Abstract
• Background and aims Boragineae is one of the main tribes of Boraginaceae, but delimitation and intergeneric classification of this group are unclear and have not yet been studied using DNA sequences. In particular, phylogenetic relationships in Anchusa s.l. still need to be elucidated in order to assess its taxonomic boundaries with respect to the controversial segregate genera Hormuzakia, Gastrocotyle, Phyllocara and Cynoglottis.
• Methods Phylogenetic relationships among 51 taxa of tribe Boragineae were investigated by comparative sequencing of the trnLUAA intron of the plastid genome and of the ITS1 region of the nuclear ribosomal DNA. Exemplar taxa from 16 genera of Boragineae and all subgenera of Anchusa s.l. were included, along with two selected outgroups from tribes Lithospermeae and Cynoglosseae.
• Key results Phylogenies generated by maximum parsimony and combined ITS1-trnL sequences support the monophyly of the tribe and a split into two clades, Pentaglottis and the remainder of Boragineae. The latter contains two large monophyletic groups. The first consists of three moderately to well-supported branches, Borago–Symphytum, Pulmonaria–Nonea and Brunnera. In the Pulmonaria–Nonea subclade, the rare endemic Paraskevia cesatiana is sister to Pulmonaria, and Nonea appears to be paraphyletic with respect to Elizaldia. The second main group corresponds to the well-supported clade of Anchusa s.l., with the megaphyllic, polyploid herb Trachystemon orientalis as sister taxon, although with low support. Anchusa s.l. is highly paraphyletic to its segregate genera and falls into four subclades: (1) Phyllocara, Hormuzakia, Anchusa subgenus Buglossum and A. subgenus Buglossoides; (2) Gastrocotyle; (3) A. subgenus Buglossellum and Cynoglottis; and (4) A. subgenus Anchusa, Lycopsis and Anchusella. All species of Anchusa subg. Anchusa, including the South African A. capensis, are included in a single unresolved clade. Anchusa subgenus Limbata is also included here despite marked divergence in floral morphology. The low nucleotide variation of ITS1 suggests a recent partly adaptive radiation within this group.
• Conclusions Molecular data show that nine of the usually accepted genera of the Boragineae consisting of two or more species are monophyletic: Anchusella, Borago, Brunnera, Cynoglottis, Gastrocotyle, Hormuzakia, Nonea, Pulmonaria and Symphytum. In addition, the tribe includes the four monotypic genera Paraskevia, Pentaglottis, Phyllocara and Trachystemon. The morphologically well-characterized segregate genera in Anchusa s.l. are all confirmed by DNA sequences and should be definitively accepted. Most of the traditionally recognized subgenera of Anchusa are also supported as monophyletic groups by both nuclear and plastid sequence data. In order to bring taxonomy in line with phylogeny, the institution of new, independent generic entities for subgenera Buglossum, Buglossellum and Buglossoides and a narrower but more natural concept of Anchusa are advocated.
Key words: Anchusa, Boraginaceae, Boragineae, ITS1, molecular systematics, phylogeny, taxonomy, trnL
INTRODUCTION
Boragineae Bercht. & J.Presl (=Anchuseae DC.) is one of the major tribes (approx. 170 species) of Boraginaceae Juss. s.s. (approx. 2000 species), a family of the euasterid I clade (Angiosperm Phylogeny Group II, 2003) recently been shown to be paraphyletic, if defined in the traditional broad sense (e.g. sensu Gürke, 1893). Hydrophyllaceae R.Br. ex Edwards are sister to a clade formed by Ehretiaceae Mart. ex Lindl. (including Lennoaceae Solms), Cordiaceae R.Br. ex Dumort. and Heliotropiaceae Schrad. (Böhle and Hilger, 1997; Ferguson, 1999; Gottschling et al., 2001, Diane et al., 2002) with all except Lennoaceae usually included as subfamilies in the Boraginaceae s.l. Relationships within and between the tribes of Boraginaceae s.s. are still not well understood, mainly due to insufficient sampling of many groups in the phylogenetic analyses so far published (e.g. Långström and Chase, 2002).
Boragineae are morphologically well characterized by faucal corolla appendages (=fornices) and by strophiolate mericarpids attached basally on a planar gynobase. These mericarpids show a more or less thickened basal annulus surrounding a plug-like scar and usually have an elaiosome for ant dispersal. The tribe is native to the Old World only and has its major centre of diversity in the Mediterranean basin and adjacent Western Asia, with only two species of Anchusa subgenus Anchusa ranging into Eastern subtropical Africa and the Cape region.
Johnston (1924) and Riedl (1963) regarded Boragineae as a natural group possibly originated from Lithospermeae, with Eritrichieae/Cynoglosseae representing the ‘neighbouring’ lineage. Recent studies of Boraginaceae based on ribosomal ITS1 and plastid atpB sequences (Gottschling et al., 2001; Långström and Chase, 2002) have corroborated this view, although taxon sampling in both analyses was not sufficient to reliably demonstrate the monophyly of the tribe.
Boragineae have been subject to conflicting treatments with regard to their circumscription and number of genera recognized depending on the weight attributed by the different authors to fruit and floral characters. In the early systems based entirely on mericarpid morphology and attachment (De Candolle, 1846; Bentham, 1876; Gürke, 1893) nine genera were recognized, including the genus Alkanna Tausch. Johnston (1924) accepted 12 genera, including Lithodora Griseb., but excluding Alkanna, whereas Melchior (1964) adopted a ‘lumping’ approach and reduced the tribe to eight genera. Steps towards an apparently more natural treatment were made by Guşuleac(1923, 1928), the most important monographer of the tribe, who reduced Boragineae to those taxa with faucal appendages in the corolla, moving Lithodora and Alkanna to Lithospermeae. In his system, the tribe consisted of 11 genera, with Elizaldia Willk. reduced to synonymy in Nonea Medik. Guşuleac's treatment was largely followed by Riedl (1963), who recognized 13 genera with a narrowly defined Anchusa L. and Elizaldia separate from Nonea.
To date, however, there is still uncertainty concerning the number and delimitation of the genera of Boragineae, mainly because little is known about the phylogenetic relationships in the tribe. While some of these genera are distinctive (e.g. Borago L., Symphytum L., Brunnera Steven and Pentaglottis Tausch), others have been historically controversial because of a weaker morphological characterization and reticulate patterns of variation.
Anchusa s.l. is the genus that has been subjected to the most variable treatments. Understood in a broad sense by most early authors, it was shown by Guşuleac to include distinct lineages that he regarded as separate genera. Guşuleac's exhaustive morphological studies (Guşuleac, 1927, 1928, 1929) resulted in the well-supported separation at genus level of species originally described under Anchusa, such as Phyllocara aucheri (DC.) Guşul., Hormuzakia aggregata (Lehm.) Guşul., Gastrocotyle hispida (Forssk.) Bunge and Pentaglottis sempervirens (L.) L.H. Bailey. He further subdivided the remainder of Anchusa (Anchusa s.s.) into six subgenera Cynoglottis, Lycopsis, Buglossum, Buglossellum, Buglossoides and (Eu) Anchusa. This elaborate system, however, was not followed by most later authors of flora treatments, who continued to adopt a traditionally broad concept of Anchusa [e.g. Chater, 1972 (Fl. Europaea); Chamberlain, 1979 (Fl. Turkey); Greuter et al., 1984 (Med-Checklist)].
Recent studies based on micromorphology, palynology and karyology (Bigazzi and Selvi, 1998, 2000, 2001; Bigazzi et al., 1999) have provided evidence for a narrow concept of Anchusa and widely supported Guşuleac's generic treatment. Furthermore, evidence has been provided for also maintaining Cynoglottis (Guşul.) Vural & Kit Tan and Anchusella Bigazzi, Nardi & Selvi, the latter originally described as Anchusa subgenus Rivinia Greuter (Greuter, 1965), as separate genera in view of their apomorphic features in reproductive structures (Vural and Tan, 1983; Bigazzi et al., 1997).
A second phylogenetically poorly known and controversial lineage is represented by Pulmonaria and Nonea, two apparently related genera each with numerous species. To this lineage belongs the enigmatic species Paraskevia cesatiana (Fenzl. & Friedr.) W. Sauer & G. Sauer (Sauer and Sauer, 1980), originally described as Anchusa cesatiana (von Friedrichsthal, 1838), but later variously combined under Pulmonaria (Boissier, 1879) and Nonea (Boissier, 1849; Guşuleac, 1929; Greuter, 1981). Nonea also has close relationships with the North African genus Elizaldia, to which it is probably paraphyletic as suggested by a recent morphological analysis (Selvi et al., 2002).
