Abstract
• Background and aims. Flowering phenology is described and the effect of flowering time on pollination success is evaluated in the deceit-pollinated tropical orchid, Myrmecophila christinae. It was expected that, due to this species' deceit pollination strategy and low observed pollinator visit rate, there would be a higher probability of natural selection events favouring individuals flowering away from the population flowering peak.
• Methods. The study covers two consecutive years and four populations of M. christinae located along the north coast of the Yucatán Peninsula. For phenological and pollination success data, a total of 110 individuals were monitored weekly in 1998, and 83 individuals in 1999, during all the flowering and fruiting season.
• Key results. The results showed significant differences in the probability of donating and receiving pollen throughout the flowering season. The probability of receiving or donating pollen increased the further an individual flowering was from the flowering peak. Regression analysis showed directional and disruptive phenotypic natural selection gradients, suggesting the presence of selection events unfavourable to flowering during flowering peak, for both male success (pollen removal) and female success (fruit production). However, the intensity and significance of the natural selection events varied between populations from year to year. The variation between seasons and populations was apparently due to variations in the density of reproductive individuals in each population and each season.
• Conclusions. As in other deceit-pollinated orchids, natural selection in M. christinae favours individuals flowering early or late in relation to population peak flowering. However, results also suggested a fluctuating regime of selective events act on flowering time of M. christinae.
Key words: Deceit pollination, flowering time, fluctuating natural selection, negative frequency-dependent selection, México, Myrmecophila christinae, Orchidaceae, tropical perennial plant, phenology, phenotypic natural selection
INTRODUCTION
Flowering phenology is one of the most important characteristics in the life history of plants because it strongly determines fitness through sexual reproduction (Ratchke and Lacey, 1985). Adaptive hypotheses generated to explain how natural selection can shape flowering phenology include aspects such as pollinator availability, escape from predation and extreme climatic conditions (e.g. Thomson, 1980; Augspurger, 1981; and see Ratchke and Lacey, 1985).
In predominantly out-crossing species of angiosperms that are strongly limited by pollinators, natural selection is expected to increase the possibility of mating through an increase in pollinator visits (Ratchke, 1983). One strategy to enhance pollinator visits is to increase a plant's attraction to insects through a temporal concentration of floral resources, which could then be maintained through stabilizing natural selection (e.g. Ollerton and Díaz, 1995; O'Neil, 1997). In contrast, in plant species with deceit-based pollination, flowering synchrony is predicted to be disadvantageous because pollination success depends on the frequency with which pollinators can be deceived (negative frequency-dependent selection) (Smithson and Macnair, 1997; Ferdy et al., 1998; Gigord et al., 2001; Castillo et al., 2002).
It is estimated that more than one-third of the Orchidaceae (approx. 10 000 species) utilize deceit pollination (Dodson and Frymire, 1961; Ackerman, 1986; Johnson et al., 1998). Evidence suggests that natural selection against synchronous flowering is common for many of these species (Fritz, 1990; Sabat and Ackerman, 1996; O'Connel and Johnston, 1998). In addition to negative frequency-dependent selection, there are two other factors in deceit-pollinated orchids that can increase selective pressure against flowering synchrony: (1) strong pollinator limitation of fruit and seed production (Primack and Hall, 1990; Calvo, 1993); and (2) high specificity in pollinator interaction (Ackerman, 1986; Nilsson, 1992).
Little is known, however, about the effect of spatio-temporal changes in the density of reproductive individuals on the pollination success of deceit-pollinated orchids (Fritz and Nilsson, 1994; Gigord et al., 2001). Evaluation of these changes is important because if pollinators discriminate against flowers that offer no reward, then pollination strategy results may vary according to the density of reproductive individuals (Ackerman, 1989; Fritz, 1990; Fritz and Nilsson, 1994; Sabat and Ackerman, 1996; Smithson and Macnair, 1997). Within this scenario it can be predicted that, in deceit pollination, natural selection does not act consistently between populations with different densities, and may not act consistently among reproductive seasons.
Myrmecophila christinae is a tropical orchid deceit-pollinated only by two solitary bees species in the coastal shrub vegetation in Yucatán, México (Rico-Gray and Thien, 1987), and is characterized by low visiting rates and very low pollination success, though it varies from year to year and between populations (fruit-set range 2–30 %) (Rico-Gray and Thien, 1987; Malo et al., 2001; Naval, 2002). In fact, manual pollination experiments have shown that seed production by M. christinae is strongly limited by lack of pollinators (Rico-Gray and Thien, 1987). Additionally, it has been observed that the population size of this species is variable due to the high landscape fragmentation. The objective of this study was to investigate the flowering phenology of four populations of M. christinae over a 2-year period, to determine if a relationship exists between the flowering time and reproductive success. The specific research questions addressed were: (1) Is the probability of donating or receiving pollen equal throughout the flowering season? (2) Is there greater pollination success for individuals that flower at a specific time during the flowering season? (3) Can phenotypic selection on flowering time be detected and is this selection consistent between reproductive seasons and between populations?
