Abstract
• Background and Aims Soybean (Glycine max) is among the many legumes that are well known for ‘hardseededness’. This feature can be beneficial for long-term seed survival, but is undesirable for the food processing industry. There is substantial disagreement concerning the mechanisms and related structures that control the permeability properties of soybean seed coats. In this work, the structural component that controls water entry into the seed is identified.
• Methods Six soybean cultivars were tested for their seed coat permeabilities to water. To identify the structural feature(s) that may contribute to the determination of these permeabilities, fluorescent tracer dyes, and light and electron microscopic techniques were used.
• Key Results The cultivar ‘Tachanagaha’ has the most permeable seed coat, ‘OX 951’ the least permeable seed coat, and the permeabilities of the rest (‘Harovinton’, ‘Williams’, ‘Clark L 67-3469’, and ‘Harosoy 63’) are intermediate. All seeds have surface deposits, depressions, a light line, and a cuticle about 0·2 µm thick overlaying the palisade layer. In permeable cultivars the cuticle tends to break, whereas in impermeable seeds of ‘OX 951’ it remains intact. In the case of permeable seed coats, the majority of the cracks are from 1 to 5 µm wide and from 20 to 200 µm long, and occur more frequently on the dorsal side than in other regions of the seed coat, a position that correlates with the site of initial water uptake.
• Conclusions The cuticle of the palisade layer is the key factor that determines the permeability property of a soybean seed coat. The cuticle of a permeable seed coat is mechanically weak and develops small cracks through which water can pass. The cuticle of an impermeable seed coat is mechanically strong and does not crack under normal circumstances.
Key words: Aleurone layer, crack, cuticle, Glycine max, hardseededness, imbibition, palisade layer, permeability, seed coat, soybean
INTRODUCTION
Seed coats can be described as being either permeable or impermeable. A permeable (normal) seed imbibes water readily when available, while an impermeable one does not take up water for days or longer. Impermeable seeds are commonly referred to as ‘hard’. ‘Hardseededness’ is the reason for seed coat-imposed dormancy (Bewley and Black, 1994), or physical dormancy (Baskin et al., 2000) in a number of families. It is biologically beneficial for long-term seed survival and can be important for wild plants (Rolston, 1978; Tran and Cavanagh, 1984). Soybean (Glycine max) and many other legumes have hard seeds (Rolston, 1978). Hardseededness in soybeans can protect against seed decay and improve agronomic qualities under certain conditions (Tyler, 1997); but hardseededness is undesirable for the food processing industry. Ideally, seeds should take up water quickly and synchronously. This trait is particularly critical when whole seeds are processed, such as for the production of soya milk, soya sauce, tofu and miso. Yet, permeable seed coats maybe susceptible to mechanical damage during pre-processing of the seeds, leading to losses. Hence, breeding programmes have been aimed at creating lines with seed coats that are fairly permeable and reasonably strong. Seed coat permeability is important to both scientific and industrial communities.
The permeability property of a seed coat should be related to its structure. A typical legume seed coat contains several specialized areas, i.e. hilum, micropyle and raphe, and the rest of the seed coat commonly known as the extrahilar region (Fig. 1). The hilum is a scar formed when the funiculus detaches from the seed at maturity. The micropyle is the pore through which the radicle emerges during seed germination. Earlier during ovule development, the micropyle is formed where the margins of the integuments meet. The raphe is a slightly depressed area on the opposite side of the hilum from the micropyle. In spite of a basic knowledge of the seed coat structure, there is substantial disagreement concerning what structural components are involved in controlling water movement into the seed.
Fig. 1.
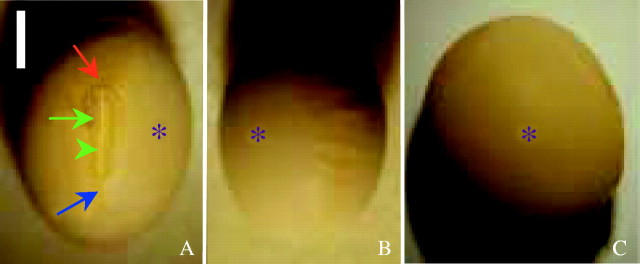
A seed of ‘Harovinton’ viewed with a dissecting microscope. (A) Ventral side. Shown are the micropyle (red arrow), raphe (blue arrow), hilum (green arrow), hilar fissure (green arrowhead), and the extrahilar region (asterisk). (B) Dorsal side. In this example the dorsal side of the seed coat is slightly wrinkled. (C) Abaxial side, with flat surface. Scale bar = 2 mm.
To understand hardseededness, a comparison of hard and normal seeds is necessary. In impermeable seeds, the hilum, micropyle and raphe must be closed to water; while in permeable seeds these regions may be the initial sites of water entry, but published results on this point are variable [Vigna unguiculata (Lush and Evans, 1980); soybean (Saio, 1976; Arechavalete-Medina and Snyder, 1981)]. In another debate, the focus has been on the extrahilar region in both permeable and impermeable seeds. Nevertheless, there is a consensus that the palisade layer, which develops from the outer epidermis of the outer ovular integument, is critical in determining the permeability property of a seed coat (Ballard, 1973; Werker, 1980/81; Tran and Cavanagh, 1984). But, the exact location and chemical nature of the control point(s) have not been identified with certainty. Suberin [Melilotus alba (Hamly, 1932)], cuticle [soybean (Arechaveleta-Medina and Snyder, 1981; Calero et al., 1981; Wolf et al., 1981)] and wax [Rhynchosia minima (Rangaswamy and Nandakumar, 1985)] were all considered, but again there are discrepancies among the conclusions. The same uncertainty exists for some special structures associated with the palisade layer, such as surface deposits (Harris, 1987; Chachalis and Smith, 2001), pits (Wolf et al., 1981) and the light line (Ballard, 1973). Surface deposits are covering materials on seed coats and are apparently derived from the endocarp. Pits are small depressions on the seed surface. A light line is a region of palisade wall material that appears brighter than the surrounding wall areas when observed with a compound microscope; this line is located near the outer surface of the palisade layer. Several more factors have also been considered in connection with the permeability property of seeds but, to date, none have been experimentally proven.
The varied results and interpretations illustrate the complexity of the issue, and the limited structural features investigated by individual researchers have not been sufficient to establish a relationship between structure and function. The present study is a detailed structural and permeability analysis of soybean seed coats, and provides new insights into the structural basis for control of water uptake.
MATERIALS AND METHODS
Soybean cultivars
Cultivars of soybean Glycine max (L.) Merr. included in the study were: ‘Tachanagaha’, ‘Harovinton’, ‘Harosoy 63’, ‘Williams’, ‘Clark L 67-3469’ (abbreviated ‘Clark-i’), and ‘OX 951’. All cultivars had yellow seed coats except for ‘Clark-i’, which had a black seed coat. Seeds were hand-harvested from plants grown during 1999, 2000 and 2001 on the experimental farms of Agriculture and Agri-Food Canada, in Harrow and/or London, Ontario. Harvested seeds were stored at room temperature.
Measurement of water uptake
Seeds used for permeability studies were harvested in 2000. All seeds were initially checked with a dissecting microscope, and only those with no visible damage were classed as ‘intact’ and were used for water uptake measurements. For each cultivar, three to five seeds were tested. Single seeds were weighed, immersed in tap water for a specific time, removed from the water, blotted with cellulose tissue, weighed again, and returned to the water. Seeds were weighed at 1-min intervals during the first 30 min, at 5-min intervals for the next 30 min, and at 15-min intervals for a further 1 h. A penultimate weight was taken at 3 h, and a final measurement at 24 h. The rate of water uptake was standardized by expressing it as weight increase (g) per gram seed (initial) weight. Seeds that did not imbibe water for a period of 24 h were classed as ‘impermeable’.
Detection of initial sites of water entry
Fluorescent tracer dyes were applied to seeds with ‘intact’ coats. For each cultivar, three to five individual seeds were observed. Following incubation for 1 min to 30 h in 0·01 % (w/v) Cellufluor® (Polysciences, Warrington, PA, USA) or berberine hemi-sulfate (Sigma, St Louis, MO, USA), the seeds were rinsed briefly with water and observed with a Zeiss epifluorescence microscope under UV light (filter set: exciter filter G 365, dichroitic mirror FT 395 and barrier filter LP 420; Carl Zeiss Canada, Don Mills, Ontario, Canada). Free-hand sections were also made from different regions of the seed coats and examined with the fluorescence microscope. Non-fluorescent dyes, 1 % fast green, 1 % safranin and 0·5 % toluidine blue O (TBO) were also tested for the same purpose. Since the dye molecules are larger than those of water, dye entry into the seed coat indicates a penetration point for water.
Scanning electron microscopy (SEM)
Surface features of dry seed coats were examined with SEM. Samples (approx. 1–2 mm2) from the abaxial and dorsal regions were excised. Samples from the ventral region were larger (approx. 2–3 mm2) and contained the hilum, micropyle and raphe, plus a narrow surrounding strip. For locations of these regions, refer to Fig. 1. For each cultivar, two to four seeds were included. All samples were coated with gold and examined with a Hitachi S570 scanning electron microscope at 15 kV.
Histochemical studies
Seeds were immersed in tap water until they were fully hydrated. For seeds with impermeable coats (from ‘OX 951’), a small wound was made at one or both ends of the seed to initiate hydration. To obtain an overall view of seed coat tissues, seed coats were cut into small pieces, briefly immersed in 0·01 % Cellufluor to stain cell walls (Hughes and McCully, 1975), gently teased apart with dissecting needles, and observed with the fluorescence microscope (as above). More detailed histochemical studies were performed on free-hand sections from the abaxial, dorsal and ventral areas of three to five seeds.