In this paper, an overview of the phylogenetics of Boragineae, as inferred from DNA sequences from both plastid and nuclear non-coding regions, is provided. Special emphasis is applied to Anchusa s.l., which has been the focus of our previous studies and for which a near-comprehensive sampling from its taxonomic and geographic range was possible. The combined use of two different markers with different evolutionary speed, the internal transcribed spacer region (ITS1) of nuclear ribosomal DNA and the more conserved trnL(UAA) intron of the plastid genome is appropriate for investigations of relationships between species and genera. The usefulness of both markers in the systematics of Boraginales has been shown in recent phylogenetic studies at different taxonomic levels (Böhle and Hilger, 1997; Gottschling and Hilger, 2001; Diane et al., 2002; Winkworth et al., 2002).
MATERIALS AND METHODS
Plant material
Silica gel preserved samples of leaf tissue from field collections or, in a few cases, from herbarium specimens were used for DNA extraction. The ingroup comprised 51 taxa (Table 1). Buglossoides arvensis and Cynoglossum amplifolium, of tribes Lithospermeae and Cynoglosseae, respectively, served as outgroups.
Table 1.
List of taxa investigated with internal DNA number, origin and voucher specimens, and GenBank accession
| Taxa |
DNA no. |
Origin and voucher** |
GenBank accession trnL/ITS1 |
|---|---|---|---|
| Anchusa aegyptiaca (L.) DC. | 695 | Cyprus: Hilger 00/1 (BSB) | AY383255/AY383294 |
| *Anchusa affinis R.Br. | 1037 | Saudi Arabia: Lavranos & Collenette 18390 (FI) | AY383279/– |
| Anchusa azurea Mill. | 619 | Cyprus: Hilger 00/18 (BSB) | AY383254/AY383293 |
| Anchusa capellii Moris | 780 | Italy, Sardinia: Bigazzi & Selvi 99.002 (FI) BSB | AY383257/AY383297 |
| Anchusa capensis Thunb. | 990 | South Africa: Orange Free State (FI) | AY383269/AY383311 |
| Anchusa cespitosa Lam. | 287 | Greece, Crete: Hilger 98/11 (FI, BSB) | AY383268/AY383310 |
| Anchusa crispa Viv. ssp. crispa | 791 | France, Corsica: Bigazzi & Selvi 99.005 (FI) | AF530603/AY071853 |
| Anchusa formosa Selvi, Bigazzi & Bacchetta | 781 | Italy, Sardinia: Bigazzi & Selvi 97.006 (FI) | AY383258/AY383299 |
| Anchusa leptophylla Roem. & Schult. | 633 | Turkey: Carle & Kürschner 4032 (BSB) | AF530604/AY383298 |
| Anchusa leucantha Selvi & Bigazzi | 862 | Greece: Bigazzi & Selvi 01.17 (FI, BSB) | AY383267/AY383309 |
| Anchusa limbata Boiss. & Heldr. | 1158 | Turkey: Bigazzi & Selvi 02.01 (FI, BSB) | AY383260/AY383301 |
| Anchusa milleri Sprengel | 623 | Israel: Hilger 21/94. (FI, BSB) | AY383256/AY383295 |
| Anchusa ochroleuca M.Bieb. | 373 | Bulgaria: Hilger 97/21 offspring (BSB) | AY383261/AY383302 |
| Anchusa officinalis L. | 672 | Germany: Hilger 2000 (BSB) | AY045703/AY045710 |
| Anchusa pusilla Guşul. | 727 | Turkey: Bigazzi & Selvi 97.018 (FI) | AY045704/AY045711 |
| Anchusa samothracica Bigazzi & Selvi | 811 | Greece: Bigazzi & Selvi 99.016 (FI, BSB) | AY383262/AY383303 |
| Anchusa strigosa Banks. & Sol. | 618 | Cyprus: Hilger 00/16 (BSB) | AY383253/AY383292 |
| Anchusa stylosa M. Bieb. | 861 | Greece: Bigazzi & Selvi 01.13 (FI, BSB) | AY383266/AY383308 |
| Anchusa thessala Boiss. & Spruner | 730 | Turkey: Bigazzi & Selvi 97.022 (FI, BSB) | AF530599/AY383296 |
| Anchusa undulata L. ssp. hybrida (Ten.) Bég. | 616 | Cyprus: Hilger 00/12 (BSB) | AY383259/AY383300 |
| Anchusella cretica (Mill.) Bigazzi, Nardi & Selvi | 667 | Italy: Bigazzi & Selvi 00.33 (FI) | AY045709/AY045716 |
| Anchusella variegata (L.) Bigazzi, Nardi & Selvi | 857 | Greece: Bigazzi & Selvi 01.10 (FI) | AY383265/AY383306 |
| Borago officinalis L. | 671 | Germany (Berlin cult.): Hilger (BSB) | AY383245/AY383283 |
| Borago pygmaea (DC.) Chater & Greuter | 375 | Germany (cult. H.Berlin-Dahlem): Hilger | AY383244/AY383282 |
| Brunnera macrophylla (Adams) I.M.Johnst. | 628 | Germany (cult. H.Berlin-Dahlem): Hilger | AY383249/AY383288 |
| Brunnera orientalis (Schenk) I.M.Johnst. | 829 | Turkey: Bigazzi & Selvi 00.28 (FI) | AY383250/AY383289 |
| Buglossoides arvensis (L.) I.M.Johnst. | 662 | Greece, Crete: Kagiampaki 4/2000 (BSB) | AY383242/AY383280 |
| Cynoglossum amplifolium A.DC. | 645 | Kenya: Fischer 5/2000 (BSB, FI) | AY383243/AY383281 |
| Cynoglottis barrelieri (All.) Vural & Kit Tan | 669 | Italy: Bigazzi & Selvi 99.019 (FI) | AY045708/AY045715 |
| Cynoglottis chetikiana Vural & Kit Tan ssp. paphalagonica (Bornm.) Vural & Kit Tan | 830 | Turkey: Bigazzi & Selvi 98.008 (FI, BSB) | AF530602/AY383307 |
| Elizaldia calycina (Roem. & Schult.) Maire ssp. multicolor (Kunze) Chater | 706 | Morocco: Lewalle 10884 (RNG) | AY383264/AY383305 |
| Gastrocotyle hispida (Forssk.) Bunge | 674 | Jordan: Baierle & al. 17.3.86 (BSB) | AY045705/AY045712 |
| Gastrocotyle macedonica (Degen & Dörfl.) Bigazzi, Hilger & Selvi | 682 | Greece: Bigazzi & Selvi 99.009 (FI, BSB) | AY045706/AY045713 |
| Hormuzakia aggregata (Lehm.) Guşul. | 693 | Israel: Bigazzi & Selvi 96.015 (FI) | AY383252/AY383291 |
| *Hormuzakia negevensis (Danin) Danin & Hilger | 664 | Israel: Danin 24.3.97 (HUJ) | AY383278/– |
| Lycopsis arvensis L. | 624 | Germany: Hilger & Werres 27.5.99 (BSB) | AY045707/AY045714 |
| Lycopsis orientalis L. | 831 | Turkey: Bigazzi & Selvi 00.10 (FI) | AY383277/AY383319 |
| Nonea lutea (Desr.) DC. | 630 | Germany (cult. H. Berlin-Dahlem): Hilger (BSB) | AY383274/AY383316 |
| Nonea pulla DC. | 661 | Germany: Hensen 28.5.00 (BSB) | AY383275/AY383317 |
| Nonea vesicaria (L.) Reichb. | 1311 | Morocco: Podlech 51525 (ITS1, M) | –/AY383304 |
| “..” | 1252 | Sicily: Bigazzi & Selvi 97.038 (trnL, FI) | AY383263/– |
| Paraskevia cesatiana (Fenzl & Friedr.) W. Sauer & G. Sauer | 859 | Greece: Bigazzi & Selvi 01.02 (FI, BSB) | AY383276/AY383318 |
| Pentaglottis sempervirens (L.) L.H.Bailey | 668 | Italy (cult.): Bigazzi & Selvi | AF530598/AY383286 |
| Phyllocara aucheri (DC.) Guşul. | 670 | Turkey: Bigazzi & Selvi 97.041 (FI) | AY383251/AY383290 |
| Pulmonaria angustifolia L. | 744 | Italy: Frey 2000 (BSB) | AY383271/AY383313 |
| Pulmonaria mollis Wulf. | 755 | Germany (cult.): Hilger (BSB) | AY383273/AY383315 |
| Pulmonaria obscura Dumort. | 681 | Germany (cult.): Hilger (BSB) | AY383270/AY383312 |
| Pulmonaria picta Rouy | 761 | Italy: Bigazzi & Selvi 91.002 (FI) | AY383272/AY383314 |
| Symphytum creticum (Willd.) Greuter & Rech.fil. | 284 | Greece, Crete: Hilger (BSB) | AY383246/AY383284 |
| Symphytum tuberosum L. | 629 | Germany (cult. H. Berlin-Dahlem): Hilger | AY383247/AY383285 |
| Trachystemon orientalis (L.) G.Don | 666 | Turkey: Bigazzi & Selvi 00.06 (FI) | AY383248/AY383287 |
Those taxa which were included only in the trnL analysis.