MATERIALS AND METHODS
Study site
The study was carried out in the strip of coastal shrub vegetation located along the north coast of the Yucatán Peninsula, in the state of Yucatán, México. The coastal shrub vegetation in Yucatán grows on a line of fixed sand dunes located 100–500 m back of the beach, with altitudes of <10 m a.s.l. The climate is hot tropical and strongly seasonal (Bso, Köppen classification) with a mean annual temperature of 25·4 °C and 469 mm annual precipitation, concentrated between June and October (Flores and Espejel, 1994). During the two years of the study (1998 and 1999), slight variations in both mean annual temperature and precipitation were recorded. Mean annual temperature and precepitation were higher in 1999 (26·2 °C and 532 mm) than in 1998 (25·1 °C and 429 mm).
The M. christinae populations on the north coast of Yucatán occur in a landscape that is severely fragmented by tourist development, which has drastically reduced the species' distribution range and abundance (Malo et al., 2001; Naval, 2002). The M. christinae populations used in the present study are located along 40 km of the highway between the city of Progreso and the town of Telchac Puerto (21°20′N, 89°30′W), and each population is separated from the next one by an average distance of 10 km. In 1998, four populations were located: Telchac, Chicxulub, Nuevo Yucatán and Mr Bú. Only three of these populations were available for study in 1999, because the area containing the Chicxulub population had been slashed and burned. Telchac covered the largest area (40 ha), followed by Chicxulub (9 ha), Nuevo Yucatán (6 ha) and Mr Bú (8 ha). Recent reports suggest a weak relationship between fruit production and the area each population occupies, and no relationship with their degree of geographic isolation (Naval, 2002).
Study species
Myrmecophila christinae var. christinae (=Schomurgkia tibicinis) (Carnevali et al., 2001) is epiphytic, growing on the trunks of palms (Cocothrinax reaedii and Thrinax radiata 1·0 and 2·5 m high) and branches of small shrubs (e.g. Pithecellobium keyense) in the coastal shrub. Myrmecophila christinae produces one or two pseudobulbs a year, although individuals with >40 pseudobulbs have been reported (Rico-Gray and Thien, 1987). The flowering period extends from March to June and fruits are produced from May to July.
The flowers of M. christinae are located at the end of 1·5–2·0 m long inflorescences. Extrafloral nectar is produced by the inflorescence and is collected by different ant species that serve as guardians (Rico-Gray and Thien, 1989; Malo et al., 2001). Each plant produces 1·8 ± 2·1 (mean ± s.d., n = 115) inflorescences, which sequentially produce between six and 15 large (8–9 cm diameter) flowers, with colours varying from creamy yellow to purplish rose. Though M. christinae is completely self-compatible, it requires pollen vectors for fruit formation. Flowers last from 6 to 10 d and are receptive from the first day of anthesis. No floral nectar or other form of reward is produced, although flowers produce a strong vanilla scent at dawn. Each flower has a pollinarium containing eight pollinia. Pollination is via food deceit of ‘naive’ (recently emerged inexperienced) bees that explore potential floral resources (Rico-Gray and Thien, 1989; Malo et al., 2001). The only known pollinators are Eulaema polychroma and Xylocopa sp. (Rico-Gray and Thien, 1987). During the study period Xylocopa sp. was relatively abundant but only two E. polychroma individuals were recorded. It was observed that some pollination by Xylocopa sp. between 0500 and 0630 h whose visits were rapid (approx. 2 s) and effective with the removal of usually the entire pollinarium.
As in the majority of orchid species, and especially those of the tropics, fruit production in M. christinae is low, though it varies from year to year and between populations (2–30 %) (Rico-Gray and Thien, 1987; Naval, 2002). Manual pollination experiments have shown that M. christinae is strongly pollen-limited (Rico-Gray and Thien, 1987). The fruits produce as many as 700 000 wind-dispersed seeds (Ortegón, 2000).
Phenology, pollen removal and pollen deposition (pollination)
For the phenological censuses, all reproductive individuals in the Chicxulub, Nuevo Yucatán and Mr Bú populations were marked. Due to the large area of the Telchac population, four 20 × 20 m quadrants were randomly placed and all reproductive individuals marked within each quadrant. Over the 2-year study period, a total of 110 individuals were monitored in 1998, and 83 individuals in 1999 (Table 1). Monitoring consisted of recording the following variables for two or three consecutive days every week for every individual: (a) the week of initial flowering; (b) the number of open flowers; (c) the number of flowers with pollinarium removal; (d) the number of flowers with pollinia deposited into stigmas (pollinated); and (e) the number of fruits.