Detection of cutin
Sections were stained for cuticle with Sudan VI, Sudan black B (Jensen, 1962) or Bismarck brown Y–azure B (Graham and Joshi, 1996). Other sections were stained with berberine–aniline blue (Brundrett et al., 1988), Sudan red 7B or fluorol yellow 088 (Brundrett et al., 1991), methods developed primarily for detecting suberin. Since cutin shares certain similarities in chemistry with suberin (Kolattukudy, 1980), a positive reaction on the surface of the palisade layer would indicate the presence of a cuticle.
Detection of cutin by isolation methods
Three procedures were used. (1) Incubation in a mixture of ammonium oxalate (1·6 %) and oxalic acid (0·4 %) at 35 °C for up to 2 weeks. This is a conventional chemical method for isolating leaf cuticles (e.g. Villena et al., 1999). (2) Treatment with 60 % or 80 % H2SO4 for up to 24 h on concavity slides. Cell walls resistant to acid digestion were considered to contain cutin (or suberin; Johansen, 1940, 190). (3) Incubation in a pectinase solution (Sigma, St Louis, MO, USA) of about 6·8 units mL−1 in citric acid (pH 4·0) for 2–6 weeks at room temperature, with the solution refreshed every 5 d. For each experiment, the isolation progress was monitored with a light microscope. At the end of each treatment, some areas of cuticle were completely detached from the palisade cells, but in other areas the cuticle was only loosened and sporadic; adhering palisade cells were removed with fine needles. The samples were washed with distilled water and examined with differential interference contrast (DIC) optics.
Detection of lignin
Free-hand sections were treated with phloroglucinol–HCl (Johansen, 1940, 194–195) and observed with a light microscope.
Detection of callose
Sections were stained with 0·001 % aniline blue in phosphate buffer at pH 8·0 (slightly modified from Currier, 1957) and examined with UV illumination as detailed above. Pectinase-isolated palisade cells (see ‘Detection of cutin by isolation methods’) were examined the same way.
Viability test for cells in seed coats
Hydrated seed coats were kept in 0·01 % disodium fluorescein (Baker, Phillipsburg, NJ, USA) in 10 mm phosphate buffer, pH 5·3 for 0·5–2 h. For dry seed coats, an incubation of 6–12 h in the dye was necessary. After thorough rinsing with the buffer, the samples were examined with a Zeiss epifluorescence microscope under blue light (filter set: exciter filter BP 546, dichroitic mirror FT 580 and barrier filter LP 590). For each cultivar, three to five seeds were examined.
Transmission electron microscopy (TEM)
Four cultivars were selected for this study: ‘Tachanagaha’, ‘Harovinton’, ‘Clark-i’ and ‘OX 951’. Samples were excised from abaxial and dorsal regions of fully hydrated seed coats of three seeds, and fixed in 3 % glutaraldehyde in 50 mm phosphate buffer (pH 6·8) for 12 h to 5 d. Further fixation took place in 1 % OsO4 in the same buffer overnight. Following dehydration in ethanol, tissues were embedded in Spurr's resin. Semithin sections (1·5 µm) were stained with 0·05 % TBO in benzoate buffer at pH 4·4, and examined with a light microscope. Ultrathin sections (85–90 nm) were stained with uranyl acetate and lead citrate and examined in a Philips CM 12 transmission electron microscope.
RESULTS
Permeabilities of soybean seed coats as indicated by imbibition rates
Among the cultivars tested, seeds of ‘Tachanagaha’ imbibed water the most quickly, i.e. their coats were very permeable. ‘Harovinton’, ‘Harosoy 63’, ‘Williams’ and ‘Clark-i’ also imbibed water rapidly and would also be classed as permeable (Fig. 2). It normally took 12–24 h for seeds of all these cultivars to achieve full hydration. In the case of ‘OX 951', 30–60 % of the seeds took up water during a period of 24 h, while the rest did not—their coats were impermeable (Fig. 2). Some of these seeds did not imbibe water even during the next few days. Of the six cultivars, four (‘Tachanagaha’, ‘Harosoy 63’, ‘Harovinton’ and ‘OX 951’) were previously tested by Mullen and Xu (2001), with similar results. Only data for the first 3 h are shown in Fig. 2 because most concern was with early imbibition.
Fig. 2.
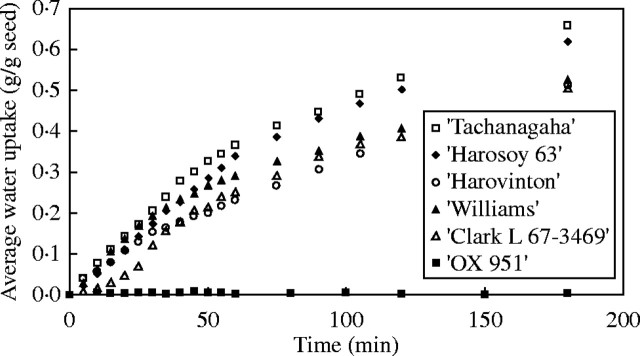
Permeabilities to water of six cultivars. All seeds were produced in 2000 and measured in 2002. Only the first 3 h of measurement is shown. Standard errors are provided for ‘Harosoy 63’ as an example. (Adding standard errors for all cultivars would otherwise obscure many other data points.)
Sites of water entry into seed coats
Wrinkles formed in the seed coat of a permeable seed, first appearing within 1–5 min of incubation, and usually on the dorsal side. These wrinkles spread toward the ventral side as imbibition proceeded. Eventually, they all disappeared when the seed was fully hydrated (by which time it was 2·2–2·4-fold its original weight).
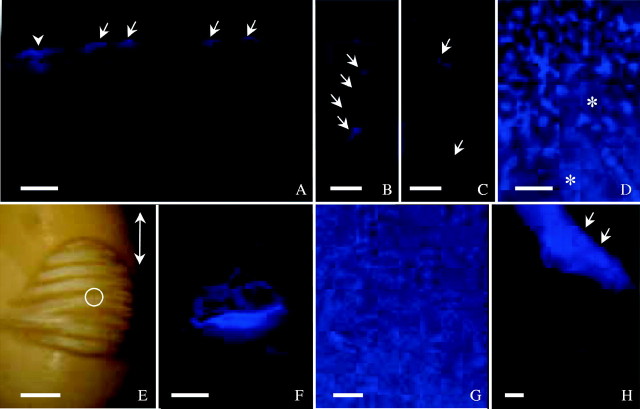
Of particular interest was the location of the initial sites of water entry into the seed coat. If such sites are also large enough to admit fluorescent, tracer dye molecules, the permeable areas can be visualized with an epifluorescence microscope following incubation in the tracer. Using Cellufluor for 1 or 2 min resulted in bright wall staining of single or clusters of palisade cells with expanded lumina (Fig. 3A–C). However, in the case of very permeable seeds, staining was sometimes observed in periclinal walls of the palisade layer even after a short treatment time, precluding detection of initial sites of water entry (Fig. 3D). Points of staining were usually on the dorsal side of the seed. Dye entry in other locations of the extrahilar region was rare but did occur. The hilum, micropyle and raphe were not sites of initial water entry. In some seeds, slight staining was detected in these areas, but observation of sections did not reveal deep penetration of the dye.
Fig. 3.
Seeds incubated in Cellufluor and observed under UV light. (A) ‘Harovinton’ seed in dye for 1 min and section made from abaxial side. Surface deposits stained (arrows), along with palisade walls subtending a crack (arrowhead). (B) and (C) ‘Tachanagaha’ seed 2 min in dye. Single cells (B) or groups of cells (C) involved in crack formation as demonstrated with the dye (arrows). (D) ‘Tachanagaha’ seed 2 min in dye, with surface deposits (Type II) stained. Areas free of large deposits were slightly stained in the outer periclinal walls of palisade layer (asterisks); this makes it difficult to detect initial sites of dye entry. (E) Initial water uptake at dorsal side of ‘OX 951’ seed after 5·5 h in dye, with wrinkles formed as water moved in. White light micrograph from a dissecting microscope. Double-headed arrow indicates the seed's long axis. A crack in the centre of the wrinkled area is circled. (F) Crack in (E) observed with UV light, illustrating intensely stained palisade walls. (G) ‘Harovinton’ seed in dye for 1 min. Heavy surface deposits at abaxial side stained. (H) ‘Harovinton’ seed in dye for 3 min, with endocarp tissue (Type III deposits, arrows) intensely stained; other deposits (Type II, lower half of micrograph) stained less intensely. Scale bars: E = 1 mm; all others = 100 µm.
In ‘OX 951’, seed coat permeability was variable. A large number of seeds did not take up water for hours or even days (see above). In accordance with this result, no staining was observed in palisade walls using Cellufluor. For those that did imbibe water, it usually entered at the dorsal side through a minute crack. Wrinkles were formed (albeit slowly) and the cracked site was stained by Cellufluor (Fig. 3E and F). In a few seeds, initial water uptake occurred elsewhere in the extrahilar region, presumably also associated with such cracks.
Berberine, another fluorescent tracer dye, was also suitable for detection of initial water entry, but non-fluorescent dyes (safranin, fast green and TBO) were not. Samples treated with the latter can only be examined with a dissecting microscope, because the seed coats were too thick for a compound microscope (with transmitted light). The limited resolution of the dissecting microscope did not allow a distinction between the stained surface deposits and cells subtending the cracks. Also, the dyes tended to accumulate on seed surface or the staining was diffuse, so that it was hardly possible to make useful observations.