BSB = Herbarium, Institut für Biologie – Systematische Botanik und Pflanzengeographie, Freie Universität, Berlin; FI = Herbarium Universitatis Florentinae, Museo di Storia Naturale, Universitá di Firenze; RNG = Herbarium, Plant Science Laboratories, University of Reading; M = Herbarium, Botanische Staatssammlung, München; HUJ = Herbarium, Department of Evolution, Systematics and Ecology, Hebrew University, Jerusalem.
DNA extraction, marker amplification and sequencing
The trnL primers (c and d) were those used by Taberlet et al. (1991), the ITS primers (P1 and P2) were those of Baldwin (1992). Genomic DNA was isolated using a modified 2 × CTAB extraction protocol [Doyle and Doyle, 1990; tissue ground in sea sand, 70 % (v/v) isopropanol substituted for the RNase step]. Approximately 40 mg of leaf tissue was used for each extraction. The DNA was amplified with Gibco BRL PCR kits. PCR products were cleaned with Qiagen QIAquick PCR purification columns, quantified with a 100 bp DNA ladder (MBI-Fermentas, St Leon-Rot, Germany), and cycle-sequenced with a GeneAmp PCRSystem 2400 (Perkin Elmer, Boston, MA, USA). A SequiThermExcel II sequencing kit (Epicentre Technologies, Madison, USA) was used with a stop-/loading-solution for terminating. Sequences were run on a GATC model 1500. Polyacrylamide gels were prepared using SequaGel-6 (National Diagnostics, Atlanta, GA, USA). The biotinylated PCR products were transferred onto a Biodyne A nylon membrane (Pall Filtron, Dreieich, Germany) and visualized by streptavidine/basic phosphatase.
Sequence alignment and phylogenetic analysis
Sequences were manually aligned with Align32 (Hepperle, 2001). Sequences are deposited in GenBank (Table 1). Parsimony analysis was performed with PAUP 4.0b1 for PC (Swofford, 1998). A heuristic search analysis was run with ‘tree-bisection-reconnection’ (TBR) branch-swapping with accelerated transformation (ACCTRAN) optimization to infer branch lengths; MULTREES option on, ADDSEQ = random, ten randomized replicates. All characters were weighted equally, and character state transitions were treated as unordered. Gaps were coded as separate characters according to the ‘simple gap coding’ method after Simmons and Ochoterena (2000). The bootstrap (BS) (Felsenstein, 1985) and jackknife (Farris et al., 1996) were performed with 100 replicates (TBR branch-swapping, ten random taxon entries per replicate and MULTREES on); search = FASTSTEP and 10 000 replicates were used in trnL analysis because of computational time limitations.
RESULTS
Analysis of the trnL region
The aligned trnL data set (available from the authors upon request) is 469 bp in length with sequences varying from 419 base pairs (bp) (Symphytum creticum) to 460 bp (Anchusa strigosa and A. azurea). In the phylogenetic analysis, 42 sites were parsimony informative, 47 were uninformative and 380 were constant. Analysis of the trnL resolved the position of Hormuzakia negevensis and Anchusa affinis, two species of which ITS1 sequences were not available.
The heuristic search yielded 3380 most-parsimonious trees of tree length (L) = 134, consistency index (CI) = 0.813 and retention index (RI) = 0.903. The strict consensus tree is shown in Fig. 1. The monophyly of Boragineae is corroborated by 78 % BS and 71 % jackknife support. The ingroup falls into eight clades, the relationships of which remain unresolved: (1) Pentaglottis, (2) Trachystemon, (3) Borago, (4) Symphytum, (5) Brunnera, (6) Phyllocara, Hormuzakia, Anchusa subgenus Buglossum and subgenus Buglossoides, (7) Gastrocotyle, Anchusella, Cynoglottis, Lycopsis, Anchusa subgenus Buglossellum, subgenus Anchusa and subgenus Limbata, (8) Nonea, Elizaldia, Pulmonaria and Paraskevia. Most of these clades can already be recognized in a condensed alignment (outgroups, identical and non-informative positions removed) which shows the insertions/deletions (indels) (Fig. 2).
Fig. 1.
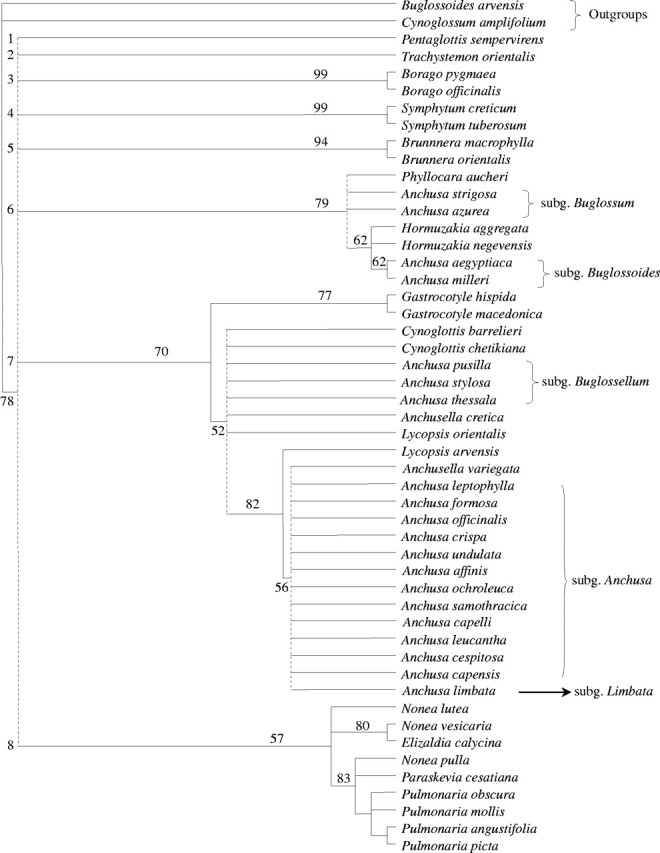
Strict consensus of the 3380 most-parsimonious trees from trnL sequence data. Tree length (L) = 134, consistency index (CI) = 0·813, retention index (RI) = 0·903. The subgenera of Anchusa s.l. are indicated. The numbers above the branches are bootstrap percentages (percentages <50 % are not shown). Main polytomies are indicated by dotted lines and the main unresolved clades are numbered (1–8).
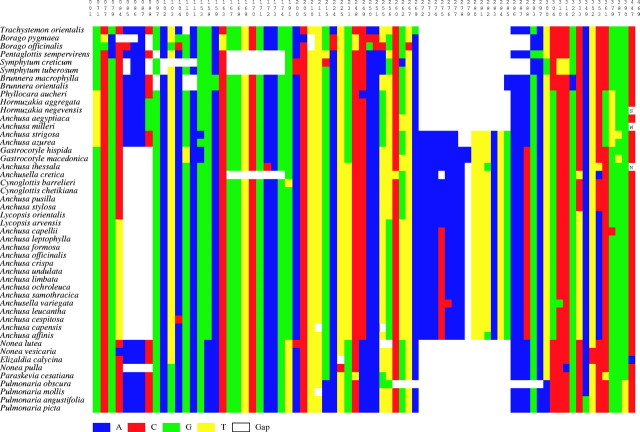
Fig. 2.
Condensed alignment scheme of trnL sequences (outgroups, identical and non-informative positions removed). Taxa are ordered according to the main insertions/deletions shared. Column numbers refer to nucleotide positions in the original alignment.
