per leaf remained constant, indicating that the final number of trichomes is established early in leaf development. Cells of glandular trichomes differentiate before those of the epidermis and produce secreted material only during the relatively short period (around 1–2 weeks) of leaf unfolding and expansion. In fully expanded leaves, glandular trichomes appeared to be at the post-secretory phase and function mainly as storage organs; they contained lipid droplets and osmiophilic material (probably phenolics). Concentrations (mg g−1 d. wt) of surface flavonoids decreased with leaf age in all taxa. However, the changes in total amount (µg per leaf) of flavonoids during leaf development were taxon-specific: no changes in B. pubescens ssp. czerepanovii, increase in B. pendula and in B. pubescens ssp. pubescens followed by the decline in the latter taxon. Concentrations of most of the individual leaf surface flavonoids correlated positively with the density of glandular trichomes within species, suggesting the participation of glandular trichomes in production of surface flavonoids.
• Conclusions Rapid decline in the density of leaf trichomes and in the concentrations of flavonoid aglycones with leaf age suggests that the functional role of trichomes is likely to be most important at the early stages of birch leaf development.
Key words: Birch, Betula pendula, Betula pubescens ssp., pubescens, Betula pubescens ssp., czerepanovii, glandular trichomes, non-glandular trichomes, flavonoid aglycones, leaf development
INTRODUCTION
Leaves of many plants are densely covered with glandular and non-glandular trichomes, which originate from epidermal cells (Werker, 2000). The indumentum of non-glandular trichomes and the lipophilic substances secreted by glandular trichomes (terpenes, lipids, waxes and flavonoid aglycones) serve as a barrier against various external factors, including herbivores and pathogens, UV-B radiation, extreme temperatures and excessive water loss (Ehleringer, 1982; Harborne, 1991; Wagner, 1991; Karabourniotis et al., 1993; Tattini et al., 2000; Werker, 2000). The development of leaf trichomes often begins at very early stages of leaf development, sometimes even before the leaf primordium can be distinguished. In some plants, the final number of trichomes is established early during leaf differentiation (Ascensão and Pais, 1987), while in others new trichomes are formed throughout all the stages of leaf development (Maffei et al., 1989; Turner et al., 2000).
In many plant species trichome density is very high in young leaves but decreases rapidly with leaf expansion (Maffei et al., 1989; Werker et al., 1993; Pérez-Estrada et al., 2000). It has been suggested that, in young leaves lacking epidermis, trichomes and their exudates may serve as a functional analogue of the epidermis in mature leaves (Karabourniotis and Fasseas, 1996) since they play a similar protective role against biotic and abiotic factors such as water deficit (Ehleringer, 1982; Mauseth, 1988), insect herbivores (Levin, 1973; Juniper and Jeffree, 1983; Wagner, 1991), phytopathogenic fungi (Allen et al., 1991), and UV-B radiation (Day et al., 1993; Karabourniotis et al., 1993; Tattini et al., 2000). At later stages of leaf development, when the formation of the epidermis is completed, the functional role of the trichomes becomes less important, and they often senesce and shed. In some cases, however, trichomes remain viable and functional in mature leaves (Werker, 2000). Moreover, the composition of exudates produced by glandular trichomes may change with leaf age (Maffei et al., 1989; Werker et al., 1993).
The leaves and young shoots of birch (Betula) bear both glandular and non-glandular trichomes on their surface. The peltate resin glands on birch stem produce a mixture of phenolics and steroidal triterpenoids, which play an important role in birch resistance against mammalian herbivores (Reichardt et al., 1984; Rousi et al., 1991). It has been shown that the number of stem resin glands of B. pendula is determined at the beginning of primary tissue growth, and that the secretory activity of glands is higher on young shoots (Lapinjoki et al., 1991; Raatikainen et al., 1992). The secondary growth of later seasons separates the resin droplets wider apart, and they gradually weather off. This loss of primary chemical defense is compensated for by the thickness and chemical constituents of the bark.
Unlike birch stem glands, birch leaf trichomes have so far received considerably less attention, although recent studies have suggested that they may play a role in birch resistance against UV-B radiation (Kostina et al., 2001), springtime frost (Prozherina et al., 2003) and insect herbivores (Rautio et al., 2002). A previous study of newly flushed leaves in three birch taxa (Betula pendula, B. pubescens ssp. pubescens and B. pubescens ssp. czerepanovii) revealed significant differences in trichome structure and density among taxa (Valkama et al., 2003). It has also been demonstrated that surface extracts of birch leaves contain flavonoid aglycones, and that composition and concentrations of these compounds differ markedly among birch species (Valkama et al., 2003). However, changes in birch leaf trichomes and flavonoid aglycones during birch leaf development have not been studied before, although they may have important consequences for birch adaptation to abiotic and biotic factors.
In the present paper, changes in trichome number and ultrastructure, and in composition, concentrations and total amount of flavonoid aglycones that occurred in leaves from budburst to maturation, in Betula pendula, B. pubescens ssp. pubescens and B. pubescens ssp. czerepanovii, are followed. In particular, the following questions are addressed: (1) At what stage of leaf development is the final number of trichomes established? (2) Are birch trichomes present on leaf surface during all stages of leaf development? (3) Do composition and concentrations of flavonoid aglycones change throughout leaf development? (4) Are changes in trichome density and concentrations of flavonoid aglycones due to changes in leaf size or to changes in trichome number per leaf and in synthesis of flavonoids?