Table 1.
Reproductive individuals (n); total number of flowers and density of reproductive individuals (DRI) and flowers (FD) for two consecutive years (1998–1999) in four Myrmecophila christinae populations located in the coastal shrub vegetation of Yucatán, México
| 1998 |
1999 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population |
n |
Total no. of flowers |
DRI (ha−1) |
FD (ha−1) |
n |
Total no. of flowers |
DRI (ha−1) |
FD (ha−1) |
||||||
| Telchac | 68 | 817 | 420 | 540 | 54 | 603 | 330 | 390 | ||||||
| Chicxulub | 12 | 149 | 75 | 330 | nd | nd | nd | nd | ||||||
| Nuevo Yucatán | 20 | 348 | 30 | 50 | 15 | 120 | 21 | 17 | ||||||
| Mr Bú | 10 | 561 | 1·2 | 70 | 14 | 270 | 1·7 | 33 | ||||||
nd, No data available.
Orchid species usually have very low flower visit frequency, which limits the possibility of making direct estimations of pollinator abundance (Ackerman, 1981; Parra-Tabla et al., 2000). This is further complicated with M. christinae because the pollinators are not easily detectable at the time of flower visits (see study species). In response, pollinarium removal and the rate at which flowers are pollinated can be used as an indirect pollinator abundance estimator. This estimation is based on the positive correlation between pollination rates and pollinator abundance reported for other temperate and tropical orchid species (Ackerman, 1981; Parra-Tabla et al., 2000; and see O'Connel and Johnston, 1998; Aragón and Ackerman, 2004).
Variation and phenotypic natural selection in flowering time
Differences in flowering initiation between populations and between years were analysed with a factorial ANOVA using the MGLH procedure in SYSTAT (SYSTAT, 1997). The specific differences between populations and years were analysed with the Tukey multiple comparison test (Zar, 1984).
Phenotypic selection analysis was done with regression methods (Lande and Arnold, 1983; Arnold and Wade, 1984), the advantages of which have been widely addressed by various authors (e.g. Endler, 1986; Johnston, 1991). This analysis correlates a phenotypic character with an individual fitness measure. In the case of M. christinae, the week of flowering initiation (WFI) was used as the phenotypic characteristic most likely to be under selection. The WFI was defined as the first week in which an individual had open flowers. Average flowering period for each M. christinae individual was similar between years (2·2 weeks ± 1·5, n = 90, and 2·09 weeks ± 1·9, n = 83, mean ± s.d., for 1998 and 1999, respectively), though it is during the first week of flowering that each plant opens between 60 % and 80 % of its flowers. The partial components for relative fitness used in this analysis were: (a) fruit production (number of mature fruits/number of flowers produced) as an estimate of female success; (b) pollinarium removal rate (number of flowers with pollinarium removed/number of flowers produced) as an index of male success; and (c) ‘total’ pollination success, estimated as (total pollinations of locality/total pollinarium removals of locality) (pollinarium removals)+fruits (Ackerman et al., 1997). This index of overall or ‘total’ success accounts for the fact that usually in tropical orchids more pollinarium removals occur than pollination events (Ackerman et al., 1997). Fruit production and pollinarium removal rates were used to determine female and male success because of the high inter-individual variation on flower production.
A great advantage in orchid species is the availability of a male success measure, which is possible because the pollen is organized into discrete packets (pollinia), making removal easily detectable (Fritz and Nilsson, 1996). This male success index has been used in other orchid species, and there is evidence of a positive correlation between removal rate and ovules fertilized (Nilsson et al., 1992).
Each partial component of relative individual fitness (wi) was defined as a ratio of the fitness value to the population mean (Lande and Arnold, 1983). The directional selection gradient (βi), which expresses the strength and sign (positive or negative) of the directional selection, was estimated using regression of each fitness component with the WFI value of each individual: wi = µ + βi (WFI) + e; where µ = regression constant, βi = directional selection gradient on the character, and e = error. The quadratic selection gradient, which expresses the strength of the stabilizing or disruptive selection, was estimated from the partial regression coefficient of each fitness component and the quadratic term of the character: wi = µ + βi (WFI) + γii (WFI)2 + e; where γii = quadratic selection gradient. The selection gradients were standardized (β′, γ′), as this allows expression of any change in the fitness value due to a change in the character under selection in terms of standard deviation units (Lande and Arnold, 1983).