The monophyly of Borago (clade 3) is supported by 99 % BS and jackknife support; B. pygmaea and B. officinalis share at least six unique indels or substitutions. In clade 4 (99 % BS support), S. creticum forms a group with S. tuberosum. The relationship between Borago and Symphytum was not seen in the consensus tree, but it received BS/jackknife support of 59 % and 56 %, respectively. A shared deletion in position 286–288 characterizes the species of these two genera. Monophyly of Brunnera (clade 5) is supported by 94 % BS; a shared deletion at position 110–111 occurs in the two species of this genus (B. macrophylla and B. orientalis).
Anchusa in a broad sense is subdivided into two clades (6 and 7). One (clade 6) received BS 79 % and 76 % jackknife, and the other (clade 7) 70 % and 61 %, respectively. Both share an insertion at position 272–285 with the exception of Phyllocara, Hormuzakia, and Anchusa subgenus Buglossoides. In clade 6, the relationships between Phyllocara, Anchusa subgenus Buglossum, Hormuzakia plus A. subgenus Buglossoides remained unresolved. The clade with the two latter taxa received 62 % BS, as did A. subgenus Buglossoides itself. Clade 7 was also supported by a shared deletion at position 85–88. The Gastrocotyle clade (77 % BS and 70 % jackknife) is sister to the remainder of clade 7, which is weakly supported (52 % BS). Within the latter, Lycopsis arvensis is sister to the terminal clade of Anchusa subgenus Anchusa (56 % BS) which also includes Anchusella variegata, Anchusa subgenus Limbata and the two African species A. capensis and A. affinis. All together, these taxa form a clade (82 % BS, 70 % jackknife) whose position is not resolved with respect to Cynoglottis, Anchusa subgenus Buglossellum, Anchusella cretica and Lycopsis orientalis. Relationships among the latter taxa also remain unresolved.
Clade 8, formed by Pulmonaria, Nonea, Elizaldia and Paraskevia, is weakly supported (57 % BS and 51 % jackknife), but a deletion at position 272–285 is shared by all the taxa of this clade. A close relationship is revealed between Elizaldia calycina and Nonea vesicaria (80 % BS and 66 % jackknife). This suggests paraphyly of Nonea relative to Elizaldia. The trnL sequences did not resolve the position of Paraskevia with respect to Nonea pulla and Pulmonaria. The monophyly of Pulmonaria is supported by 83 % BS and 75 % jackknife.
Analysis of the ITS1 region
The topology of the trees based on ITS1 was almost identical to that resulting from the combined ITS1–trnL analysis (with lower resolution and support to the clades), and is therefore not presented or discussed separately. The position numbers in the next section refer to the positions in ITS1.
Combined ITS1–trnL analysis
The combined ITS1–trnL data set (ITS1 positions 1–295, trnL 296–764, alignment available from the authors upon request) is 764 bp in length, with ITS1 sequences varying from 270 bp (Borago pygmaea, Phyllocara aucheri and Anchusa thessala) to 277 bp (Pentaglottis sempervirens). In the phylogenetic analysis, 183 sites were parsimony informative, 97 were uninformative and 484 were constant. As expected, ITS1 sequences are more variable than the trnL intron and thus gave a better resolution in part; however, the two markers gave substantially congruent trees, with the exception of the positions of Anchusella cretica and Nonea pulla (see below).
The heuristic search found six most-parsimonious trees, L = 740, CI = 0·605 and RI = 0·787, one of which is shown in Fig. 3. Boragineae are relatively weakly supported as a monophyletic group with 79 % BS and jackknife support. No shared indels for the whole ingroup were found in the ITS1, but some indels characterize the ingroup plus Cynoglossum amplifolium or the ingroup plus Buglossoides arvensis.
Fig. 3.
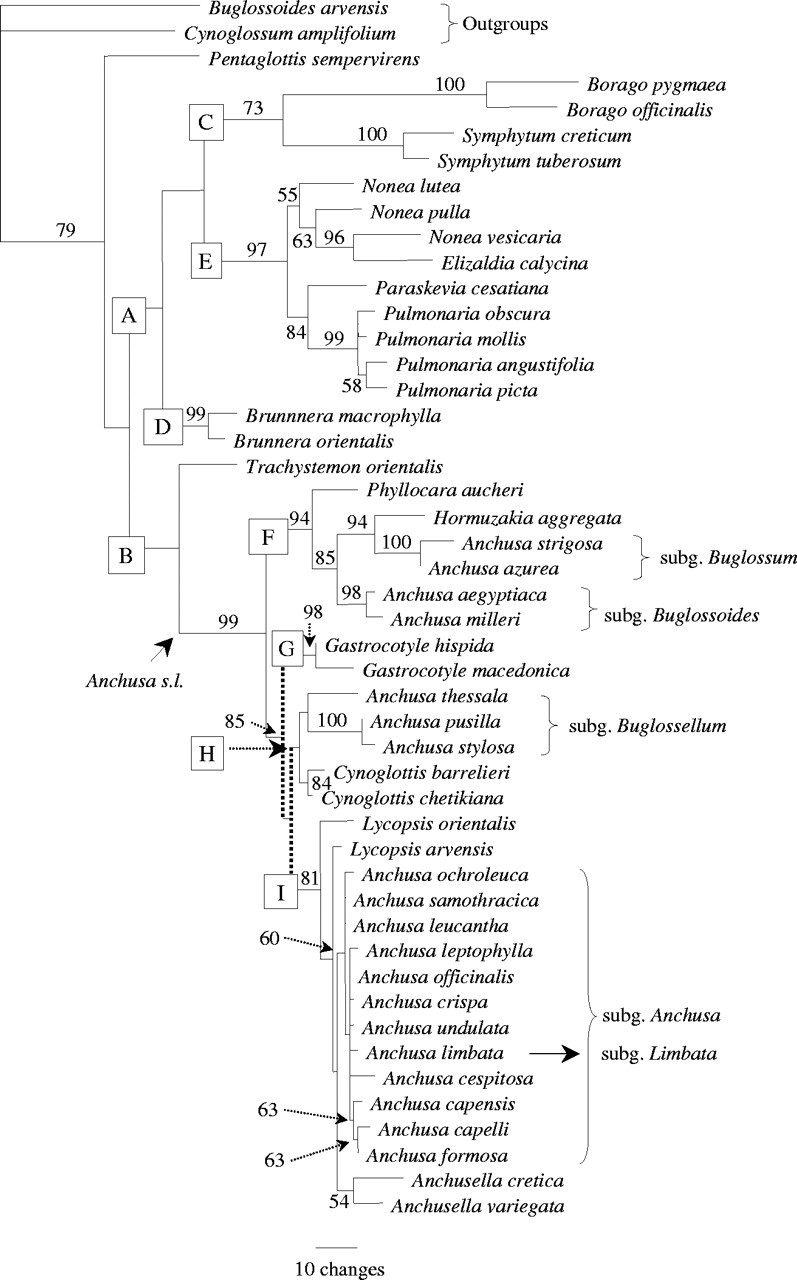
One of the six most-parsimonious trees from ITS1-trnL sequences. L = 740, CI = 0·605, RI = 0·787. The subgenera of Anchusa s.l. are indicated. Letters on the branches indicate the main clades discussed in the text; numbers indicate bootstrap percentages (percentages < 50 % are not shown). Branch lengths are estimated under ACCTRAN. The branches collapsing in the strict consensus tree (not shown) are indicated by dotted lines.
Nine major clades can be recognized (A–I). Pentaglottis is sister to the remainder of the tribe, which is then divided into two clades, A and B (though with BS and jackknife support <50 %). Clade A consists of three moderately to strongly supported branches: Borago plus Symphytum (C), Brunnera (D) and Nonea/Elizaldia/Paraskevia/Pulmonaria (E). Brunnera is sister to clades C and E but BS support for these two nodes is <50. Within clade C (73 % BS and 70 % jackknife), Borago and Symphytum are both supported by 100 % BS and jackknife. The monophyly of Borago is also supported by deletions at positions 83 and 120. A deletion at position 43 is restricted to clade E. Two points emerge in clade E. Firstly, Elizaldia is nested in Nonea, with which it shares a 1 bp substitution at position 111, and the close relationship between E. calycina and N. vesicaria is confirmed by a shared insertion in ITS1 at position 101 (96 % BS and 94 % jackknife). In contrast to trnL alone, N. pulla is here part of the weakly supported Nonea clade. Secondly, Paraskevia is sister to Pulmonaria (84 % BS and jackknife). The monophyly of Pulmonaria is supported by ITS1 insertions at positions 72–73 and 174, and by 99 % BS and jackknife.