MATERIALS AND METHODS
Experimental trees
The birch (Betula L.) trees used in this study represent five different clones of B. pendula, five provenances of B. pubescens ssp. czerepanovii and two seed origins of B. pubescens ssp. pubescens. Details on tree origin are provided in Valkama et al. (2003). The trees, growing in the Botanical Garden of the University of Turku (60°26′N, 22°10′E), were aged 10 (B. pendula), 20 (B. pubescens ssp. czerepanovii) and 21 years (B. pubescens ssp. pubescens) at the time of the study.
Leaf samples
Leaves from short shoots were collected at the following stages of leaf development:
Stage 1: 1-cm-long leaves (7 May for B. pendula; 14 May for B. pubescens ssp. pubescens; 15 May for B. pubescens ssp. czerepanovii)
Stage 2: 2-cm-long leaves (11 May for B. pendula; 17 May for B. pubescens ssp. pubescens; 20 May for B. pubescens ssp. czerepanovii)
Stage 3: 3-cm-long leaves (20 May for B. pendula; 22 May for B. pubescens ssp. pubescens; 27 May for B. pubescens ssp. czerepanovii)
Stage 4: 4-cm-long leaves (28 May for B. pendula; 29 May for B. pubescens ssp. pubescens; 5 June for B. pubescens ssp. czerepanovii)
Stage 5: fully expanded leaves (7 June for B. pendula; 6 June for B. pubescens ssp. pubescens; 12 June for B. pubescens ssp. czerepanovii)
Stage 6: mature leaves (3 July for B. pendula; 5 July for B. pubescens ssp. pubescens; 10 July for B. pubescens ssp. czerepanovii).
Density and total number of trichomes per leaf
Three leaves from each of the three to five trees per each clone, provenance or seed origin were sampled at each stage of leaf development. Samples were prepared as described by Valkama et al. (2003) and trichome densities were counted under a light microscope from three frames (1·3 × 1·3 mm2) per leaf. The density of non-glandular trichomes (leaf hairs) was studied only in B. pubescens ssp. pubescens because in B. pendula leaf hairs are sparse and in B. pubescens ssp. czerepanovii they have uneven distribution within leaf and high variation within and between leaves, trees and provenances. The total number of trichomes per leaf was calculated by multiplying the number of trichomes per mm2 by the leaf area.
Microscopy
One leaf was collected from short shoots of three trees per each of clone, provenance or origin at stages 1, 3 and 5 of leaf development. Samples for light, transmission and scanning electron microscopy were fixed and studied as described by Valkama et al. (2003). The presence of small plastids, vacuoles, lipid droplets and osmiophilic material was recorded from each sample to estimate seasonal changes in trichome structure and the secretion of exudates.
Extraction, analysis and identification of leaf surface flavonoids
Ten leaves from each clone, provenance or origin were collected for each stage of leaf development and then frozen and stored at −80 °C. Five leaves from each tree were used for chemical analyses, while the remaining five leaves were dried for 48 h at 70 °C for measurement of the percentage of leaf dry weight (d. wt). Leaf surface flavonoids were extracted and analysed as described by Valkama et al. (2003). Extraction efficiency was 97 %. Total concentrations of flavonoid aglycones were calculated as a sum of concentrations of individual flavonoids for each stage of leaf development.
Statistics
The statistical analyses were carried out using SPSS 10·0 for Windows. The chi-square test followed by the Mann–Whitney U test was used to compare ultrastructural parameters among stages of leaf development. For analysis of the density and the total number of trichomes, the General Linear Model (GLM) repeated-measures procedure was used with leaf side (upper or lower) and stage of leaf development as within-subject factors, and taxa (for glandular trichomes) or origin (for non-glandular trichomes) as between-subject factors. Clone-, provenance- or origin-specific means were used for this analysis.
Following the established practice in the ecological literature, the term ‘concentration’ is used to refer to the mass of a particular compound per unit of leaf mass (mg g−1 d. wt; Koricheva, 1999), although the chemically correct term would be ‘content’. The mass of a compound per leaf (µg per leaf) is referred to as ‘amount’ (Riipi et al., 2002). The total amount of flavonoid aglycones per leaf was calculated as the total concentration of flavonoid aglycones multiplied by the mean dry mass of the leaf. The total concentration of flavonoid aglycones per mm2 was calculated as the total amount of flavonoid aglycones per leaf divided by the mean leaf area. Pearson's product-moment correlation coefficients were calculated between the average density of glandular trichomes on the upper and lower leaf sides and concentrations of individual compounds per mm2 for each species. Clone-specific means for B. pendula (n = 5) or provenance/origin-specific means for both subspecies of B. pubescens (n = 7) were used for those correlations.