Surface features of seeds
All cultivars examined were quite similar to each other in the overall structure of their seed coats, each having a micropyle, a hilum, a raphe and an extrahilar region, as documented for soybean and other legumes (Fig. 1). In dry seeds, the hilum fissure was slightly open as observed with a scanning electron microscope (Fig. 4A); sometimes the outer end of the tracheid bar was visible, a feature not obvious under light microscopes. The micropyle was either open (Fig. 4A) or closed except in ‘OX 951’ which consistently had a closed micropyle (Fig. 4B). The raphe was slightly depressed, continuous with the rest of the seed coat and without outgrowths (Fig. 4C). The raphe was rarely cracked open slightly (Fig. 4D).
Fig. 4.
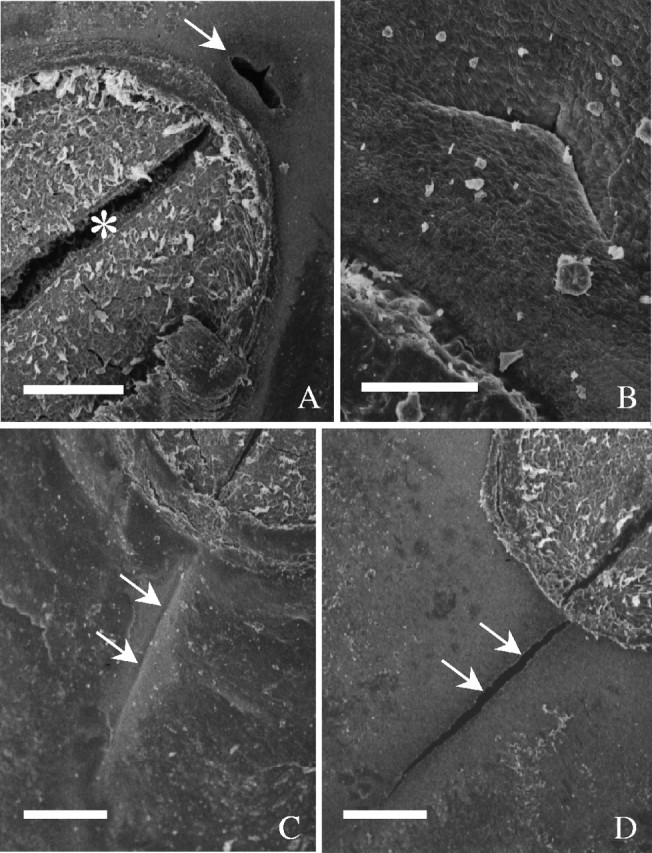
SEM of the ventral side of seeds. (A) ‘Clark-i’. The micropyle is open (arrow) and the hilum fissure is slightly open (asterisk). (B) Closed micropyle in ‘OX 951’. (C) Raphe in ‘OX 951’, marked by arrows. (D) Raphe in ‘Clark-i’ slightly open (arrows). Scale bars: A, C and D = 200 µm; B = 50 µm.
Surface deposits and cuticle
All seeds examined had covering materials on their surfaces. Here one cultivar (‘Clark-i’) is documented in some detail and the rest are described briefly for the purpose of comparison. In a given seed, the abaxial and dorsal sides of its coat were not noticeably different with respect to their endocarp deposits, but there was variability between cultivars.
The surface was not evenly covered. ‘Clark-i’ seeds had a few small regions devoid of covering materials and, thus, the cuticle was exposed. This cuticle was rugulose (Fig. 5A). On fractured seed coats, the cuticle was about 0·2 µm thick. Large areas were covered with a thin layer of amorphous material (Fig. 5B and C), which was often accompanied by additional deposits, scattered (Fig. 5D) or abundant (Fig. 5E). In the latter case, the deposits appeared either randomly distributed or in a honeycomb-like pattern. Very rarely, there were scale-like, amorphous deposits directly on the cuticle (Fig. 5F). There were crystal-like substances that were aligned predominantly parallel to the honeycomb ‘walls’ and were piled up to varied heights (Fig. 5G and H). In regions with limited accumulation of the crystal-like substances, there was a better chance to observe their orientations (Fig. 5I). In large deposits, crystal-like substances were covered by amorphous layers (Fig. 5J). All types of deposits could be seen in a single seed, but varied among individual seeds.
Fig. 5.
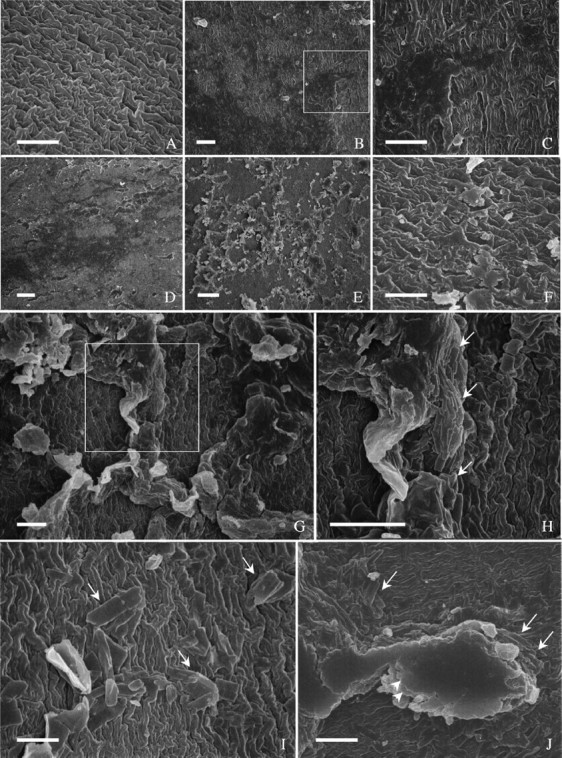
Surface features of ‘Clark-i’ observed with SEM. (A) An area beside the hilum, free of surface deposits. Cuticle rugulose pattern. (B) Dorsal side, with thin layer of surface deposits. (C) Enlarged from boxed area in B, illustrating amorphous deposits. (D) An area at dorsal side covered with a thin layer of surface deposits, on top of which are a few additional large deposits. (E) An area beside the hilum, with heavy large deposits in the form of a honeycomb on a thin layer of amorphous deposits. (F) An area at abaxial side, with scale-like deposits directly on cuticle. (G) Abaxial side, with heavy deposits. (H) Enlarged from boxed area in G, showing crystal-like accumulations (arrows). (I) Abaxial side with a few crystals (arrows). (J) Abaxial side, with an accumulation of crystals (arrows) on which are layers of amorphous materials (arrowheads). Scale bars = 5 µm.
‘Harovinton’ seeds had areas covered with an amorphous material that was so thin that the rugulose pattern of the cuticle was not obscured (Fig. 6A and B). Large areas were covered by additional deposits (Fig. 6C and D), which were similar to those in ‘Clark-i’ (Fig. 5C–F). In addition, fragments of endocarp tissues were observed where endocarp cells were sometimes discernable (Fig. 6E and F). These tissues were morphologically variable (Fig. 6E and F); they apparently detached from different depths of the endocarp. In ‘Harosoy 63’, surface deposits were similar to those in ‘Clark-i’ and ‘Harovinton’. Some areas were covered by a thick layer of amorphous materials. In ‘Tachanagaha’, both a thin layer of covering material and additional deposits were present (Fig. 6G–M), but were not as conspicuous as in other cultivars. In ‘Williams’, the majority of the seed surface was covered by a layer of amorphous material, but there were few additional deposits.
Fig. 6.