Within clade B (62 % BS and 63 % jackknife) Trachystemon is sister to the main lineage of Anchusa s.l., the monophyly of which is strongly supported (99 % both indices and a shared deletion at position 50 of ITS1). ITS1 provides better resolution in this large group than trnL. A key point is that the genus Anchusa, even when intended in a strict sense, is grossly paraphyletic. In fact, all of its subgenera (except A. subgenus Limbata) are sister groups of well-established genera rather than to Anchusa subgenus Anchusa. Within clade F (94 % BS and jackknife), the monotypic genus Phyllocara is sister to the three subclades of Hormuzakia, Anchusa subgenus Buglossum (A. strigosa and A. azurea, 100 %, both indices), and Anchusa subgenus Buglossoides (A. aegyptiaca and A. milleri, 98 % both indices). Clade F is sister to the three clades G, H and I (85 % BS). Clade G (98 % both support indices) corresponds to Gastrocotyle, and is sister to clades H and I, though this relationship is not confirmed in the strict consensus tree (not shown). Clade H (51 % BS) comprises the two subclades of Anchusa subgenus Buglossellum (A. thessala, A. stylosa and A. pusilla) and Cynoglottis (84 % BS, 73 % jackknife). Anchusa stylosa and A. pusilla share an ITS1 deletion in position 132 and cluster together with 100 % BS and jackknife support. Clade I (81 % BS and 76 % jackknife) is formed by the two species of Lycopsis (not forming a clade), Anchusella plus Anchusa subgenus Limbata and all the taxa of Anchusa subgenus Anchusa. In contrast to trnL alone, Anchusella is monophyletic, though with low support, and sister to the rest of clade I, comprising all the taxa of subgenus Anchusa plus subgenus Limbata (60 % BS and 56 % jackknife). Species level relationships within this group remain largely unresolved. The Sardinian endemics A. capellii and A. formosa form a moderately supported (63 % BS) terminal clade sister to the South African A. capensis.
DISCUSSION
Infratribal relationships
Both nuclear and plastid DNA markers used in this analysis support the monophyly of Boragineae. Our results are largely congruent with the views of Johnston (1924), Guşuleac (1923, 1928) and Popov (1953: 207), who regarded the tribe as a ‘natural’ group of ‘ancient Mediterranean’ origin. No discrepancy occurs with respect to more recent studies based on ITS1 (Gottschling et al., 2001) and plastid atpB (Långström and Chase, 2002) that focused on higher taxonomic levels of Boraginales.
To facilitate discussion, the backbone of the trees for Boragineae has been summarized and the distribution of 15 systematically relevant morphological characters plotted onto the nine major clades found in the ITS1–trnL analysis (Fig. 4).
Fig. 4.
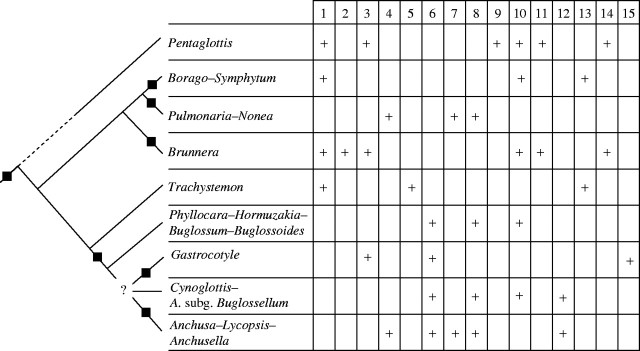
Distribution of 15 morphological characters plotted onto the nine major clades of ITS1-trnL phylogeny of the Boragineae. A square on the clade indicates bootstrap support >85 %. A cross indicates that all or the majority of the taxa of the clade possess the morphological character. Habit: (1) only perennial. Inflorescence and flower: (2) cymes always ebracteate; (3) corolla brachymorphic (with short tube and rotate to subrotate limb); (4) zygomorphy present in some taxa; (5) corolla scales in two series; (6) granular thickenings of cutin on the trichomes of the faucal scales; (7) stylar polymorphism present in some taxa; (8) stigma papillae with typical lageniform shape. Fruit: (9) mericarpids excentrically stalked at the base; (10) mericarpids erect or suberect in all or some of the taxa. Pollen: (11) grains small (P < 15 μm); (12) grains with prolate shape; (13) mesocolpia with supratectum gemmae; (14) grains with (3)-4-(5) apertures; (15) apertures rhomboidal with conical processes on the colpus membrane.
The resolution of the deepest nodes of the phylogeny of the tribe remain poorly supported. In the trnL tree, eight main clades form an unresolved polytomy, whereas in the ITS1–trnL tree Pentaglottis sempervirens is sister to the remainder of the tribe with low support (BS and jackknife <50 %). In the Boragineae subtree from atpB sequences published by Långström and Chase (2002), Pentaglottis clusters with Nonea in a clade whose sister group is Anchusa, whereas Borago is sister to the remainder of the tribe.
Pentaglottis sempervirens (≡ Caryolopha sempervirens). This is the only member of the tribe native to the Atlantic region of south-west Europe. It possesses autoapomorphies in fruit morphology, such as excentrically stalked mericarpids, and in the form of the stigma, with a subconical receptive surface with densely crowded, granulose papillae (Guşuleac, 1928; Bigazzi and Selvi, 2000). Because of this and its unique karyotype of 2n = 22 small chromosomes, Pentaglottis had already been suggested to take an isolated position in Boragineae (Britton, 1951). Guşuleac(1928: 403) spoke of a ‘very ancient Brunnera–Caryolopha (Pentaglottis) type, which survives only in these two genera’. The present results would support an isolated position for Pentaglottis, despite low BS support in the combined analysis.
The ITS1–trnL analysis indicates an early split of the tribe into two main lineages, the first (A) with three subclades (C, D and E), and the second (B) corresponding to Anchusa s.l. plus the monotypic genus Trachystemon (62 % BS). With the exception of Nonea and Elizaldia, the taxa of clade A are mainly perennial and mesophytic, and some of them are restricted to humid forest habitats of Pleistocene refugial areas (e.g. Brunnera, Borago pygmaea). On the contrary, xerophytism, less marked habitat specificity and annual growth are widespread in the taxa of clade B (except Trachystemon).
Borago–Symphytum (C). The relationship between these two genera is supported by 73 % BS assigned to clade C and by a 2-bp deletion in the trnL sequence. Morphologically, Borago and Symphytum share significant features, such as the (8)9–10(11)-aperturate pollen grains with a gemmate tectum, and the stigmatic receptive surface with skittle-like papillae, lacking the elaborated apical disk of most other Boragineae (Bigazzi and Selvi, 1998, 2000). Ecologically, predominance of mesophytism is another distinguishing aspect of this clade and supports a common ancestry of the two genera. On the other hand, numerous characters separate them in inflorescence, flower and fruit morphology. Among these, in Symphytum the cymes are ebracteate and the corolla is almost tubular with elongated, triangular scales, while in Borago cymes are bracteate and the corolla is rotate to campanulate with short scales. The latter is further characterized by pollen with branched columellae and thick exine, and there are two autapomorphic 1 bp deletions in the ITS1 sequence. The two genera are allopatric (except the widespread weed Borago officinalis), Symphytum being mainly south-east European–western Asiatic and Borago south-west Mediterranean. The support for the relationship between these two genera, though moderate, is in line with Guşuleac's opinion (Guşuleac, 1928) of a ‘Paleoborago’ ancestor shared by Borago, Symphytum and Procopiania. The latter genus was instituted by the same author to accommodate S. creticum (Procopiania cretica), a south Aegean species with floral morphology intermediate between Borago and Symphytum due to the corolla lobes being longer than the tube and the exserted stamens. In more recent times, Procopiania was accepted by some authors (Riedl, 1963; Pawlowski, 1971; Chater, 1972; Stearn, 1986) but not by others (Runemark, 1967; Wickens, 1969) who included it in Symphytum. The data presented here showed that S. creticum is nearly identical to S. tuberosum in both ITS1 and trnL sequences.