Changes in concentrations and total amount of surface flavonoids during leaf development were analysed separately in B. pubescens and B. pendula, since the composition of epicuticular flavonoids differs between the two species. The GLM repeated-measures procedure was used with stage of leaf development as the within-subject factor and subspecies (for B. pubescens) or clone (for B. pendula) as the between-subject factor. Changes in the total concentrations (mg g−1 d. wt) and total amount of total flavonoid aglycones per leaf were examined by means of graphical vector analysis (Koricheva, 1999). This method makes it possible to distinguish whether shifts in concentrations are due to changes in compound synthesis or in biomass accumulation.
RESULTS
Density and total number of glandular trichomes
The density of glandular trichomes decreased throughout leaf development in all birch taxa examined, although to a different extent depending on stage of leaf development and taxon, as indicated by significant Stage × Taxa interaction (F10,30 = 8·5, P < 0·001; Fig. 1). For example, trichome density declined significantly in B. pendula from stage 1 to stage 3, whereas in B. pubescens ssp. pubescens it decreased significantly from stage 1 to stage 2. During later stages of leaf development, trichome density declined slightly and non-significantly in these taxa. In B. pubescens ssp. czerepanovii, it declined non-significantly for all stages of leaf development. Moreover, during leaf expansion, trichome density on the lower leaf side was higher in B. pubescens ssp. pubescens than in ssp. czerepanovii. However, when leaves reached their final size, there was no difference in glandular trichome density among birch taxa (Fig. 1).
Fig. 1.
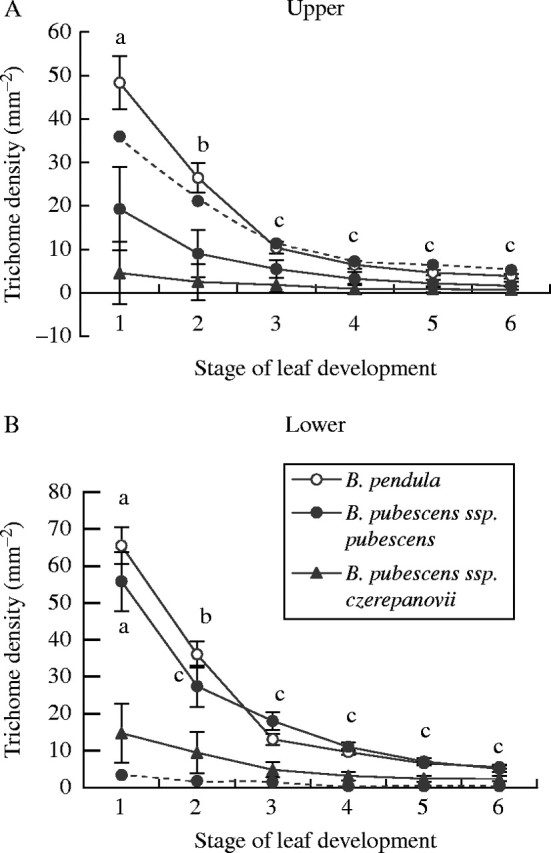
Density of glandular (solid lines) and non-glandular trichomes (dotted lines, for B. pubescens ssp. pubescens only) on upper (A) and lower (B) sides of birch leaves during leaf development. Symbols represent clone-specific means for B. pendula (n = 5), provenance-specific means for B. pubescens ssp. czerepanovii (n = 5) or origin-specific means for B. pubescens ssp. pubescens (n = 2) ± s.e. Symbols marked by different letters are significantly different among stages of leaf development at P < 0·05.
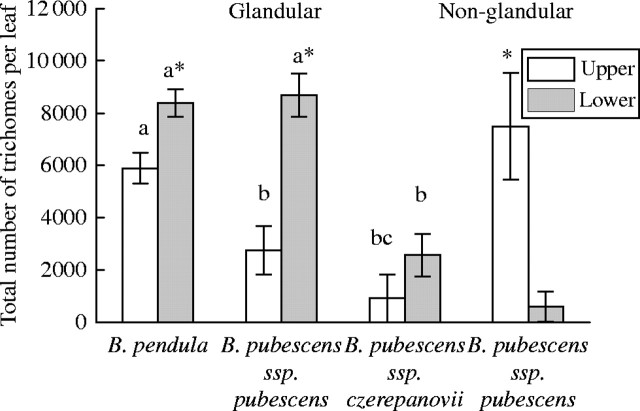
Unlike trichome density, the total number of glandular trichomes per leaf did not change during leaf development in any of the birch taxa studied (F5,30 = 2·3, P = 0·065), although it varied significantly among taxa (F2,6 = 15·7, P = 0·004) and between leaf sides depending on taxon (F2,6 = 13·3, P = 0·006, Fig. 2). For example, on the upper leaf surface, the total number of glandular trichomes was higher in B. pendula than in both subspecies of B. pubescens. On the lower leaf surface, it was higher in B. pendula and in B. pubescens ssp. pubescens compared with that of ssp. czerepanovii. In addition, the total number of glandular trichomes was significantly higher on the lower leaf side than on the upper leaf side in B. pendula and B. pubescens ssp. pubescens, whereas the difference in their number was non-significant between leaf sides in ssp. czerepanovii (Fig. 2).
Fig. 2.