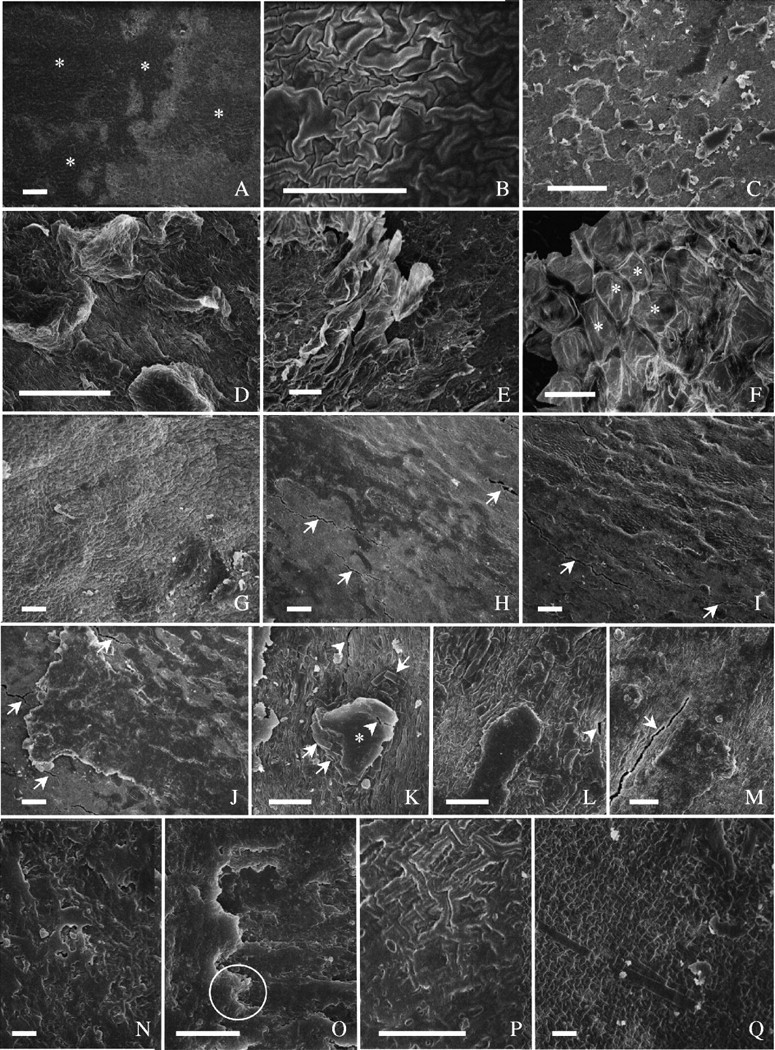
Surface features of seed coat. (A–F) ‘Harovinton’: (A) area beside hilum, covered with a thin layer of amorphous deposits of Type I (asterisks); (B) enlarged from (A)—the covering is so thin that the morphology of the underlying cuticle is not fully obscured; (C) abaxial side—heavy deposits (Type II) on top of the thin layer, appearing as isolated or forming a honeycomb pattern; (D) dorsal side, with heavy deposits (Type II); (E) abaxial side, with a fragment of endocarp tissue (Type III deposits) on top of other deposits; (F) similar to E, but cells of endocarp tissue readily recognizable (asterisks). (G–M) ‘Tachanagaha’: (G) large area beside hilum, nearly free of surface deposits; (H) dorsal side, with a thin layer of amorphous materials (cracks are marked with arrows); (I) abaxial side; some large deposits on top of a thin layer of amorphous materials; with cracks (arrows); (J) dorsal side—enlarged view of amorphous deposits—arrows point to cracks; (K) abaxial side, with accumulation of crystals (arrows) and amorphous deposits (asterisk)—cracks both in deposit material and in cuticle (arrowheads); (L) at dorsal side, with similar deposit materials as in K—a crack (arrowhead) in cuticle; (M) dorsal side, with crack (arrow) in cuticle with little deposit. (N–Q) ‘OX 951’: (N) abaxial side, with surface deposits irregular; (O) enlarged from an area similar to N, showing multilayered deposits (circle); (P) dorsal side, with a thin layer of deposit material; (Q) beside the hilum, with little deposit material and a few single crystals. Scale bars: A, C, G, K–N, O and Q = 10 µm; B, D and P = 5 µm; E, F and H–J = 50 µm.
‘OX 951’ seeds were almost entirely covered with surface deposits that largely appeared as amorphous; as a result, the morphology of the underlying cuticle was typically obscured (Fig. 6N and O). Frequently the deposits were heavy, but there were small areas with little covering material (Fig. 6P and Q) where a few isolated crystals were observed (Fig. 6Q).
Summarizing these observations, the endocarp deposits can be categorized as three types: Type I—a layer of amorphous material in immediate contact with the palisade cuticle (e.g. Figs 5B and 6A); Type II—large deposits of amorphous/crystalline materials overlying Type I deposits (e.g. Figs 5F and 6C); and Type III—occasional occurrence of endocarp fragments on seed coat surface, as observed in ‘Harovinton’ (Fig. 5E and F).
None of the deposits was stained by Sudan dyes, but it is interesting that they reacted differently to Cellufluor. Type III deposits could be stained readily (Fig. 3H), Type II deposits also concentrated the dye but not as intensely (Fig. 3D and G), and Type I deposits apparently did not pick up the dye.
Depressions and cracks
Depressed areas on seed surface, commonly called ‘pits’ in the literature, were normally shallow, but occasionally extended deep into the seed coat (Fig. 7A–L). There were more depressions in the vicinity of the hilum than elsewhere, but their densities were apparently a characteristic of the individual genotypes (Wolf et al., 1981). Among the cultivars examined in the present work, depressions were numerous in ‘Tachanagaha’ (Fig. 7A–D), ‘Williams’ (Fig. 7E–H) and ‘Harosoy 63’ (Fig. 7I), but rare in ‘Clark-i’, ‘Harovinton’ (Fig. 7J) and ‘OX 951’ (Fig. 7K and L). In the last three cultivars, deep depressions were seldom seen (Fig. 7L). Some depressions had cracks on the surface (Fig. 2C, H and I), except in ‘OX 951’.
Fig. 7.
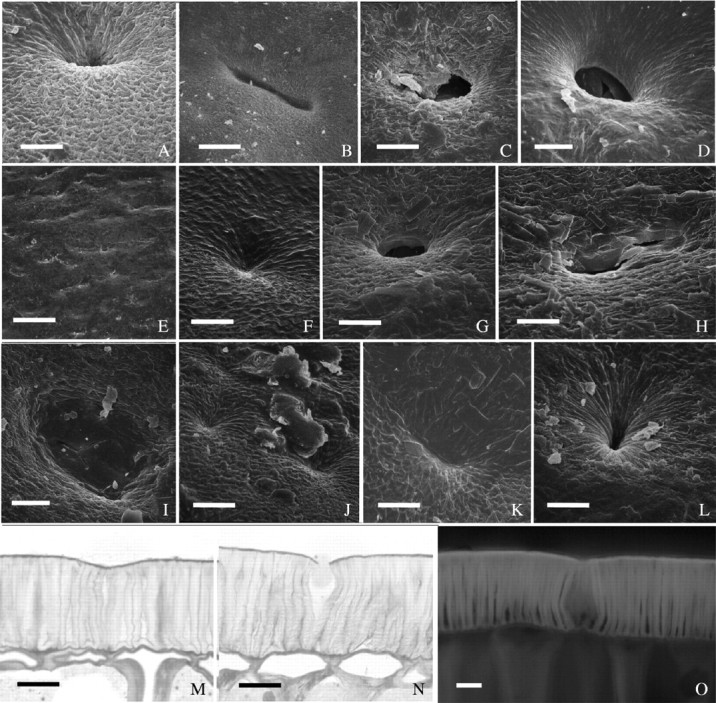
Examples of depressions on seed coats. (A–D) ‘Tachanagaha’: (A) small circular depression near hlium of unknown depth; (B) long depression near hilum; (C) circular depression near hilum, with cracked deposit material; (D) circular depression at abaxial side, accompanied by some deposit material. (E–H) ‘Williams’: (E) dorsal side—low magnification image depicting the numerous, shallow depressions; (F) shallow depression near hilum; (G) a rare case of deep depression, at dorsal side; (H) depression at dorsal side, with broken cuticle where deposit material is minimal. (I) ‘Harosoy 63’: depression at dorsal side with small cracks in cuticle. (J) ‘Harovinton’: shallow depressions. (K and L) ‘OX 951’: (K) depression near hilum, without cracks; (L) as in K, but deeper. (M and N) ‘Williams’: palisade layer with depressions at abaxial side in semithin sections stained with TBO: (M) anticlinal walls of palisade cells in the depression are thin and twisted; (N) as in M, but outer periclinal walls in depression region cracked open. (O) ‘OX 951’— free-hand section from abaxial side of hydrated seed coat under UV light. Dimmer autofluorescence in anticlinal walls of the depression region than other regions. Scale bars: A–D and F–L = 10 µm; E = 100 µm; M–O = 20 µm.
In ‘intact’ seeds selected (see MATERIALS AND METHODS), small cracks in the cuticle were almost always visible with the scanning electron microscope, especially in the five permeable cultivars. These cracks were narrow splits (Fig. 8A–E), predominantly perpendicular to the seed's long axis. Most cracks were in the range of 1–5 µm wide and 20–200 µm long. They were usually present on the dorsal side and less often on the abaxial side of a seed. Cracks frequently occurred on flat areas of a seed coat (Figs 6H–M and 8A–D), but there were cases where they existed in depressions (see above). ‘Tachanagaha’ had more cracks than others. Quantification of cracks was not attempted owing to their unpredictable locations and frequencies.
Fig. 8.
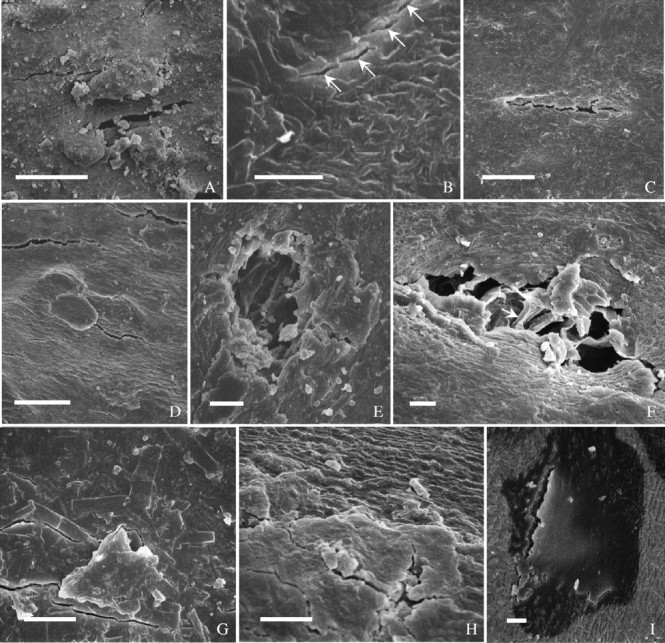
Cracks in seed coats. (A) Cracks at abaxial side in ‘Harovinton’. (B–D) Dorsal side in ‘Williams’: (B) cracks involve single cells (arrows); (C) a crack occurs where surface deposits are missing; (D) cracks close to each other. (E) Crack at dorsal side in ‘Harosoy 63’, occurring where deposits are missing. (F) ‘Tachanagaha’: an example of severe damage in seed coat. Arrow pointing to anticlinal wall of palisade cell. (G–I) ‘OX 951’: (G) cracks in heavy deposits at dorsal side; (H) cracks near edge of deposits at dorsal side; (I) crack near hilum in surface deposits and into palisade walls. Scale bars: A, C and D = 50 µm; B and E–I = 10 µm.