One disadvantage of regression methods is that the relative fitness measure must not be transformed to avoid misinterpretation (Lande and Arnold, 1983). This restriction can lead to statistical problems such as non-normal residuals distribution and consequent difficulty in estimating the significance of the selection gradients (Mitchell-Olds and Shaw, 1987). Thus the significance of the regression coefficients was estimated using a jackknife procedure (Mitchell-Olds and Shaw, 1987). In cases where a significant relationship was found, the result was corroborated with non-parametric correlations (Spearman rank-correlation) using bootstrap procedures (SYSTAT, 1997). The final significance of the models for each year and population was generated using the sequential Bonferroni correction (Rice, 1988).
RESULTS
Phenology and pollen removal and deposition rates (pollination)
The 1998 flowering period lasted 12 weeks (26 March to 13 June) in all the populations, but the 1999 flowering period was only 9 weeks long (13 April to 12 June). The total number of monitored flowers was 1865 and 993 in 1998 and 1999, respectively (Table 1). The density of reproductive individuals and flowers varied widely between populations and years (Table 1). Evident flowering peaks were noted only in the Telchac population in 1998 and in the Telchac and Mr Bú populations in 1999 (Fig. 1).
Fig. 1.
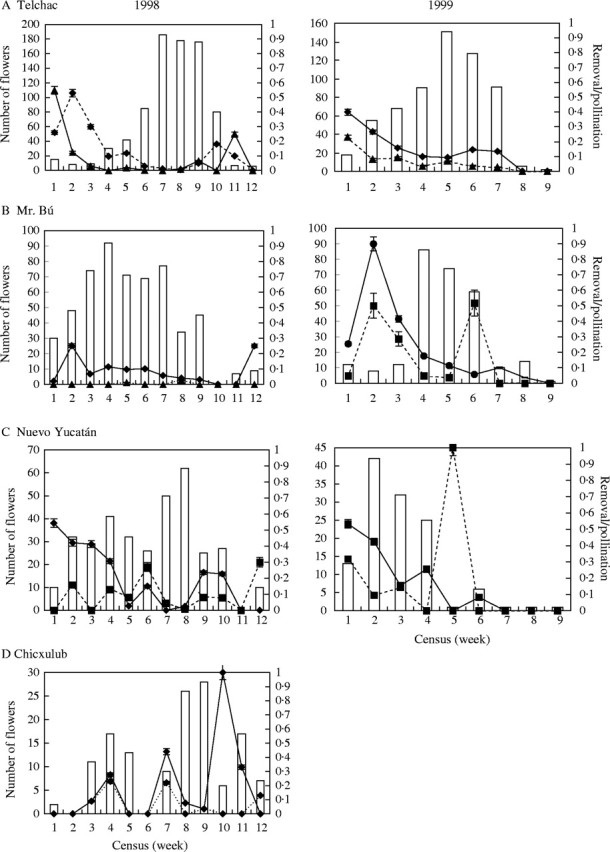
Flowering phenology of four Myrmecophila christinae populations in the coastal shrub vegetation of the state of Yucatán, México, in 1998 and 1999: (A) Telchac, (B) Mr Bú, (C) Nuevo Yucatán and (D) Chicxulub. In 1998 the first census with flowers recorded is March 26, and in 1999 the first census with flowers recorded is April 13. Bars represent total number of open flowers for each census visit. Solid lines represent average value of pollinium removal rate (± s.d.), and dotted lines represent average value of pollinated flower rate (± s.d.). Please note difference in scale for number of flowers in each site and year.
The frequencies of flowers with their pollinarium removed or in which pollination was observed were generally lower during flowering peak or greatest flower abundance in 1998 (Fig. 1). Significant differences between censuses were observed in 1998 for the proportion of flowers in which pollinaria were removed and deposited in the Telchac and the Nuevo Yucatán populations (H ≥ 22·4, P < 0·01 in all cases; non-parametric Kruskall–Wallis test). In 1999, the highest pollinarium removal and deposition frequencies were generally at the beginning of flowering and lowest at peak flowering (Fig. 1). Significant differences for pollinarium removal between censuses were observed in the Telchac and the MrBú populations (H ≥ 24·35, P < 0·01 in both cases; non-parametric Kruskall–Wallis test), and significant differences for pollinarium deposition were observed in the Nuevo Yucatán and the Mr Bú populations (H ≥ 22·9, P < 0·01 in both cases non-parametric Kruskall–Wallis test).