Total number of glandular and non-glandular trichomes on leaves of different birch taxa. Bars represent clone-specific means for B. pendula (n = 5), provenance-specific means for B. pubescens ssp. czerepanovii (n = 5) or origin-specific means for B. pubescens ssp. pubescens (n = 2) ± s.e. Bars marked by different letters are significantly different among taxa within leaf side at P < 0·05. Bars marked by asterisks are significantly different among leaf sides within taxon at P < 0·05.
The density and total number of non-glandular trichomes were significantly higher (P < 0·001) on the upper leaf side than on the lower leaf side (Fig. 1, dotted line, and Fig. 2). During leaf development, the density of non-glandular trichomes significantly decreased (F5,25 = 14·32, P < 0·001 ), but the total number of non-glandular trichomes did not change (F5,25 = 1·32, P = 0·290).
Structural changes in glandular trichomes
Light microscopy showed that glandular trichomes were already fully developed and differentiated into cortical and medullar cells in 1-cm-long birch leaves, which still lacked differentiated palisade and spongy parenchyma and epidermal cells (Fig. 3A). At this stage of leaf development, glandular trichomes had a spherical shape and intact cuticle (Fig. 3D). In 3-cm leaves, there was differentiation between palisade and spongy parenchyma, and the epidermis was already formed (Fig. 3B). In the cortical cells of glandular trichomes, central vacuole occupied almost the whole volume of the cytoplasm and the cuticle was slightly detached from the cells (Fig. 3B). When the leaf reached its final size, trichomes exhibit features of the post-secretory phase: flattened shape, degenerated cytoplasm and detached cuticle of some trichomes (Fig. 3C and E).
Fig. 3.
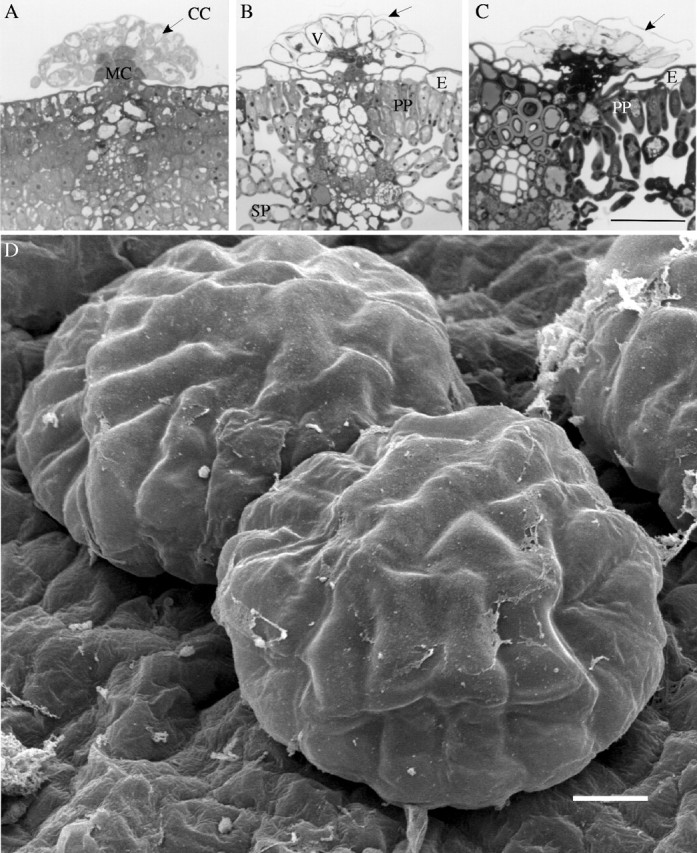
Light (A–C) and SEM (D and E) micrographs of glandular trichomes of B. pendula at different stages of leaf development. (A and D) 1-cm leaves. Young glandular trichomes have a spherical shape, their cells are differentiated into cortical and medullar cells and are covered by intact cuticle. No differentiation between palisade and spongy parenchyma could be distinguished, and epidermis is not completely developed. (B) 3-cm leaf. Cuticle is slightly detached from cortical cells. Vacuole occupied almost the whole volume of the cytoplasm. Leaf mesophyll is differentiated into palisade and spongy parenchyma, epidermis is already formed. (C and E) Fully expanded leaves. Aged trichomes are flattened, covered with detached cuticle (arrows). Scale bars: A, B and C = 50 µm; D and E = 10 µm. CC, cortical cells; MC, medullar cells; PP, palisade parenchyma; SP, spongy parenchyma; E, epidermis; V, vacuole.