Cracks varied in depth. They were typically through the outer periclinal walls of palisade cells as well as the cuticle, and a few extended deep into the palisade layer (Fig. 8F) or even cell layers underneath. But, it was not easy to determine their exact depths with a scanning electron microscope. Nevertheless, cracks were readily detectable with Cellufluor as sites of intense staining of the adjacent wall material (Fig. 3B, C and F). There was an indication that cracks were more likely to occur where surface deposits were thin or missing (Figs. 6M, and 8C and E). In contrast to the results obtained from the five cultivars with permeable seed coats, in impermeable seeds of ‘OX 951’ cuticular cracks were absent. In some seeds a few cracks were observed, but they were through the surface deposits and did not extend as far as the cuticle (Fig. 8G and H). In this cultivar, about half of the seeds took up water albeit slowly; such seeds did have one or more cuticular cracks that extended into the palisade (Fig. 8I).
Anatomy and histochemistry of seed coats
For the purpose of an anatomical study, the seed coat can be simplified as consisting of a hilum and an extrahilar region. In the hilum, there was a counterpalisade layer in addition to a palisade layer (Fig. 9A). In the counterpalisade layer, the inner periclinal walls were lignified more heavily in the few cells around the hilar fissure than in cells farther away (Fig. 9A). In the palisade layer adjacent to the lignified counterpalisade cells, the outer periclinal walls stained intensely with berberine–aniline blue, emitting a yellowish green fluorescence under UV light (Fig. 9B). Underneath the hilum was the tracheid bar (Fig. 9A and B). The extrahilar region was made of, from outside to inside, the following layers: palisade, hourglass, parenchyma tissue, aleurone and compressed endosperm tissue (Fig. 9C and D). The focus of the present anatomical study was on the abaxial region of the seed coat; other regions were investigated less extensively since preliminary observations showed that there was no fundamental variation across the extrahilar area. Highlighted below are, for the most part, novel features of soybean seed coats. Special attention was paid to features that may contribute to the permeability properties, such as cuticle and surface deposits on the palisade surface.
Fig. 9.
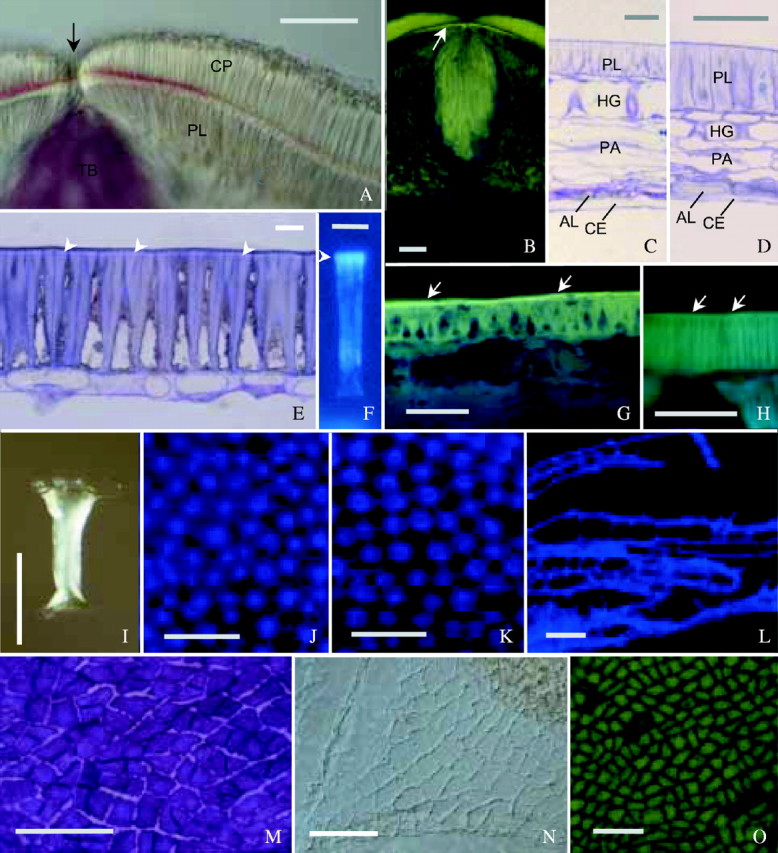
Structure and histochemistry of seed coats. (A) Free-hand section through hilum of ‘OX 951’, treated with phloroglucinol–HCl. Inner periclinal walls of counterpalisade layer (CP) are red, indicating lignin. Reaction most intense in a region around hilar fissure (arrow). Tracheid bar (TB) heavily lignified, while palisade layer (PL) not. (B) Section of ‘Clark-i’ through hilum stained with berberine–aniline blue. Light line is demonstrated by fluorescence (arrow), being most intense in the region below hilar fissure. Counterpalisade layer and tracheid bar also heavily stained. (C) Semithin section from abaxial region in ‘Clark-i’, stained with TBO. All cell layers are illustrated: palisade layer (PL), hourglass layer (HG), compressed parenchyma (PA), aleurone layer (AL) and compressed endosperm (CE). Cells of AL have thick outer periclinal walls and thin inner periclinal walls. (D) Dorsal region in ‘Harovinton’, processed as in (C): hourglass cells (HG) short and compressed parenchyma (PA) narrow. (E) Abaxial region in ‘Clark-i’, processed as in C: light line evident (arrows). (F) Macrosclereid of ‘Harovinton’, isolated by pectinase and stained with aniline blue. Secondary wall is fluorescent, with the light line region (arrowhead) more intense. (G) Free-hand section from ‘OX 951’ stained with berberine–aniline blue. Palisade cuticle visible (arrows) overlying the light line. (H) Section from ‘Harovinton’ stained as in G, with palisade cuticle barely visible (arrows). (I) Osteosclereid of ‘Harovinton’ isolated by pectinase and observed with DIC optics: wide outer end, narrower inner end and thickened wall in middle region. (J and K) Hourglass layer of ‘Harosoy 63’, partially macerated seed coat, stained with Cellufluor: (J) outer end, without intercellular spaces; (K) inner end, with intercellular spaces. (L) Portion of compressed parenchyma of ‘Harosoy 63’, with branched cells. Treated as in J. (M–O) Aleurone layer: (M) peeled-off preparation from hydrated ‘Harovinton’ seed coat, stained with TBO, depicting dense protoplasts and unevenly thickened anticlinal walls; (N) cuticle from outer periclinal side of ‘Harovinton’, isolated by sulphuric acid and observed with DIC optics; (O) vitality staining of ‘Harosoy 63’ with disodium fluorescein. Those cells that accumulated the dye were alive, and the few that did not were dead. Scale bars: A–D and G–I = 50 µm; E and F = 10 µm; J–O = 100 µm.
Palisade layer
This tissue consisted of macrosclereids that developed from the outer epidermis of the outer integument. There were no intercellular spaces and the cell walls were uniquely constructed. There was only a primary wall at the outer periclinal end (Fig. 9E), but also a thin secondary wall at the inner periclinal end. The anticlinal walls were unequally thickened: in addition to thickening along the entire length, there were longitudinal thickening bars. Toward the outside, these bars were thick and were packed together so that the cell lumina were extremely small or absent (Fig. 9E). Under UV light, all secondary walls emitted a dim, whitish blue autofluorescence. In a hydrated seed coat, palisade cells were swollen so that cell lumina expanded and some outer tangential walls were broken.
In cross-sections of seed coats, depressions in the palisade layer had thin anticlinal walls that were slightly squeezed. These walls were not able to fully recover their shapes in hydrated seed coats (Fig. 7M and N). Also, they were not as bright as those in other areas under UV light, suggesting less modification with phenolic substances (Fig. 7O).
The cuticle was not easy to detect by staining methods. Staining with Sudan VI, Sudan black B, Bismarck brown Y–azure B (or –TBO), Sudan red 7B or fluorol yellow 088 did not clearly demonstrate a cuticle in any cultivar. With the berberine–aniline blue procedure, ‘OX 951’ displayed a yellowish-green cuticle under UV light (Fig. 9G) and other cultivars gave a weak staining (e.g. in ‘Tachanagaha’; Fig. 9H). On a fractured seed coat observed with SEM, the cuticle was sometimes distinguishable from the outer periclinal walls (Fig. 10A and B). In ultrathin sections, ‘OX 951’ exhibited a well-defined, dense cuticle (Fig. 10D). Similar results were obtained for ‘Harovinton’ and ‘Clark-i’. The cuticle in ‘Tachanagaha’ showed loose and thin regions (Fig. 10E).
Fig. 10.