Seasonal variation in pollinarium removal and pollinarium deposiion in 1998 was different in our study populations. High rates of pollinarium removal were recorded during flowering initiation and near flowering termination in the populations of Telchac, Nuevo Yucatán and Mr Bú (Fig. 1). The Chicxulub population displayed removal peaks throughout the flowering period (Fig. 1). Pollinaria deposited in the Telchac population were similar to pollinarium removal with high rates at flowering initiation and termination (Fig. 1). The pattern in the remaining populations was unclear, with a particularly low pollinarium deposition in the Mr Bú population (Fig. 1).
In contrast, pollinarium removal patterns in 1999 were similar among the populations (Fig. 1). The highest removal rate was seen at flowering initiation (Fig. 1). Pollinarium deposition in the Telchac population during this season was similar to its removal rate. No clear pattern was seen in the Nuevo Yucatán and Mr Bú populations but high pollinarium deposition was recorded near flowering initiation and termination (Fig. 1).
Overall the low pollinarium removal and rate at which flowers were pollinated suggest a low but variable pollinator abundance between sites and years. The data of all populations show negative tendencies in the relationship between the total number of open flowers and pollinarium removal in 1998 (r = −0·41, P = 0·005, R2 = 0·18), and open flowers and pollination rate (r = −0·25, P = 0·06, R2 = 0·06). However, in 1999 none of the relationships were significant (r ≤ 0·13, P ≥ 0·08 in both cases).
Variation in the flowering time (WFI)
Significant differences were seen between populations in the week of flowering initiation (WFI) (F2,201 = 37·7, P < 0·001), though there were no significant differences between years or in the year × population interaction (1998: F1,201 = 0·037, P = 0·8; 1999: F3,201 = 0·18, P = 0·9). In 1998, the Telchac population had a later (higher) average week of flowering initiation, whereas in 1999 it was only later than the Nuevo Yucatan population (P < 0·01, Tukey-test). Of the total number of flowering individuals in 1998, only 47 flowered the following year (36 in Telchac, five in Nuevo Yucatán and six in Mr Bú).
Phenotypic selection for flowering time (WFI)
The phenotypic selection analysis showed significant selection gradients in three of the four populations for 1998 and 1999 (Table 2). The results for all the estimated fitness components in the Telchac population showed selection events unfavorable to initial flowering during the population flowering peak. The analysis suggested a significant directional selection for ‘total’ and female fitness (total: F1,66 = 6·01, P = 0·017, R2 = 0·08; female: F1,66 = 4·68, P = 0·03, R2 = 0·07), showing that individuals with earlier flowering had greater fitness (Table 2). Significant quadratic gradients were detected for male fitness (F2,65 = 6·21, P = 0·003, R2 = 0·16), suggesting disruptive selection for this trait (Table 2). Negative directional selection gradients were seen in Telchac in 1999, indicating natural selection in favour of early flowering (male fitness: F1,55 = 8·55, P = 0·005, R2 = 0·13; female fitness: F1,55 = 10·91, P = 0·0016, R2 = 0·16; total fitness: F1,55 = 10·85, P < 0·002, R2 = 0·19). Significant quadratic gradients were detected for ‘total’ fitness for this year as well (F2,65 = 7·4, P = 0·002, R2 = 0·23), suggesting disruptive selection (Table 2).
Table 2.
Effect of week of flowering initiation (WFI; flowering time) on total, female and male success in four Myrmecophila christinae populations
| 1998 |
1999 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Population |
β′ |
γ′ |
β′ |
γ′ |
||||
| Telchac | ||||||||
| Total fitness | −0·28*(0·09) | 0·16 (0·04) | −0·42* (0·19) | 0·95*(0·08) | ||||
| Female fitness | −0·257* (0·123) | +0·45 (0·04) | −0·407** (0·162) | +0·42 (0·08) | ||||
| Male fitness | −0·19 (0·07) | +1·81*** (0·02) | −0·37** (0·118) | +0·76 (0·05) | ||||
| Chicxulub | ||||||||
| Total fitness | +0·08 (0·05) | +0·02 (0·13) | nd | nd | ||||
| Female fitness | −0·53* (0·08) | −0·02 (0·02) | nd | nd | ||||
| Male fitness | +0·87*** (0·06) | +0·86 (0·014) | nd | nd | ||||
| Nuevo Yucatán | ||||||||
| Total fitness | −0·32 (0·28) | 0·79 (0·112) | −0·07 (0·51) | −0·25 (0·28) | ||||
| Female fitness | −0·101 (0·25) | −0·542 (0·06) | +0·06 (0·51) | +0·026 (0·5) | ||||
| Male fitness | −0·38 (0·173) | +2·011* (0·06) | −0·37 (0·174) | −0·74 (0·17) | ||||
| Mr Bú | ||||||||
| Total fitness | −0·118 (0·18) | 1·67 (0·115) | −0·33 (0·22) | −0·42 (0·21) | ||||
| Female fitness | +0·02 (0·2) | −0·11 (0·173) | +0·38 (0·14) | −2·01 (0·083) | ||||
| Male fitness | −0·23 (0·2) | −0·62 (0·033) | −0·67*** (0·08) | −0·04 (0·06) | ||||
The standardized coefficients for directional (β′) and quadratic (γ′) selection for the two consecutive years are presented.