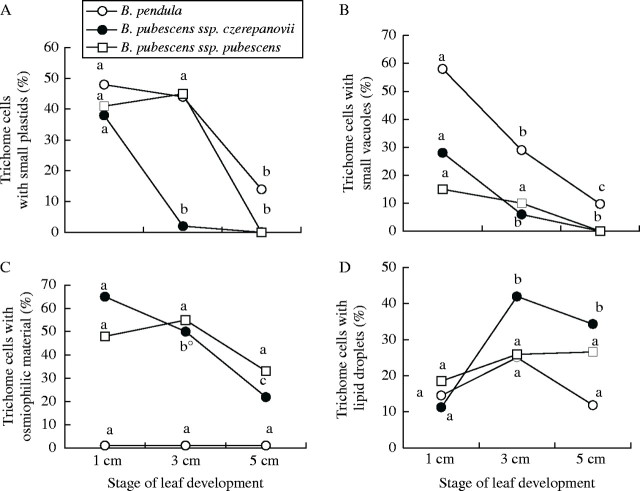
TEM observations revealed further developmental changes and among-taxa differences in glandular trichome ultrastructure. The proportion of cells with small plastids in B. pendula and B. pubescens ssp. pubescens was high in 1–3-cm leaves, but decreased drastically in fully expanded leaves (Fig. 4A). In B. pubescens ssp. czerepanovii, glandular cells with small plastids were observed only in 1-cm leaves, but not in older leaves (Fig. 4A). The proportion of glandular cells with small vacuoles declined during leaf development in all birch taxa (Fig. 4B). The proportion of glandular cells with osmiophilic material decreased significantly in B. pubescens ssp. czerepanovii and tended to decline in ssp. pubescens, whereas, in B. pendula, trichome cells lacked osmiophilic material during all stages of leaf development (Fig. 4C). In contrast, the proportion of cells with lipid droplets increased with leaf age in B. pubescens ssp. czerepanovii, while no changes were observed in B. pendula and B. pubescens ssp. pubescens (Fig. 4D).
Fig. 4.
Ultrastructural changes in leaf glandular trichomes of Betula species during leaf development. Proportion of trichome cells with small plastids (A), small vacuoles (B), osmiophilic material (C) and lipid droplets (D). Different letters indicate significant difference among stages of leaf development (P < 0·05, Mann–Whitney U test). Letter followed by ° indicates marginally significant difference (0·05 < P < 0·1).
Epicuticular flavonoids
The composition of flavonoid aglycones did not change during leaf development. The same 12 and 6 compounds, which were present in newly flushed leaves of B. pubescens and B. pendula, respectively (Valkama et al., 2003), occurred on the birch surface regardless of the leaf developmental stage. In contrast, concentrations (mg g−1 d. wt and mg mm−2) of flavonoid aglycones decreased during leaf development in all taxa examined (Fig. 5). Two compounds, flavonol methyl ether 2 and flavanone methyl ether, occurred at highest concentrations in both subspecies of B. pubescens (Fig. 5A and B). In ssp. czerepanovii, concentrations of these compounds decreased considerably during the first three stages of leaf development, but only slightly during later stages (Fig. 5B), whereas, in ssp. pubescens, concentrations of these compounds decreased moderately until stage 3, but markedly during later stages (Fig. 5A). In B. pendula, concentrations of tetrahydroxyflavone dimethyl ether decreased five-fold from stage 1 (1-cm leaves) to stage 6 (mature leaves), while the concentrations of other compounds changed only slightly (Fig. 5C).
Fig. 5.
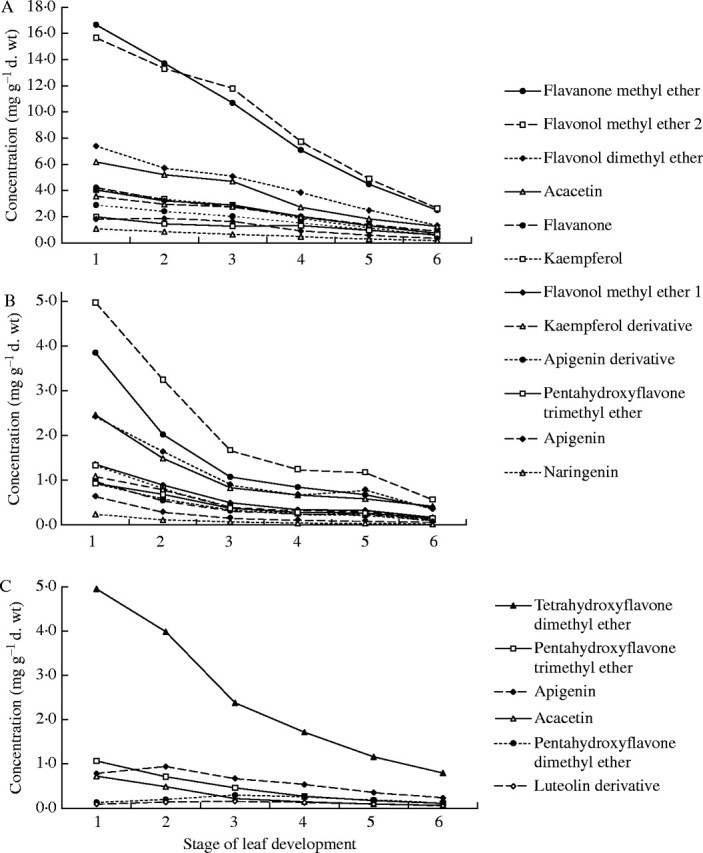
Changes in concentrations of individual leaf surface flavonoids during leaf development in B. pubescens ssp. pubescens (A), B. pubescens ssp. czerepanovii (B) and B. pendula (C). Each symbol represents clone-specific means for B. pendula (n = 5), provenance-specific means for B. pubescens ssp. czerepanovii (n = 5) and ssp. pubescens (n = 2).