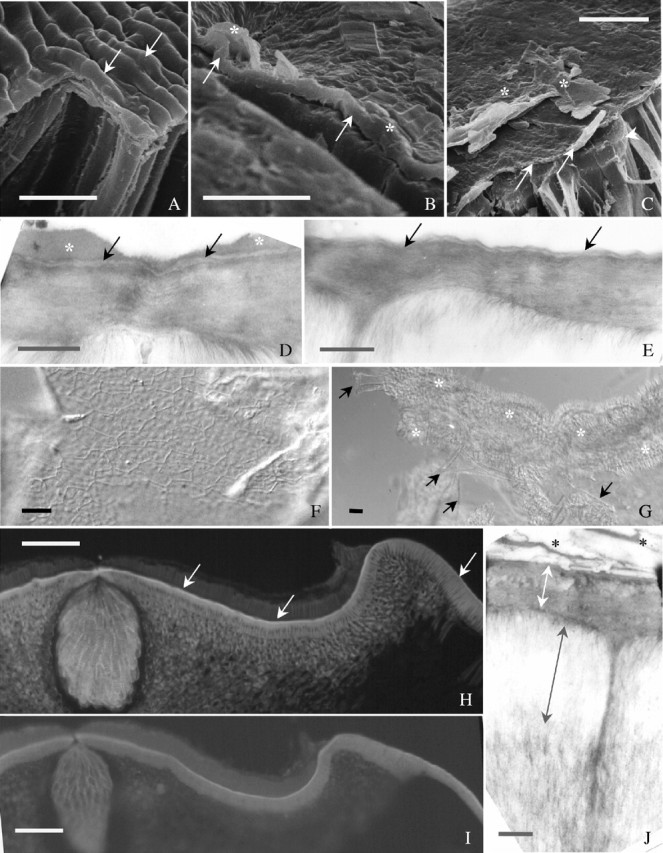
Palisade cuticle and light line. (A–C) Dry seed coat fractured and observed with SEM: (A) a region near hilum in ‘Williams’—cuticle (arrows) tightly adheres to periclinal walls; (B) abaxial side in ‘Harovinton’—surface deposits (asterisks), and palisade cuticle (arrows) are clearly seen; (C) ‘OX 951’, with surface deposits (asterisks) on cuticle (arrows)—outer end of the anticlinal wall in one cell visible (arrowhead), corresponding to the light line region. (D) Abaxial side in ‘OX 951’ observed with TEM. Palisade cuticle (arrows) conspicuous and near uniformly dense. There are surface deposits (asterisks). (E) Abaixial side in ‘Harovinton’ observed with TEM. Cuticle has loose regions (arrows). (F) Palisade cuticle of ‘OX 951’ isolated by pectinase. Large sheets of cuticle easily obtained. (G) Palisade cuticle of ‘Tachanagaha’ isolated by pectinase. Cutilce normally in small pieces (arrows), along with palisade cells (asterisks). (H) Section from hilum region of hydrated seed coat of ‘OX 951’. The light line is fluorescent (arrows) following staining with berberine-aniline blue. (I) Section from ‘Tachanagaha’ stained with aniline blue, illustrating a light line. (J) Palisade layer of ‘Clark-i’ observed with TEM. Light line appears as electron-lucent (black double-head arrow). The periclinal wall is marked with white double-head arrow. There are surface deposits (asterisks, Type II). Scale bars: A and C = 5 µm; B = 2 µm; D, E and J = 0·5 µm; F and G = 10 µm; H and I = 100 µm.
The palisade cuticle isolated by either concentrated sulphuric acid or pectinase varied according to the cultivar. Large sheets of cuticle were obtained from ‘OX 951’ (Fig. 10F). But normally only small pieces of cuticle were obtained from other cultivars; this was especially the case with ‘Tachanagaha’ (Fig. 10G). Although appearing structurally similar, the cuticle from ‘OX 951’ was especially mechanically strong, because it did not break easily when touched with dissecting needles. The ammonium oxalate–oxalic acid procedure failed to isolate cuticles from any cultivar.
A light line was a constant feature of all cultivars. It appeared as a narrow band near the outer end of the palisade layer under white light illumination (Fig. 9E). In sections stained with berberine–aniline blue, the light line, along with the cuticle, emitted an intense yellowish-green fluorescence under UV light while the rest of the palisade walls were less bright (Fig. 10H). The light line was particularly evident in the palisade layer above the tracheid bar (Fig. 9B). By using the aniline blue stain for callose under UV light, the light line appeared as a greenish-blue region that was brighter than the rest of the layer in either sections (Fig. 10I) or enzyme-isolated cells (Fig. 9F). The staining pattern was similar to that obtained with berberine–aniline blue. With SEM, the light line was found to be where the secondary thickening bars were tightly appressed to each other. In ultrathin sections, the light line was represented by the outer ends of the secondary walls as an electron-lucent region (Fig. 10J), apparently more obvious in ‘Clark-i’ and ‘Harovinton’ than in the other cultivars.
Hourglass layer
This layer was made of osteosclereids, or hourglass cells (Fig. 9C, D and I). Cells were longest (in the anticlinal direction) near the hilum, became shorter farther away, and were shortest in the dorsal region (Fig. 9C and D). In partially macerated seed coats, this layer was found to have a sealed outer face and a mesh-like inner face (Fig. 9J and K).
Parenchyma
The few layers underneath the hourglass layer were severely compressed in a dry seed coat, but the cells could partially regain their original shapes when hydrated (Fig. 9C and D). All cells were connected to their neighbours by branches (Fig. 9L).
Endosperm
The endosperm was represented by an aleurone layer and a mass of compressed tissue. The aleurone was the outermost endosperm layer and was characterized by thick outer periclinal walls, thin inner periclinal walls, and unevenly thickened anticlinal walls (Fig. 9C and M). There was a cuticle abutting the outer periclinal walls; this inner cuticle could be isolated by either sulphuric acid (Fig. 9N) or pectinase. Aleurone cells had dense protoplasts and were the only living cells in the entire seed coat (Fig. 9M and O). No difference was detected among the cultivars with regard to endosperm structure.
DISCUSSION
The aim of the present work was to locate the structural features of soybean seed coats that determine their permeability properties. Among the six selected cultivars, seed coats from five were variously permeable (‘Tachanagaha’, ‘Harovinton’, ‘Williams’, ‘Clark-i’ and ‘Harosoy 63’), as the seeds imbibed water quickly upon immersion in water (Fig. 2). Seed coats of ‘OX 951’ were variable in their permeability, about half allowing a slow rate of water uptake and the other half being impermeable for 1 or more days. This range of permeability of the cultivars provided an ideal opportunity to seek correlating anatomical features.
Cell layers of the seed coat
The cells of the soybean seed coat exhibited tremendous heterogeneity in structure. The cells in the palisade layer were tightly packed (Fig. 9E). The hourglass layer was aerenchymatous (Fig. 9C, D and K) and, thus, cannot make a seal to water flow. In fact, some legumes have intercellular spaces in the outer end as well as in the inner end of the layer [e.g. Melilotus (Jha and Pandey, 1989); Pisum sativum (van Dongen et al., 2003)] and are more porous than in soybean. Hourglass cells in the dorsal region were shorter than in other regions (Fig. 9C and D) and could be overlooked if samples were not properly prepared (Pereira and Andrews, 1985; McDonald et al., 1988). Deeper into the seed coat was a region of dead parenchyma tissue. The only living cell layer was the aleurone (Fig. 9M and O) that was bordered by a mass of compressed endosperm tissue.
The aleurone layer is able to mobilize its reserves (McCleary and Matheson, 1974, 1975, 1976) and protect the embryo (Matsui et al., 1996) during germination, but it is not known whether it plays any role in controlling the imbibition process. There is a cuticle on the aleurone. Developmentally, this cuticle is initially on the inner surface of the inner integument and later adheres to the aleurone layer when the integument degenerates (Chamberlin et al., 1994). But this cuticle does not constitute a barrier to water. This is because (a) there was no noticeable difference between permeable and impermeable cultivars in this feature, (b) wounding to convert an impermeable seed to a permeable one did not need to be as deep as in the aleurone layer (e.g. Arechavaleta-Medina and Snyder, 1981), and (c) the aleurone is located deep in the seed coat; therefore, it cannot affect the initial water uptake by the external layers. Nevertheless, the unique construction (specialized walls and living protoplasts) of the aleurone may exert some control over the rate of water flow into the embryo. A steady and even water uptake by the embryo would assure its optimum performance during imbibition; otherwise, imbibition damage would occur (Taylor et al., 1992).
The structural features discussed so far have convinced us that efforts in locating a water barrier should be focused on the palisade layer. There are several issues to consider.
Surface deposits
Three types of surface deposits in soybean have been described, but these may not have the same origin. There is no doubt about the endocarp origin of Type III deposits (Fig. 6E and F): the endocarp tissue is ripped off the fruit wall and stays on the seed, as described earlier for soybean (Wolf et al., 1981; Vaughan et al., 1987) and some other legumes (Newell and Hymowitz, 1978). Early work also produced strong evidence that Type II deposits were derived from the endocarp, based on a morphological comparison of the deposits and the endocarp (Wolf et al., 1981; Newell and Hymowitz, 1978). The ontogenetic identity of Type I deposits is hard to determine. The possibility cannot be excluded that the palisade layer contributes to it. A developmental study would clarify this matter.