The standard errors for coefficients estimated with the jackknife method are shown in parentheses (see text).
Bold type indicates the significant coefficients according to the sequential Bonferroni correction (Rice, 1988)
nd, No data available.
P < 0·05
P < 0·01
P < 0·001.
Significant directional selection events were observed in Chicxulub in 1998 for the female (F1,12 = 4·43, P = 0·05, R2 = 0·29) and male success components (F1,12 = 34·73, P < 0·001, R2 = 0·76) (Table 2). The selection coefficient for the female component was negative, indicating a greater advantage for individuals that initiated flowering at the beginning of the flowering season. For the male component, however, the coefficient was positive, suggesting a greater advantage for initial flowering towards the end of the flowering season (Table 2).
In 1998, a significant quadratic effect was seen in male fitness (F2,17 = 5·21, P = 0·017, R2 = 0·38) in the Nuevo Yucatan population, caused by the greater advantage of individuals that flowered towards the beginning or end of the flowering season (Table 2). Negative directional selection in male success was only detected in Mr Bú in 1999 (F1,44 = 11·65, P = 0·0042, R2 = 0·45), suggesting that individuals that flowered at the beginning of the flowering season were favoured.
DISCUSSION
This study showed that, for M. christinae, the probability of donating or receiving pollen is not equal throughout the flowering season. It was also shown that it is possible to detect phenotypic natural selection events acting on the time of initial flowering by using the pollination success components, and that these events can vary spatially and temporally.
The adaptive value of flowering time has been shown for a number of species (Augspurger, 1981; Dieringer, 1991; Widén, 1991; Ollerton and Lack, 1992; Gómez, 1993; Domínguez and Dirzo, 1995; O'Neil, 1997). In xenogamous species the advantage of synchronous flowering in pollination may be caused by an increase in pollinator attraction, higher gamete removal and deposition rates and greater opportunities for mating. This appears to be the case in species that offer some kind of floral reward and have a promiscuous pollination system (e.g. Augspurger, 1981; Domínguez and Dirzo, 1995). However, in species that use deceit pollination and have a specialized pollination system one can predict that natural selection will act against synchronous flowering because pollination success depends on the pollinator's ability to discriminate between flower types that offer reward and those that do not (Fritz and Nilsson, 1996; Smithson and Macnair, 1997).
Data on the relationship between flowering time and pollination success in tropical and temperate orchids using food deceit pollination support the prediction that individuals that flower earlier or later from the population peak are favoured (Fritz, 1990; Fritz and Nilsson, 1996; Sabat and Ackerman, 1996). Results for the only orchid species that uses deceit pollination, and for which the effect of natural selection on flowering time has been explicitly estimated, show that individuals that flower early or late have greater success (Sabat and Ackerman, 1996; O'Connel and Johnston, 1998; Maad, 2000). In Cypripedium acaule, however, it was observed that microhabitat (i.e. canopy openness) was a more important factor in explaining differences in fruit production than day of initial flowering (O'Connell and Johnston, 1998). In Tolumnia variegata, flower density at a focal level (i.e. per host tree) notably affected fruit production, despite intense disruptive selection events during flowering (Sabat and Ackerman, 1996).
In M. christinae the observed results are consistent with the prediction that in deceit-pollinated species natural selection favours individuals with early or late flowering. The result of this selection, however, varied from year to year and between populations. A number of different factors have been suggested as important in explaining differences in pollination success between and within populations in orchid and other plant species. These include disturbance and isolation at a population level (Aizen and Feinsinger, 1994; Groom, 1998; Parra-Tabla et al., 2000), floral display and size at an individual level (Fritz, 1990; Fritz and Nilsson, 1996; Ollerton and Lack, 1998), pollinator availability (Ackerman, et al., 1997) and microhabitat (Johnson and Bond, 1992; O'Connel and Johnston, 1998).
In M. christinae, however, no relationship was observed between the area occupied by a population and its degree of geographic isolation, and the population's fruit production (Malo et al., 2001; Naval, 2002). Similarly, microhabitat is a factor that does not affect plant accessibility to pollinators in M. christinae (Ortegón, 2000), in contrast to the observed effect of surrounding vegetation at an individual level in C. acaule (O'Connel and Johnston, 1998). Neither is there a clear relationship between floral display and pollination success for M. christinae, and at least within a reproductive season there appears to be no resource limitations (Ortegón, 2000).