Changes in total concentrations (mg g−1d.wt) and in total amount (µg per leaf) of flavonoid aglycones varied depending on the taxon, as indicated by significant Leaf stage × Taxa interaction (F10,30 = 48·6, P < 0·001 and F10,30 = 8·04, P < 0·001, respectively). In B. pubescens ssp. pubescens, the decrease in total concentrations of flavonoid aglycones in expanding leaves was accompanied by an initial increase in the total amount of flavonoids (Fig. 6A). The total amount was stabilized in stage 3 (22 May) and stage 4 (29 May) and then significantly decreased in mature leaves (5 July; Fig. 6A). In B. pubescens ssp. czerepanovii, total concentrations of flavonoid aglycones decreased with leaf development from 29·2 ± 3·9 mg g−1 d. wt in 1-cm leaves to 3·2 ± 0·8 mg g−1 d. wt in mature leaves (P < 0·05), but the total amount of flavonoids did not change (295 ± 51 µg per leaf in 1-cm leaves and 248 ± 54 µg per leaf in mature leaves; P > 0·05). Finally, in B. pendula the decrease in total concentrations of flavonoid aglycones was accompanied by a gradual increase in their total amount until leaves reached their full size (7 June) and then by non-significant decline in mature leaves (3 July; Fig. 6B).
Fig. 6.
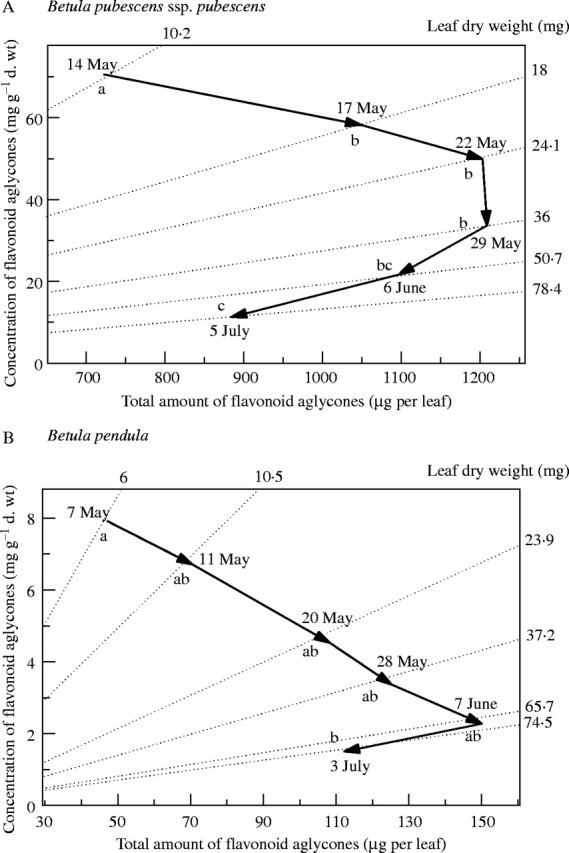
Changes in total concentrations and total amount of surface flavonoids and in leaf dry weight during leaf development in B. pubescens ssp. pubescens (A) and B. pendula (B). Dates correspond to stages 1–6 of leaf development (see Materials and methods). For each sampling date, mean concentration of flavonoid aglycones is plotted against corresponding total amount per leaf. Diagonal lines indicate mean leaf dry weight at each sampling date. Direction of vector from earlier sampling date to later one indicates changes in concentrations and total amounts of flavonoids between the two dates. For example, vertical shifts indicate that there are no changes in the total amount of flavonoids per leaf and changes in concentrations are caused by changes in leaf biomass. On the other hand, a horizontal shift to the right indicates that the rate of flavonoid synthesis is matched by changes in leaf biomass so that no changes in concentrations occur. Different letters indicate significant difference among stages of leaf development for total amount of flavonoid aglycones at P < 0·05.
Correlations between glandular trichome density and epicuticular flavonoids
In both subspecies of B. pubescens, glandular trichome density and concentrations of all 12 individual compounds per mm2 were highly correlated through all stage of leaf development (r = 0·77–0·99, P < 0·001, n = 7). In B. pendula, concentrations of all of the compounds except apigenin and pentahydroxyflavone trimethyl ether were correlated with glandular trichome density through all stages of leaf development (r = 0·62–0·92, P < 0·01, n = 5).
DISCUSSION
The results presented here demonstrate that leaf trichomes in all birch taxa are differentiated at a very early stage of leaf development, prior to the differentiation of epidermis and mesophyll cells. During leaf development, the density of trichomes decreased in all Betula taxa studied. This decline was due to growth dilution in expanding leaves, since the total number of trichomes per leaf remained constant and neither the formation of new trichomes nor trichome shedding at later stages of growth occurred. Similarly, the number of resin glands on shoots of B. pendula is determined at the beginning of primary tissue growth, and the formation of additional new glands at later stages of growth is highly unlikely (Lapinjoki et al., 1991).