The surface deposits exhibited some inter-cultivar variations in morphology, but a chemical characterization would be more desirable for a functional consideration. Unfortunately, this subject has not been well investigated. In mungbean (Watt et al., 1977) and soybean (Hahalis et al., 1996), staining with Cellufluor suggested the presence of cellulose in the deposits. In the present work, it was found that the main component of Type III deposits was cellulose (Fig. 3H), Type II deposits may contain considerable non-cellulosic material in addition to cellulose, and Type I deposits had no or little cellulose (Fig. 3G). Harris (1987) postulated that surface deposits reduced seed coat permeability in soybean based on the fact that deposits were light in a permeable cultivar (‘Hardee’) and heavier in an impermeable one (‘Brachett’). Do the surface deposits constitute a hydrophobic layer? A classic way of approaching this issue has been using organic solvents. Wolf et al. (1981) noticed differential responses of surface deposits to different solvents, but no clear correlation with permeabilities was established. Arechavaleta-Medina and Snyder (1981) found that soaking hard soybean seeds for 24 h in hexane, chloroform or acetone did not make them permeable, but treatment in methanol or ethanol did. Wax was implied by some authors (Williams, 1950; Calero et al., 1981; Ragus, 1987), but this idea was not confirmed by the results of the present study and a preliminary chemical analysis (unpubl. res.). Recently, a hydrophobic protein was detected on the surface of soybean seeds, and its amount is usually parallel to the density of surface deposits (Gijzen et al., 1999, 2003). The crystal-like deposits are probably formed from this hydrophobic protein. But the present study did not indicate a relationship between the protein and seed coat permeability, because some of the permeable seed coats had heavy deposits (e.g. ‘Harovinton’ and ‘Clark-i’).
Light line
The light line has traditionally been regarded as resulting from the difference in the refraction of light due to a change in chemical composition of palisade walls (e.g. M. alba; Hamly, 1932, 1935). It was also suggested that the light line is a region where the microfibrils changed from a longitudinal to a transverse orientation (Scott et al., 1962; Werker, 1980/81) but this was not the case in soybean (Fig. 10J). The light line is not merely an optical phenomenon caused by chemical modifications (see below), but is a real structure; it is where the secondary walls are tightly appressed to one another (Fig. 10C).
According to many studies light line is responsible for the impermeability of seed coats (see Hyde, 1954), but this idea has been largely abandoned since both permeable and impermeable seeds have a light line (see Werker, 1980/81). Nevertheless, some more recent studies tended to revitalize the old idea. Harris (1987) reported a more prominent light line in a hardseeded cultivar of soybean (‘Brachett’) than in a soft-seeded one (‘Hardee’). Yet, there has been no experimental proof that the light lines are causal factors for the permeability differences. Some authors detected callose in the light lines of impermeable seeds [Trifolium ssp. (Bhalla and Slattery, 1984); Stylosanthes scabra (Serrato-Valenti et al., 1993)] and considered this to be the reason for hardseededness, but proof has been lacking. It is important to understand that callose simply cannot be a barrier to water because, being a β-1,3-glucan, it is a hydrophilic substance. Furthermore, since callose does not constitute a complete sheath covering the palisade layer (Fig. 10J). Even a complete callose sheath normally acts as a semipermeable membrane (readily allowing movement of water but not of solutes), as in the endosperm of Cucumis melo (Yim and Bradford, 1998). Electron-dense (osmiophilic) and electron-lucent light lines were observed in pea (Harris, 1983) and Indigofera parviflora (Manning and van Staden, 1987a), respectively; in neither case were the wall modifications discussed in connection with the permeability properties of the seed coats. Staining of the light line was detected with either berberine–aniline blue or aniline blue alone (Fig. 9B), indicating phenolics and callose (Brundrett et al., 1988), but again there was no noticeable difference between permeable and impermeable cultivars in the staining patterns. At the ultrastructural level, there was some difference in staining intensity of the light lines among the cultivars, but this observation was not in line with the observed permeabilities (see Results). It seems highly unlikely that the light line plays a role in controlling seed coat permeability to water.
Cuticle of the palisade layer
The only structural feature that clearly and consistently correlates with seed coat permeability is that of the cuticle covering the palisade layer. Previously, Arechavaleta-Medina and Snyder (1981) converted impermeable soybean seeds to permeable ones by carefully scraping off a small area of the cuticle, but were unable to explain how the cuticles made intact permeable and impermeable seeds different, because these cuticles were virtually identical at the light microscopic level. In the present study, the use of SEM revealed small cracks in the cuticles of permeable seeds from five soybean cultivars but not in the cuticles of impermeable ones (from ‘OX 951’). Some seeds of ‘OX 951’ did take up water; now it is concluded that this was because they had minute cuticular cracks (Fig. 3E and F). Further, the positions of the cuticular cracks coincide with the initial sites of hydration, which typically were on the dorsal side of the seed (Fig. 3A–F). It is well known that major damage to a seed coat will result in a highly permeable localized area so that the seed will hydrate quickly. According to the present study, many similar but slower events are occurring on a micro-scale over a larger area in an uninjured and apparently intact seed as water enters through the naturally occurring small cracks in the cuticle. This phenomenon is completely different from permeability brought about by opening the strophiole (or lens), an outgrowth on the raphe in some other legumes (Martin and Watt, 1944; Ballard, 1973; Kelly and van Staden, 1987; Manning and van Staden, 1987b; Baskin, 2003).
To the best of our knowledge, this is the first detailed report of these small, cuticular cracks associated with the palisade layer of legume seeds. Why have they not been noticed before? The main reason is their small size (1–5 µm wide and 20–200 µm long). The cracks are beyond the limit of resolution of the dissecting microscope and cannot be visualized with a compound microscope either (the intact seed coats being too thick for light to penetrate). They were apparent in the present study only with the use of SEM. Their presence was also demonstrated with the use of fluorescent dyes (Cellufluor and berberine), but not with non-fluorescent dyes (safranin, fast green and TBO). The high sensitivity of fluorescent dyes is essential to highlight the staining of minute wall areas subtending the cuticular cracks during the initial stage of hydration. Non-fluorescent dyes [fast green (Powell and Matthews, 1979); food colouring dyes (Arechavaleta-Medina and Snyder, 1981)] can be used to detect macroscopic injuries in seed coats, but are not recommended for detection of the small cracks normally present in permeable seed coats.
It is not known what causes the small cuticular cracks to form. They are apparently not an artifact of SEM specimen preparation because they can also be detected with fluorescent tracer dyes, their presence correlates with seed coat permeability to water, and their distribution correlates with the sites of initial water uptake. It can only be speculated that stresses, such as fluctuations in temperature and relative humidity, on the palisade layer during seed desiccation and storage are responsible. The dorsal side is probably more susceptible to such stresses because the seed coat is heavily curved (Fig. 1B) and is very thin in this area (Fig. 9D). Formation of depressions might be expected to put a strain on the palisade layer but, strangely, they do not lead to the production of many cracks (Fig. 7A–L), the majority of cracks being found in flat regions. These observations explain why there is no perfect correlation between the presence of depressions and seed coat permeability (Wolf et al., 1981; Harris, 1987). A developmental study will be required to trace the origins of the cracks.
Why are cracks more prevalent in some cultivars than in others? The thickness of the cuticle is almost constant (about 0·2 µm), and its surface features per se are not fundamentally different among the cultivars. Based on the present investigation it can be said that, in general, the strength of the cuticle is critical. Both light and electron microscopic studies suggested that seeds of ‘OX 951’ possessed a denser cuticle than other cultivars (Figs. 9G and H, and 10D and E). The greater strength of the cuticle of ‘OX 951’ became apparent when this layer was isolated; it tore much less readily than those of other (permeable) cultivars. Is there any functional connection between surface deposits and cuticular cracks? In permeable cultivars, there was an indication that areas with deposits have fewer cracks than in bare areas (Figs 6M and 8C). But surface deposits alone did not make a seed impermeable because some cracks were deep enough to include the cuticle as well (Fig. 8A and D). In ‘OX 951’, the seed coats would be impermeable when the few cracks are superficial (Fig. 8G and H) and would be permeable when they extend through the cuticle (Fig. 8I).
In conclusion, the results of the present study have documented the presence of hitherto unknown small cracks that normally occur in the cuticle covering the palisade layer of soybean seed coats. The presence or absence of these cracks appears to control the permeability property of the seed coat to water. Thus, the condition of hardseededness in soybean can be at least partially attributed to a strong cuticular structure that is resistant to cracking. At the same time, the cuticle of an impermeable seed is not unbreakable; this idea could explain how the seed coats of many wild plants ‘soften’ over extended periods of time.