Changes in flower density between populations and from year to year probably explain, at least partially, the absence of a consistent pattern of selection events acting on flowering phenology in M. christinae. In other deceit-pollinated orchid species it has been observed that pollination rates are lower in large populations with high flower density, perhaps because of a decrease in the success of the deceit strategy (Fritz and Nilsson, 1994, 1996; Sabat and Ackerman, 1996). Worth note in the present study is that the M. christinae population with the highest flower density (Telchac) had the greatest number of significant selection gradients, though this was the population with the largest sample size.
Another non-exclusive explanation of the differences in selection regimes between sites and years could be variation in M. christinae pollinator abundance. Recent experiments in pollinator limitations during a reproductive season in 12 populations (including Telchac, Mr Bú and Nuevo Yucatán) have confirmed not only that this species is strongly limited by pollinators (Rico-Gray and Thien, 1987), but also that the degree of limitation within a single season is equal between populations (Naval, 2002). The pollinarium removal and rate of flower pollination observed in this study, suggests that, as in other orchid species (e.g. Ackerman 1981; O'Connel and Johnston, 1998; Parra-Tabla et al., 2000), M. christinae pollinator abundance is low and varies greatly between years. It is evident, that low pollinator availability is a factor that can favour selection events acting on flowering phenology (Fritz and Nilsson, 1996; Ackerman et al., 1997; O'Connel and Johnston, 1998).
Thus, negative frequency-dependent selection events can be predicted for deceit-pollinated species, be it through a direct pollinator learning response and/or variations in pollinator abundance (Sabat and Ackerman, 1996; Ackerman et al., 1997; Smithson and Macnair, 1997; Ferdy et al., 1998; Maad, 2000). It is probably in this way that negative frequency-dependent selection cyclically modifies the phenological pattern of flowering in deceit-pollinated species.
In conclusion, the results for M. christinae reported here and those previously reported for other deceit-pollinated orchids, suggest that natural selection plays an important role in shaping flowering phenology in these species. Nonetheless, because the effect of natural selection on flowering phenology appears to depend on factors such as density of reproductive individuals and pollinator abundances, it is quite likely that fluctuating selective regimes exist within the distribution ranges of these species.
Supplementary Material
Acknowledgments
We thank James Ackerman, Conchita Alonso, Azucena Canto, Cesar Domínguez, Luis Eguiarte, Leandro Freitas, Peter Feinsinger, Carlos Herrera, Don Levin, Mónica Medrano and two anonymous reviewers for useful comments on the manuscript. Jorge Leirana and Miguel Carbajal helped us during data collections. Financial support was provided by CONACyT (3076P-N9608), and Secretaría de Estado de Educación y Universidades through a fellowship for a sabbatical year to V.P.-T. (SAB2002-0109). Special thanks are due to Pit Feinsinger for his advice and encouragement.
LITERATURE CITED
- Ackerman JD. 1981. Pollination biology of Calypso bulbosa var. occidentalis (Orchidaceae): a food-deception system. Madroño 28: 101–110. [Google Scholar]
- Ackerman JD. 1986. Mechanisms and evolution of food-deceptive pollination systems in orchids. Lindleyana 1: 108–113. [Google Scholar]
- Ackerman JD. 1989. Limitations to sexual reproduction in Encyclia krugii (Orchidaceae). Systematic Botany 14: 101–109. [Google Scholar]
- Ackerman JD, Meléndez-Ackerman EJ, Salguero-Faria J. 1997. Variation in pollinator abundance and selection on fragrance phenotypes in a epiphytic orchid. American Journal of Botany 84: 1383–1390. [PubMed] [Google Scholar]
- Aizen MA, Feinsinger P. 1994. Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 75: 330–351. [Google Scholar]
- Aragón, S, Ackerman JD. 2004. Does flower color variation matter in deception pollinated Psychilis monensis (Orchidaceae)?. Oecologia 138: 405–413. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection: applications. Evolution 38: 720–734. [DOI] [PubMed] [Google Scholar]
- Augspurger CK. 1981. Reproductive synchrony of a tropical shrub: experimental studies of on effects of pollinators and seed predators on Hybanthus prunifolius (Violaceae). Ecology 62: 775–788. [Google Scholar]
- Calvo RN. 1993. Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74: 1033–1042. [Google Scholar]