Brunnera (D). This is a well-defined genus with three rhizomatous species in humid forests of Asia Minor, Caucasus and western Siberia. Morphological autapomorphies are the ebracteate inflorescences, small pollen grains with spinulose equatorial band and stigmas with irregularly cuspidate papillae. Karyologically, B. macrophylla and B. orientalis possess complements of 2n = 12 small chromosomes (the lowest number in the tribe known to date) with low heterochromatin content (approx. 4 %; Britton, 1951; Bigazzi and Selvi, 2001). The monophyly of Brunnera is confirmed by strong BS and jackknife support in both ITS1 and trnL analsyses, but its phylogenetic position remains unclear. Popov (1953) argued that Brunnera evolved through hybridization events between primary members of the Boragineae and Myosotideae and that it is a relict member of the Tertiary forest floras of the Euxine and western Siberian phytochoria (see also Edmondson, 1978). There is no evidence for this hybridization hypothesis, but the molecular data given here suggest it is a sistergroup to clades C and E though with BS <50 %. However, its position within clade A does not support the relationship with the genus Cynoglottis (clade B) which was formerly supposed due to resemblance in flower and fruit morphology (Guşuleac, 1928; Vural and Tan, 1983).
Pulmonaria–Nonea (E). Monophyly of this clade is weakly supported in the trnL analyses but confirmed by ITS1 sequences. The widely accepted assumption (e.g. Johnston, 1924) of a close relationship between Pulmonaria and Nonea is corroborated by molecular data. In the combined analysis, they are sister groups when treated in a wide sense. Morphologically, there are no characters exclusive to this large group. It is proposed to keep these two genera separate in line with traditional taxonomy, in contrast to Johnston (1924) and Greuter (1981). Monophyly of Pulmonaria is supported by two ITS1 insertions and 99 % BS and jackknife support. Nonea is morphologically and karyologically heterogeneous (Selvi and Bigazzi, 2002) and a wider species sampling of this genus is required for a better understanding of its infrageneric relationships.
Two other important points emerge in clade E. Firstly, both ITS1 and trnL sequences demonstrate that Nonea and Elizaldia, which differ only by the exserted stamens in Elizaldia, together form a monophyletic group. Elizaldia calycina is nested in Nonea and forms a terminal clade with N. vesicaria. This matches morphological evidence (see Selvi et al., 2002), geographical patterns and chromosome data. In fact, E. calycina and N. vesicaria are sympatric over most of the Mediterranean belt of North Africa and are the only taxa in the group with 2n = 2x = 30, a complement possibly originated via amphidiploidy from annual ancestors with x = 7 and x = 8 (Grau, 1971; Luque, 1995). Secondly, the ITS1 sequences indicate that the tetraploid species Paraskevia cesatiana, known only from three isolated localities in the mountains of the Greek Peloponnese (Sauer and Sauer, 1980), is sister to Pulmonaria, with which it forms a well-supported clade in the combined tree. Our results are substantially in line with Sauer's opinion (Sauer, 1987: 273) that Paraskevia may share an Early Tertiary ancestor with Pulmonaria, and the conservation of plesiomorphic characters (Selvi et al., 2002) may be linked to its long geographic isolation in the Peloponnese. Paraskevia differs substantially from Pulmonaria in its non-rhizomatous root system, the absence of heterostyly and the prefloral development of foliage leaves.
Trachystemon orientalis. This is a large-leaved, rhizomatous herb with a hexaploid chromosome complement (2n = 6x = 54, pers. obs.). It occurs in humid forests along the southern Black Sea. The trnL phylogeny does not resolve its relationships, whereas ITS1 sequences indicate a sister group relationship to Anchusa s.l. (clade B), but with weak support (62 % BS). Morphologically, there is no evidence for such a relationship. Trachystemon orientalis is characterized by striking autapomorphies, such as the corolla with two series of scales and contorted lobes, the pubescent filaments and the cystolithic trichomes of the adaxial leaf surface (Selvi and Bigazzi, 2001). Based on the corolla with short tube, long lobes and exserted stamens, Guşuleac (1928) suggested a close relationship with Borago and Procopiania. This assumption receives support from pollen (multiaperturate grains with gemmate tectum) and stigma characters (receptive surface with simple papillae lacking apical disk) which are common to these two genera (Bigazzi and Selvi, 1998, 2000). Thus, the discrepancy between molecular and morphological data suggests that additional analyses are needed to ascertain the affinities of Trachystemon.
Anchusa s.l. (F–I). Both trnL and ITS1 show considerable phylogenetic divergence in Anchusa s.l., whose monophyly is supported by 99 % BS in the combined analysis. Four main lineages emerge in this group, with clade F (94 % BS) as sister to a monophyletic group (85 % BS) consisting of the clades G, H and I.
Clade F. Clade F highlights relationships which were not previously suspected. It is a morphologically heterogeneous group mainly composed of south-east Mediterranean species with x = 8 as base chromosome number. The monotypic genus Phyllocara, described to accomodate the annual Anatolian species Anchusa aucheri DC. (Guşuleac, 1928), is sister to the rest of this group. Morphologically it is an isolated species due to unique traits in its inflorescence, flower and pollen morphology (for full description, see Bigazzi et al., 1999). The other two subclades correspond to Anchusa subgenus Buglossoides and to Hormuzakia plus Anchusa subgenus Buglossum. No common morphological characters distinguish these taxa from the rest of Anchusa, but they share a distinctive 6-bp insertion in the trnL sequences. Hormuzakia aggregata, a psammophytic species of arid habitats, also has autapomorphies (the congested-aggregate inflorescence and the helmet-shaped mericarpids; Guşuleac, 1928; Bigazzi et al., 1999). The trnL sequences show that the position of Hormuzakia negevensis, known only from a narrow area in the Negev desert, falls with H. aggregata and Anchusa subgenus Buglossoides; the two species of the latter form an independent clade with moderate BS support. The close affinity between H. aggregata and H. negevensis is supported by the helmet-shaped nutlets unique to these taxa (Danin, 1995, 2000). A relationship between Hormuzakia and Anchusa subgenus Buglossoides was suggested by Guşuleac (1928), who believed in a common ancestry from a Tertiary ‘Buglossoides’ type.
Clade G. Clade G corresponds to Gastrocotyle, a strongly supported genus with two disjunct, annual species (G. hispida and G. macedonica) characterized by striking synapomorphies in inflorescence, pollen and stigma morphology (Selvi and Bigazzi, 2000; Bigazzi et al., 2002). The sister group of Gastrocotyle remains unclear, but there is no molecular evidence for a close relationship with Hormuzakia as argued by Guşuleac (1928).
Clade H. In clade H the annual taxa of Anchusa subgenus Buglossellum and Cynoglottis are sister groups, although with low BS support. The low support received by A. subgenus Buglossellum is due to the sequence divergence of A. thessala. This is the only species of Anchusa with base chromosome number x = 6 (Markova and Goranova, 1995) and erect mericarpids like Cynoglottis. The monophyly of the latter genus is supported by the brachymorphic corollas (with short tube and rotate limb) and the small pollen grains like Brunnera and Pentaglottis (Vural and Tan, 1983), and by x = 9, a base chromosome number which is not found in Anchusa (Bigazzi and Selvi, 2001).
Clade I. In clade I Lycopsis and Anchusella, with zygomorphic flowers and annual habit, are sister to Anchusa subgenus Limbata and A. subgenus Anchusa, with consistently actinomorphic flowers and biennial/perennial habit. All these taxa have the base chromosome number x = 8.
Our phylogenetic reconstruction suggests that floral zygomorphy has appeared repeatedly in Boragineae, maybe as an insect-pollination specialization syndrome. This condition occurs, in slightly different forms, in the distant clades of Anchusa (I) and, partly, Nonea (E) (Selvi et al., 2002). Johnston (1924) was already aware that tendency to zygomorphy occurs several times in Lithospermeae (e.g. Echium and Echiochilon), Cynoglosseae (e.g. Caccinia) and Boragineae, and consequently he attached little taxonomic importance to this character in his treatment of the Old World Boraginoideae. Lycopsis is characterized by corollas with a sigmoid tube and almost planar limb but it does not receive strong support (BS < 50 %). Anchusella has a straight tube and strongly oblique limb, but it is not monophyletic in the trnL analysis and is weakly supported even in the combined analysis. Such a weak support may be due to the deletion shown by A. cretica from position 166–173, which is probably not homologous with that in the Symphytum clade (Fig. 2). However, monophyly of Anchusella is corroborated by other outstanding morphological autoapomorphies such as the unbranched cymes, the corniculate stigma with embricate papillae, the pollen with spinulose aperture margins and, above all, the androecium with only two fertile stamens (Greuter, 1965; Bigazzi et al., 1997).