Acknowledgments
The following individuals are gratefully acknowledged: Mr Dale Weber (University of Waterloo, Canada) for support with electron microscopy; Dr John Mullen [Greenhouse and Processing Crops Research Centre, Agriculture and Agri-Food Canada, Harrow, ON (currently Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, Ottawa)] for his gift of soybean seeds, some of which were used in this study; Dr Mark Bernards (University of Western Ontario, London, Canada), Dr Derek Bewley (University of Guelph, Canada), Dr John Lott (McMaster University, Hamilton, Canada), Drs John van Herk and Henry Olechowski (W.G. Thompson & Sons, Blenheim, Canada), and Mr Matt McLean (Ontario Soybean Growers, Chatham, Canada) for their constructive discussions; Jamie Riske and Chris Meyer (University of Waterloo, Canada) for their technical help; Drs John Dickie (Royal Botanic Gardens, Kew, London, UK) and Robert Yaklich (ARS–USDA, Beltsville, MD, USA) for their insightful reviews of the manuscript. This work was supported by a Strategic Grant from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Current address: Department of Biology, Nipissing University, North Bay, Ontario, Canada P1B 8L7
LITERATURE CITED
- Arechavaleta-Medina F, Snyder HE. 1981. Water imbibition by normal and hard soybeans. Journal of American Oil Chemical Society 1981: 976–979. [Google Scholar]
- Ballard LAT. 1973. Physical barriers to germination. Seed Science & Technology 1: 285–303. [Google Scholar]
- Baskin CC. 2003. Breaking physical dormancy in seeds—focussing on the lens. New Phytologist 158: 229–232. [Google Scholar]
- Baskin JM, Baskin CC, Li X. 2000. Taxonomy, anatomy and evolution of physical dormancy. Plant Species Biology 15: 139–152. [Google Scholar]
- Bewley JD, Black M. 1994.Seeds: physiology of development and germination. New York: Plenum Press, 147–197. [Google Scholar]
- Bhalla PL, Slattery HD. 1984. Callose deposits make clover seeds impermeable to water. Annals of Botany 53: 125–128. [Google Scholar]
- Brundrett MC, Enstone ED, Peterson CA. 1988. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146: 133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. 1991. Efficient lipid staining in plant material with Sudan red 7B or fluorol yellow 088 in polyethylene fglycol-glycerol. Biotechnic & Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- Calero E, West S, Hinson K. 1981. Water absorption of soybean seeds and associated causal factors. Crop Science 21: 926–932. [Google Scholar]
- Chachalis D, Smith ML. 2001. Seed coat regulation of water uptake during imbibition in soybean [Glycine max (L.) Merr.]. Seed Science & Technology 29: 401–412. [Google Scholar]
- Chamberlin MA, Horner HT, Palmer RG. 1994. Early endosperm, embryo, and ovule development in Glycine max (L.) Merr. International Journal of Plant Science 155: 421–436. [Google Scholar]
- Currier HB. 1957. Callose substance in plant cells. American Journal of Botany 44: 478–488. [Google Scholar]
- Gijzen M, Miller S, Kuflu K, Buzzell RI, Miki BLA. 1999. Hydrophobic protein synthesized in the pod endocarp adheres to the seed surface. Plant Physiology 120: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen M, Weng C, Kuflu K, Woodrow L, Yu K, Poysa V. 2003. Soybean seed lustre phenotypes and surface protein cosegregate and map to linkage E. Genome 46: 659–664. [DOI] [PubMed] [Google Scholar]
- Graham ET, Joshi PA. 1996. Plant cuticle staining with Bismarck brown Y and azure B or toluidine blue O before paraffin extraction. Biotechnic & Histohemistry 71: 92–95. [DOI] [PubMed] [Google Scholar]
- Hahalis D, Cochrane MP, Smith ML. 1996. Water penetration sites in the testa of soybeans (Glycine max L. Merril) during seed imbibition. Science of Legumes 3: 218–226. [Google Scholar]
- Hamly DH. 1932. Softening of the seeds of Melilotus alba Botanical Gazette 93: 345–374. [Google Scholar]
- Hamly DH. 1935. The light line in Melilotus alba Botanical Gazette 96: 755–757. [Google Scholar]
- Harris WM. 1983. On the development of macrosclereids in seed coats of Pisum sativum L. American Journal of Botany 70: 1528–1535. [Google Scholar]
- Harris WM. 1987. Comparative ultrastructure of developing seed coats of ‘hard-seeded’ and ‘soft-seeded’ cultivars of soybean, Glycine max (L.) Merr. Botanical Gazette 148: 324–331. [Google Scholar]
- Hughes J, McCully ME. 1975. The use of an optical brightener in the study of plant structure. Stain Technology 50: 319–329. [DOI] [PubMed] [Google Scholar]
- Hyde EOC. 1954. The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Annals of Botany 18: 241–256. [Google Scholar]
- Jensen WA. 1962.Botanical histochemistry. San Francisco: W. H. Freeman, 256–269. [Google Scholar]
- Jha SS, Pandey AK. 1989. Seed coat structure in Melilotus (Fabaceae). Phytomorphology 39: 221–229. [Google Scholar]
- Johansen DA. 1940.Plant microtechnique. New York: McGraw-Hill. [Google Scholar]
- Kelly MK, van Staden J. 1987. The lens as the site of permeability in the papilionoid seed, Aspalathus linearis Journal of Plant Physiology 128: 395–404. [Google Scholar]
- Kolattukudy PE. 1980. Biopolyester membranes of plants: cutin and suberin. Science 208: 990–1000. [DOI] [PubMed] [Google Scholar]
- Lush WM, Evans LT. 1980. The seed coats of cowpea and other grain legumes: structure in relation to function. Field Crops Research 3: 267–286. [Google Scholar]
- McCleary BV, Matheson NK. 1974. α-d-Galactosidase activity and galactomannan and galactosylsucrose oligosaccharide depletion in germinating legume seeds. Phytochemistry 13: 1747–1757. [Google Scholar]
- McCleary BV, Matheson NK. 1975. Galactomannan structure and β-mannanase and β-mannanosidase activity in germinating legume seeds. Phytochemistry 14: 1187–1194. [Google Scholar]
- McCleary BV, Matheson NK. 1976. Galactomannan utilization in germinating legume seeds. Phytochemistry 15: 43–47. [Google Scholar]
- McDonald MR Jr, Vertucci CW, Roos EE. 1988. Seed coat regulation of soybean seed imbibition. Crop Science 28: 987–992. [Google Scholar]
- Manning JC, van Staden J. 1987. The functional differentiation of the testa in seed of Indigofera parviflora (Leguminosae: Papilionoideae). Botanical Gazette 148: 23–34. [Google Scholar]
- Manning JC, van Staden J. 1987. The role of the lens in seed imbibition and seedling vigour of Sesbania punicea (Cav.) Benth. (Leguminosae: Papilionoideae). Annals of Botany 59: 705–713. [Google Scholar]
- Martin JN, Watt JR. 1944. The strophiole and other seed structures associated with hardness in Melilotus alba L. and M. officinalis Willd. Iowa State College Journal of Science 18: 457–469. [Google Scholar]
- Matsui M, Uenaka T, Toyosawa I, Fukuda M. 1996. Role of soybean aleurone layer in water uptake in seeds. Nippon Nõgeikagaku Kaishi 70: 663–669. [Google Scholar]
- Mullin WJ, Xu W. 2001. Study of soybean seed coat components and their relationship to water absorption. Journal of Agricultural Food Chemistry 49: 5331–5335. [DOI] [PubMed] [Google Scholar]
- Newell CA, Hymowitz T. 1978. Seed coat variation in Glycine Willd. subgenus Glycine (Leguminosae) by SEM. Brittonia 30: 76–88. [Google Scholar]
- Pereira LAG, Andrews CH. 1985. Comparison of non-wrinkled and wrinkled soybean seedcoats by scanning electron microscope. Seed Science & Technology 13: 853–859. [Google Scholar]
- Powell AA, Matthews S. 1979. The influence of testa condition on the imbibition and vigour of pea seeds. Journal of Experimental Botany 30: 193–197. [Google Scholar]
- Ragus LN. 1987. Role of water absorbing capacity in soybean germination and seedling vigour. Seed Science & Technology 15: 285–296. [Google Scholar]
- Rangaswamy NS, Nandakumar L. 1985. Correlative studies on seed coat structure, chemical composition, and impermeability in the legume Rhynchosia minima Botanical Gazette 146: 501–509. [Google Scholar]
- Rolston MP. 1978. Water impermeable seed dormancy. Botanical Review 44: 365–396. [Google Scholar]
- Saio K. 1976. Soybeans resistant to water absorption. Cereal Foods World 21: 168–173. [Google Scholar]
- Scott FM, Bystrom BG, Bowler E. 1962.Cercidium floridum seed coat: light and electron microscopic studies. American Journal of Botany 49: 821–833. [Google Scholar]
- Serrato-Valenti G, Cornara L, Ferrando M, Modenesi P. 1993. Structural and histochemical features of Stylosanthes scabra (Leguminosae; Papilionoideae) seed coat as related to water entry. Canadian Journal of Botany 71: 834–840. [Google Scholar]
- Taylor AG, Prusinski J, Hill HJ, Dickson MD. 1992. Influence of seed hydration on seedling performance. HortTechnology 2: 336–344. [Google Scholar]
- Tran VN, Cavanagh AK. 1984. Structural aspects of dormancy. In: Murray DR, ed. Seed physiology, Vol. 2. Germination and reserve mobilization. Sydney: Academic Press, 1–44. [Google Scholar]
- Tyler JM. 1997. Effect of impermeable seed coat on germination of seed for early maturing soybean. Seed Technology 19: 45–50. [Google Scholar]
- van Dongen JT, Ammerlaan AMH, Wouterlood M, van Aelst AC, Borstlap AC. 2003. Structure of the developing pea seed coat and the post-phloem transport pathway of nutrients. Annals of Botany 91: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DA, Bernard RL, Sinclair JB, Kunwar IK. 1987. Soybean seed coat development. Crop Science 27: 759–765. [Google Scholar]
- Villena JF, Domínguez E, Stewart D, Heredia A. 1999. Characterization and biosynthesis of non-degradable polymers in plant cuticles. Planta 208: 181–187. [DOI] [PubMed] [Google Scholar]
- Watt EE, Poehlman JM, Cumbie BG. 1977. Origin and composition of a texture layer on seeds of mug bean. Crop Science 17: 121–125. [Google Scholar]
- Werker E. 1980. Seed dormancy as explained by the anatomy of embryo envelopes. Israel Journal of Botany 29: 22–44. [Google Scholar]
- Williams LF. 1950. Structure and genetic characteristics of the soybean. In: Markley KS, ed. Soybeans and soybean products, Vol. 1. New York: Interscience Publishers, 111–134. [Google Scholar]
- Wolf WJ, Baker FL, Bernard RL. 1981. Soybean seed-coat structural features: pits, deposits and cracks. Scanning Electron Microscopy 1981 (III): 531–544. [Google Scholar]
- Yim K-O, Bradford KJ. 1998. Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon. Plant Physiology 118: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]