Similarly to trichome density, total concentrations (mg g−1 d. wt and mg mm−2) of surface flavonoid aglycones decreased considerably during leaf development. In B. pubescens ssp. czerepanovii and B. pendula, this decrease was largely due to growth dilution in expanding leaves for all stages of leaf development, since the amount of flavonoid aglycones (µg per leaf) either did not change (in B. pubescens ssp. czerepanovii) or increased with leaf age (in B. pendula). However, in B. pubescens ssp. pubescens, growth dilution occurred only during the first three stages of leaf development, whereas in mature leaves the decrease in the total concentration of flavonoid aglycones was observed together with the decline in their total amount per leaf. This might be due to reduction in their synthesis together with simultaneous degradation of the compounds or transformation into insoluble, cell-wall-bound forms. This indicates that flavonoid synthesis is at its highest level in young versus older leaves in all taxa studied, whereas its duration may vary among birch taxa. Moreover, glandular trichome density is strongly correlated with concentrations of most of the individual flavonoid aglycones (per mm2) through leaf development, suggesting the participation of glandular trichomes in production of surface flavonoids. The localization of flavonoid aglycones within glandular trichome cells was frequently demonstrated for other plant species (for references, see Wollenweber, 1984).
Furthermore, ultrastructural features of birch glandular trichomes change throughout leaf development. Cells of young birch glandular trichomes are characterized by many traits indicating high metabolic activity: the presence of numerous small plastids, small vacuoles and osmiophilic material, which is likely to represent phenolics (Valkama et al., 2003). All the above features declined rapidly with leaf age. Therefore, it seems likely that the secretory phase of birch trichomes is relatively short (about 1–2 weeks) and coincides with leaf expansion. The release of the secreted material in young leaves probably occurs by the passage of exudates droplets through the intact cuticle, since no cuticular rupture was observed in young trichomes. A similar mode of secretion of exudates, which contains flavonoid aglycones, was suggested for Salvia blepharophylla (Bisio et al., 1999).
By the time the birch leaf reaches its full size and the formation of epidermis is completed, the glandular trichomes exhibit features of the post-secretory phase (flattened shape, degenerated cytoplasm and detached cuticle). At this phase, trichomes function mainly as storage organs allowing release of exudates on leaf surface by means of culicular rupture, since high amounts of lipid droplets have been observed in their cells and total amount of surface flavonoids was the highest in fully expanded leaves of B. pendula.
Ultrastructural observations indicated that other types of chemical compounds were produced in glandular trichomes in addition to flavonoid aglycones. Osmiophilic material, which is likely to represent phenolics containing o-dihydroxy groups (Nielson and Griffith, 1978), was not secreted with exudates, but stored inside glandular trichome cells in both subspecies of B. pubescens (Valkama et al., 2003). During leaf development, the amount of osmiophilic material declined, nevertheless, 20–40 % of cells in aged trichomes possessed it. Similarly, the presence of phenolics within hair cells has been reported for Olea leaves (Karabourniotis et al., 1992). It is possible that phenolics, occurring in glandular trichomes of B. pubescens, may be as important in birch resistance against herbivores as foliar phenolics (Kause et al., 1999; Haukioja et al., 2002).
To summarize, the final number of birch trichomes is already established in newly flushed leaves and does not change during leaf development. In contrast, the density of trichomes and concentrations of epicuticular flavonoid aglycones exhibit a rapid decline with leaf age. These results support the suggestion by Karabourniotis and Fasseas (1996) that the role of leaf trichomes is particularly significant during the early stage of leaf development when leaves still lack differentiated epidermis. At later stages of leaf development, glandular trichomes cease to synthesize exudates and function mainly as storage organs allowing release of exudates.
Acknowledgments
We thank Marjo Helander, Kari Saikkonen and Matti Sulkinoja for the permission to use their experimental trees at the Botanical Garden of the University of Turku. Lauri Pelliniemi, Ritva Niemi and the staff at the Laboratory of Electron Microscopy provided advice and technical assistance with TEM and SEM, and Juha Järvenpää and Tuuli Luomahaara helped with the chemical analyses, Tatjana Saarinen and Tuija Koivisto helped with preparation of samples for light microscope and counting trichome density. The study was financed by the Academy of Finland.