Neither ITS1 nor trnL support the subgenus rank for the endemic Anchusa limbata. This species, known only from a single locality in south-west Anatolia (Bigazzi et al., 2003), was separated as the monotypic subgenus Limbata Chamberlain & R Mill in view of its unique corolla with much reduced lobes and exserted scales (Chamberlain, 1977). Guşuleac (1928) tentatively referred it to genus Hormuzakia, but the present analysis shows that A. limbata is instead closely related to members of Anchusa subgenus Anchusa. The two subgenera together form a moderately supported clade, in which lack of good resolution of species-level relationships may indicate a recent, rapid and partly adaptive radiation in (semi)arid habitats of the Mediterranean and continental Europe. This is in line with the considerable morphological affinity, the usually perennial (rarely biennial) habit, the base chromosome number x = 8 and the low incidence of polyploidy. Some species groups in this clade show stylar polymorphism, i.e. the infraspecific occurrence of long-styled and short-styled populations associated with the control of self-incompatibility. Like floral zygomorphy, stylar polymorhism appears to be an advanced character and also occurs in Pulmonaria (clade E), thus providing another example of parallel evolution in the tribe. However, in A. officinalis, A. leucantha and A. undulata ssp. hybrida heterostyly is imperfect because style length is not clearly associated with the position of anthers in the corolla tube (Phillip and Schou, 1981; Selvi, 1998; Selvi and Bigazzi, 2003). Stylar polymorphism has not been documented for the species of A. subgenus Anchusa that form a weakly supported terminal clade, the Sardinian endemics A. capellii and A. formosa, and the South African A. capensis. Early divergence and common ancestry of the Sardinian endemics were hypothesized on the basis of morphological and karyological features (Selvi and Bigazzi, 1998), although the position of A. crispa, a third Corso-Sardinian endemic, is unresolved in our phylogeny. Another marker will be used to examine the monophyly of this group. Another point in need of further investigation is the South African–Mediterranean disjunction of A. affinis and A. capensis, both members of Anchusa subgenus Anchusa. At the moment, no explanation can be advanced for the relationship between A. capensis and the Sardinian endemics suggested by ITS1 sequences, and the position of A. affinis from Eritrea and Saudi Arabia remained unresolved in the trnL analysis.
Taxonomic consequences
Taxonomically, the main aspects emerging from the present study are:
Elizaldia and Nonea form a monophyletic group and the relationship between N. vesicaria and E. calycina is strongly supported. This confirms the results of a morphological analysis published recently (Selvi et al., 2002). Further studies on this group are in progress, but at this moment there is no evidence for maintaining Elizaldia separate from Nonea.
From both morphological and molecular data, there is sufficient evidence for keeping Paraskevia separate from its sister taxon Pulmonaria at generic level.
Anchusa s.l. is a strongly supported monophyletic group, but treating it as a single genus would mean neglecting remarkable morphological and molecular divergence. Both lines of evidence allow us to accept Anchusa only in a narrow sense, keeping Phyllocara, Hormuzakia, Gastrocotyle and Cynoglottis (all originally described as species of Anchusa) as separate genera. Lycopsis and Anchusella are more closely related to Anchusa subgenus Anchusa but morphological aspects also support for both the genus rank. Anchusa s.s. in Guşuleac's concept is paraphyletic due to the position of the subgenera Buglossum, Buglossoides and Buglossellum. Therefore, our data indicate that a taxonomic splitting of Anchusa is needed in order to recognize the monophyletic groups. Nevertheless, the circumscription of the new genera and the identification of their diagnostic characters is not straightforward and further phylogenetic analyses including morphological data are required. For example, the straight, erect mericarpid of Anchusa subgenus Buglossum is one of the characters upon which Guşuleac (1927, 1929) based this taxon, but the present analysis suggests that this type of mericarpid may have originated repeatedly as it is present in other distantly related taxa of Boragineae (in some species of Nonea, Anchusa thessala, Cynoglottis and, slightly modified, in Brunnera).
Based on the combined ITS1–trnL analysis, nine of the usually accepted genera of the Boragineae consisting of two or more species are monophyletic: Anchusella, Borago, Brunnera, Cynoglottis, Gastrocotyle, Hormuzakia, Nonea, Pulmonaria and Symphytum. In addition, the tribe includes the four monotypic genera Paraskevia, Pentaglottis, Phyllocara and Trachystemon. Our data do not support the monophyly of Lycopsis. The relationships and taxonomic status of Symphytum creticum could be better resolved through a wider taxon sampling of Symphytum. Finally, further studies will aim at providing morphological evidence for a more natural subdivision of Anchusa to bring taxonomy in line with phylogeny.
Acknowledgments
We thank the herbaria curators of BSB and M for providing us with leaf material and A. Biesek and C. Müller (Berlin) for technical assistance. E. Nardi (Firenze), M. Weigend (Berlin), R. Olmstead (Seattle), Dr M. Fay (Kew) and an anonymous reviewer provided very useful comments and discussion on an early version of the manuscript. This work has been partly funded by M.I.U.R. 40 % 2003 and University of Firenze.
LITERATURE CITED
- Angiosperm Phylogeny Group II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Baldwin BG. 1992. Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the Compositae. Molecular Phylogenetics and Evolution 1: 3–16. [DOI] [PubMed] [Google Scholar]
- Bentham G. 1876.Boragineae In: Bentham G, Hooker JD, eds. Genera plantarum 2. London: Reeve & Co., 832–865. [Google Scholar]
- Bigazzi M, Selvi F. 1998. Pollen morphology in the Boragineae Bercht & J Presl (Boraginaceae) in relation to the taxonomy of the tribe. Plant Systematics and Evolution 213: 121–151. [Google Scholar]
- Bigazzi M, Selvi F. 2000. Stigma form and surface in the tribe Boragineae (Boraginaceae): micromorphological diversity, relationships with pollen and systematic relevance. Canadian Journal of Botany 78: 388–408. [Google Scholar]
- Bigazzi M, Selvi F. 2001. Karyotype morphology and cytogeography in Brunnera and Cynoglottis (Boraginaceae). Botanical Journal of the Linnean Society 136: 365–378. [Google Scholar]
- Bigazzi M, Duman H, Selvi F. 2003.Anchusa limbata (Boraginaceae): contribution to the knowledge of an enigmatic species from SW Turkey. Candollea 58: 339–349. [Google Scholar]
- Bigazzi M, Hilger HH, Selvi F. 2002. Evidence from nuclear and chloroplast DNA for the placement of Anchusa macedonica in the genus Gastrocotyle (Boraginaceae). Webbia 57: 173–180. [Google Scholar]
- Bigazzi M, Nardi E, Selvi F. 1997.Anchusella, a new genus of Boraginaceae from the Central-Eastern Mediterranean. Plant Systematics and Evolution 205: 241–264. [Google Scholar]
- Bigazzi M, Selvi F, Fiorini G. 1999. A reappraisal of the generic status of Gastrocotyle, Hormuzakia and Phyllocara in the light of micromorphological and karyological evidence. Edinburgh Journal of Botany 56: 229–251. [Google Scholar]
- Böhle U-R, Hilger HH. 1997. Chloroplast DNA systematics of ‘Boraginaceae’ and related families: a goodbye to the old and familiar concept of five subfamilies. Scripta Botanica Belgica 15: 30. [Google Scholar]
- Boissier E. 1849.Diagnoses Plantarum orientalium novarum 1, no. 11. Paris: Ducloux, 95–96. [Google Scholar]
- Boissier E. 1879.Flora Orientalis 4. Geneva: Georg, 162–170. [Google Scholar]
- Britton D. 1951. Cytogenetic studies on the Boraginaceae. Brittonia 7: 233–266. [Google Scholar]
- Chamberlain DF. 1977.Anchusa In: Davis PH, ed. Materials for a flora of Turkey XXXIV: Boraginaceae, Gentianaceae, Solanaceae. Notes Royal Botanic Garden Edinburgh35: 298–299. [Google Scholar]
- Chamberlain DF. 1979.Anchusa L. In: Davis PH, ed. Flora of Turkey and the East Aegean Islands 6. Edinburgh: Edinburgh University Press, 388–402. [Google Scholar]
- Chater AO. 1972.Anchusa L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, eds. Flora Europaea 3. Cambridge: Cambridge University Press, 106–109. [Google Scholar]
- Danin A. 1995. A new Anchusa from Israel. Edinburgh Journal of Botany 52: 333–336. [Google Scholar]
- Danin A. 2000. The nomenclature news of Flora Palaestina. Flora Mediterranea 10: 101–172. [Google Scholar]
- De Candolle ALPP. 1846. Tribus Borrageae In: De Candolle ALPP, ed. Prodromus systematis naturalis regni vegetabilis 10. Paris: Masson; Leipzig: Michelsen, 1–56. [Google Scholar]
- Diane N, Förther H, Hilger HH. 2002. A systematic analysis of Heliotropium, Tournefortia and allied taxa of the Heliotropiaceae (Boraginales) based on ITS1 sequences and morphological data. American Journal of Botany 89: 287–295. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Edmondson JR. 1978.Brunnera Steven. In: Davis PH., ed. Flora of Turkey and the East Aegean Islands 6. Edinburgh: Edinburgh University Press, 387–388. [Google Scholar]
- Farris JS, Albert VA, Källersjö M, Lipscomb D, Kluge AG. 1996. Parsimony jackknifing outperforms neighbor-joining. Cladistics 12: 99–124. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Ferguson DM. 1999. Phylogenetic analysis and relationships in Hydrophyllaceae based on ndhF sequence data. Systematic Botany 23: 253–268. [Google Scholar]
- Gottschling M, Hilger HH. 2001. Phylogenetic analysis and character evolution of Ehretia and Bourreria (Ehretiaceae, Boraginales) and their allies based on ITS1 sequences. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 123: 249–268. [Google Scholar]
- Gottschling M, Hilger HH, Wolf M, Diane N. 2001. Secondary structure of the ITS1 transcript and its application in a reconstruction of the phylogeny of Boraginales. Plant Biology 3: 629–636. [Google Scholar]
- Grau J. 1971. Cytologische Untersuchungen an Boraginaceae. II. Mitteilungen der Botanischen Staatssammlung München 9: 177–194. [Google Scholar]
- Greuter W. 1965. Beiträge zur Flora der Südägäis. Candollea 20: 167–218. [Google Scholar]
- Greuter W. (ed.) 1981. Med-Checklist Notulae, 3. Willdenowia 11: 23–37. [Google Scholar]
- Greuter W, Burdet, HM, Long G. 1984. Med-Checklist 1: Pteridophyta, 2nd edn, Gymnospermae, Dicotyledones (Acanthaceae-Cneoraceae). Geneva: Conservatoire et Jardin Botaniques, 69–72. [Google Scholar]
- Gürke M. 1893.Borraginaceae In Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien IV (3a). Leipzig: Engelmann, 71–131. [Google Scholar]
- Guşuleac M. 1923. Beiträge zur Systematik der Anchuseae. Publicaţiunile Societăţii Naturaliştilor din Bucureşti 6: 79–92. [Google Scholar]
- Guşuleac M. 1927. Die europäischen Arten der Gattung Anchusa Linné. Buletinul Facultalai Stiinte Cernãuti 1: 73–123. [Google Scholar]
- Guşuleac M. 1928. Die monotypischen und artenarmen Gattungen der Anchuseae (Caryolopha, Brunnera, Hormuzakia, Gastrocotyle, Phyllocara, Trachystemon, Procopiania und Borago). Buletinul Facultalai Ştiinte Cernãuti 2: 394–461. [Google Scholar]
- Guşuleac M. 1929. Species Anchusae generis Linn. hucusque cognitae. Feddes Repertorium 26: 286–322. [Google Scholar]
- Hepperle D. 2001.Multicolor Sequence Alignment Editor. Neuglobsow, Germany: Institute of Freshwater Ecology and Inland Fisheries. [Google Scholar]
- Johnston IM. 1924. Studies in the Boraginaceae III. 1.The Old World genera of the Boraginoideae Contributions of the Gray Herbarium of Harvard University 73: 42–78. [Google Scholar]
- Långström E, Chase MW. 2002. Tribes of Boraginoideae (Boraginaceae) and placement of Antiphytum, Echiochilon, Ogastemma and Sericostoma: a phylogenetic analysis based on atpB plastid DNA sequence data. Plant Systematics and Evolution 234: 137–153. [Google Scholar]
- Luque T. 1995. Karyology of Nonea Medicus (Boraginaceae) in Spain; relationships between genera of Boragineae Barbier & Mathez (Anchuseae DC.). Botanical Journal of the Linnean Society 117: 321–331. [Google Scholar]
- Markova M, Goranova V. 1995. Mediterranean chromosome number reports no. 435–473. Flora Mediterranea 5: 289–317. [Google Scholar]
- Melchior H. 1964. Boraginaceae. In: Melchior H, ed. A. Englers Syllabus der Pflanzenfamilien edn. 12, 2. Berlin: Borntraeger, 431–434. [Google Scholar]
- Pawlowski B. 1971. De genere Procopiania Guşuleac – Rodzaj Procopiania Guşuleac. Fragmenta Floristica Geobotanica 17: 39–58. [Google Scholar]
- Phillip M, Schou O. 1981. An unusual heteromorphic incompatibility system. Distyly, self-incompatibility, pollen load and fecundity in Anchusa officinalis (Boraginaceae). New Phytologist 89: 693–703. [Google Scholar]
- Popov MG. 1953. Anchuseae DC. In: Komarov VL, ed. Flora SSSR, vol. 19. Moskva-Leningrad: Akademii Nauk SSSR, 207–263. [Google Scholar]
- Riedl H. 1963.Anchusa subgen. Chamanchusa subgen. nov. und das System der Borraginoideae-Anchuseae Österreichische Botanische Zeitschrift 110: 543–546. [Google Scholar]
- Runemark H. 1967. Studies in the Aegean flora. XI. Procopiania (Boraginaceae) included into Symphytum Botaniska Notiser 120: 84–94. [Google Scholar]
- Sauer W. 1987. The Pulmonaria dacica group: its affinities with central and south-east European allies and with the genus Paraskevia (Boraginaceae). Plant Systematics and Evolution 155: 257–276. [Google Scholar]
- Sauer W, Sauer G. 1980.Paraskevia gen. nov. mit P. cesatiana comb. nov. (Boraginaceae), eine endemische Gattung Griechenlands. Phyton 20: 285–306. [Google Scholar]
- Selvi F. 1998. Floral biometrics in the Anchusa undulata L. group (Boraginaceae) from the central-eastern Mediterranean. Botanical Journal of the Linnean Society 128: 251–270. [Google Scholar]
- Selvi F, Bigazzi M. 1998.Anchusa L. and allied genera (Boraginaceae) in Italy. Plant Biosystems 132: 113–142. [Google Scholar]
- Selvi F, Bigazzi M. 2000. Removal of Anchusa macedonica (Boraginaceae) from Anchusa Evidence from phenetics and karyotype analysis. Taxon 49: 765–778. [Google Scholar]
- Selvi F, Bigazzi M. 2001. Leaf surface and anatomy in Boraginaceae tribe Boragineae with respect to ecology and taxonomy. Flora 196: 269–285. [Google Scholar]
- Selvi F, Bigazzi M. 2002. Chromosome studies in Turkish species of Nonea (Boraginaceae): the role of polyploidy and descending dysploidy in the evolution of the genus. Edinburgh Journal of Botany 59: 405–420. [Google Scholar]
- Selvi F, Bigazzi M. 2003. Revision of genus Anchusa (Boraginaceae-Boragineae) in Greece. Botanical Journal of the Linnean Society 142: 431–454. [Google Scholar]
- Selvi F, Papini A, Bigazzi M. 2002. Systematics of Nonea (Boraginaceae-Boragineae): new insights from phenetic and cladistic analyses. Taxon 51: 719–730. [Google Scholar]
- Simmons MP, Ochoterena H. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Stearn WT. 1986. The Greek species of Symphytum (Boraginaceae). Annales Musei Goulandris 7: 175–220. [Google Scholar]
- Swofford DL. 1998.PAUP*. Phylogenetic Analysis Using Parsimony (and other methods) vers. 4.0. Sunderland, MA: Sinauer. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- von Friedrichsthal E. 1838.Reise in den südlichen Theilen von Neu-Griechenland. Beiträge zur Charakteristik dieses Landes. Leipzig: Engelmann. [Google Scholar]
- Vural M, Tan K. 1983. New taxa and records from Turkey. Notes from the Royal Botanic Garden Edinburgh 41: 65–75. [Google Scholar]
- Wickens GE. 1969. A revision of Symphytum in Turkey and adjacent areas. Notes from the Royal Botanic Garden Edinburgh 29: 157–180. [Google Scholar]
- Winkworth RC, Grau J, Robertson AW, Lockhart PJ. 2002. The origins and evolution of the genus Myosotis L. (Boraginaceae). Molecular Phylogenetics and Evolution 24: 180–193. [DOI] [PubMed] [Google Scholar]