LITERATURE CITED
- Allen EA, Hoch HC, Sreadman JR, Stavely RJ. 1991. Influence of leaf surface features on spore deposition and the epiphytic growth of phytopathogenic fungi. In: Andrews JH, Hirano SS, eds. Microbial ecology of leaves. New York: Springer-Verlag, 87–110. [Google Scholar]
- Ascensão L, Pais MSS. 1987. Glandular trichomes of Artemisia campestris (ssp. Maritima): ontogeny and histochemistry of the secretory product. Botanical Gazette 148: 221–227. [Google Scholar]
- Bisio A, Corallo A, Gastaldo P, Romussi G, Ciarallo G, Fontana N, De Tommasi N, Profumo P. 1999. Glandular hairs and secreted material in Salvia blepharophylla Brandegee ex Epling grown in Italy. Annals of Botany 83: 441–452. [Google Scholar]
- Day TA, Martin G, Vogelman TC. 1993. Penetration of UV-B radiation in foliage: evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environmental 16: 735–741. [Google Scholar]
- Ehleringer J. 1982. The influence of water stress and temperature on leaf pubescence development in Encelia farinoza American Journal of Botany 69: 670–675. [Google Scholar]
- Harborne JB. 1991. Flavonoids pigments. In: Rosenthal GA, Berenbaum MR, eds. Herbivores. Their interactions with secondary plant metabolites. San Diego: Academic Press 389–429. [Google Scholar]
- Haukioja E, Ossipov V, Lempa K. 2002. Interactive effects of leaf maturation and phenolics on consumption and growth of a geometrid moth. Entomologia Experimentalis et Applicata 104: 125–136. [Google Scholar]
- Juniper BE, Jefree CE. 1983.Plant surfaces. London: Edward Arnold. [Google Scholar]
- Karabourniotis G, Fasseas C. 1996. The dense indumentum with its polyphenol content may replace the protective role of the epidermis in some young xeromorphic leaves. Canadian Journal of Botany 74: 347–351. [Google Scholar]
- Karabourniotis G, Papadopoulos K, Papamarkou M, Manetas Y. 1992. Ultraviolet-B radiation absorbing capacity of leaf hairs. Physiologia Plantarum 86: 414–418. [Google Scholar]
- Karabourniotis G, Kyparissis A, Manetas Y. 1993. Leaf hairs of Olea europaea L. protect underling tissue against UV-B radiation damage. Environmental and Experimental Botany 33: 341–345. [Google Scholar]
- Kause A, Ossipov V, Haukioja E, Lempa K, Hanhimäki S, Ossipova S. 1999. Multiplicity of biochemical factors determining quality of growing birch leaves. Oecologia 120: 102–112. [DOI] [PubMed] [Google Scholar]
- Koricheva J. 1999. Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119: 467–473. [DOI] [PubMed] [Google Scholar]
- Kostina E, Wulff A, Julkunen-Tiitto R. 2001. Growth, structure, stomatal responses and secondary metabolites of birch seedlings (Betula pendula) under elevated UV-B radiation in the field. Trees 15: 483–491. [Google Scholar]
- Lapinjoki SP, Elo HA, Taipale HT. 1991. Development and structure of resin glands on tissues of Betula pendula Roth. during growth. New Phytologist 117: 219–223. [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defence. Quarterly Review of Biology 48: 3–15. [Google Scholar]
- Maffei M, Chialva F, Sacco T. 1989. Glandular trichomes and essential oils in developing peppermint leaves. New Phytologist 111: 707–716. [DOI] [PubMed] [Google Scholar]
- Mauseth JD. 1988.Plant anatomy. Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- Nielson AJ, Griffith WP. 1978. Tissue fixation and staining with osmium tetroxide: the role of phenolic compounds. Journal of Histochemistry and Cytochemistry 26: 138–140. [DOI] [PubMed] [Google Scholar]
- Pérez-Estrada LB, Canto-Santan Z, Oyama K. 2000. Variation in leaf trichomes of Wigandia urens: environmental factors and physiological consequence. Tree Physiology 20: 629–632. [DOI] [PubMed] [Google Scholar]
- Prozherina N, Freiwald V, Rousi M, Oksanen E. 2003. Interactive effect of springtime frost and elevated ozone on early growth, foliar injuries and leaf structure of birch (Betula pendula). New Phytologist 159: 623–636. [DOI] [PubMed] [Google Scholar]
- Raatikainen OJ, Taipale HT, Pelttari A, Lapinjoki SP. 1992. An electron microscope study of resin production and secretion by the glands of seedlings of Betula pendula Roth. New Phytologist 122: 537–543. [DOI] [PubMed] [Google Scholar]
- Rautio P, Markkola A, Martel J, Tuomi J, Härmä E, Kuikka K, Siitonen A, Leal Riesco I, Roitto M. 2002. Developmental plasticity in birch leaves: defoliation causes a shift from glandular to nonglandular trichomes. Oikos 98: 437–446. [Google Scholar]
- Reichardt PB, Bryant JP, Clausen TP, Wieland GD. 1984. Defence of winter-dormant Alaska paper birch against snowshoe hares. Oecologia 65: 58–69. [DOI] [PubMed] [Google Scholar]
- Riipi M, Ossopov V, Lempa K, Haukioja E, Koricheva J, Ossipova S, Pihlaja K. 2002. Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 130: 380–390. [DOI] [PubMed] [Google Scholar]
- Rousi M, Tahvanainen J, Uotila I. 1991. A mechanism of resistance to hare browsing in winter dormant European white birch (Betula pendula). American Naturalist 137: 64–82. [Google Scholar]
- Tattini M, Gravano E, Pinelli P, Mulinacci N, Romani A. 2000. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytologist 148: 69–77. [DOI] [PubMed] [Google Scholar]
- Turner GW, Gershenzon J, Croteau RB. 2000. Distribution of peltate glandular trichomes on development leaves of peppermint. Plant Physiology 124: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama E, Salminen J-P, Koricheva J, Pihlaja K. 2003. Com-parative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Annals of Botany 91: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ. 1991. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. 1993. Glandular hairs, secretory cavities, and the essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Annals of Botany 71: 43–50. [Google Scholar]
- Wollenweber E. 1984. The systematic implication of flavonoids secreated by plants. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes. New York, London: Plenum, 53–69. [Google Scholar]