- Carnevali FCG, Tapia-Muñoz L, Jiménez-Machorro R, Sánchez-Saldaña L, Ibarra-González L, Ramírez IN, Gómez MP. 2001. Notes on the flora of the Yucatán Península. II. A synopsis of the orchid flora of the Mexican Yucatán Península and a tentative checklist of the Orchidaceae of the Yucatán Península biotic province. Harvard Papers in Botany 5: 383–466. [Google Scholar]
- Castillo RA, Cordero C Domínguez CA. 2002. Are reward polymorphisms subject to frequency-and density-dependent selection? Evidence from a monoecious species pollinated by deceit. Journal of Evolutionary Biology 15: 544–552. [Google Scholar]
- Dieringer G. 1991. Variation in the individual flowering time and reproductive success of Agalinis strictifolia (Scrophulariaceae). American Journal of Botany 778: 497–503. [Google Scholar]
- Dodson CH, Frymire GP. 1961. Natural pollination of orchids. Missouri Botanical Garden Bulletin 49: 133–152. [Google Scholar]
- Domínguez CA, Dirzo R. 1995. Rainfall and flowering synchrony in a tropical shrub: variable selection on the flowering time of Erythroxylum havanense Evolutionary Ecology 9: 204–216. [Google Scholar]
- Endler JA. 1986.Natural selection in the wild. Princeton, NJ: Princeton University Press. [Google Scholar]
- Ferdy JB, Gouyon PH, Moret J, Godelle B. 1998. Pollinator behavior and deceptive pollination: learning process and floral evolution. American Naturalist 152: 698–705. [DOI] [PubMed] [Google Scholar]
- Flores GS, Espejel I. 1994.Tipos de vegetación de la Península de Yucatán. Universidad Autónoma de Yucatán, Sostenibilidad Maya, Mérida Yucatán. [Google Scholar]
- Fritz AL. 1990. Deceit pollination of Orchis spitzelii (Orchidaceae) on the Island of Gotland in the Baltic: a suboptimal system. Nordic Journal of Botany 9: 577–587. [Google Scholar]
- Fritz AL, Nilsson LA. 1994. How pollinator-mediated mating varies with population size in plants. Oecologia 100: 451–462. [DOI] [PubMed] [Google Scholar]
- Fritz AL, Nilsson LA. 1996. Reproductive success and gender variation in deceit-pollinated orchids. In: Lloyd DG, Barrett SCH, eds. Floral biology. Studies on floral evolution in animal-pollinated plants, New York: Chapman and Hall, 319–338. [Google Scholar]
- Gigord LDB, Macnair RM, Smithson A. 2001. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soò. Proceedings of the National Acadamy of Sciences 98: 6253–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez JM. 1993. Phenotypic selection on flowering synchrony in a high mountain plant, Hormathophylla spinosa (Cruciferae). Journal of Ecology 81: 605–613. [Google Scholar]
- Groom MJ. 1998. Alle effects limit population viability of an annual plant. American Naturalist 151: 487–496. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Bond WJ. 1992. Habitat dependent pollination success in a cape orchid. Oecologia 91: 455–457. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. 1998. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). American Journal of Botany 85: 402–411. [PubMed] [Google Scholar]
- Johnston MO. 1991. Natural selection on floral traits in two species of Lobelia with different pollinators. Evolution 45: 1468–1479. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold R. 1983. The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- Maad J. 2000. Phenotypic selection in hawk moth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution 54: 112–123. [DOI] [PubMed] [Google Scholar]
- Malo J, Leirana-Alcocer J, Parra-Tabla V. 2001. Population fragmentation, florivory and the effects of flower morphology alterations on the population success of Myrmecophila christinae (Orchidaceae). Biotropica 33: 529–534. [Google Scholar]
- Mitchell-Olds T, Shaw RG. 1987. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41: 1149–1161. [DOI] [PubMed] [Google Scholar]
- Naval C. 2002.Efecto de la fragmentación del hábitat en poblaciones de Myrmecophila christinae (Orchidaceae) en la zona costera del estado de Yucatán. MSc Thesis, UADY, México. [Google Scholar]
- Nilsson LA. 1992. Orchid pollination biology. Trends in Ecology and Evolution 7: 255–259. [DOI] [PubMed] [Google Scholar]
- Nilsson LA, Rabakonandrianina E, Pettersson B. 1992. Exact tracking of pollen transfer and mating in plants. Nature 360: 666–667. [Google Scholar]
- O'Connel LM, Johnston MO. 1998. Male and female pollination success in a deceptive orchid, a selection study. Ecology 79: 1246–1260. [Google Scholar]
- Ollerton J, Díaz A. 1995. Evidence for stabilising acting on flowering time in Arum maculatum (Araceae): the influence of phylogeny on adaptation. Oecologia 119: 340–348. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Lack AJ. 1992. Flowering phenology: and example of relaxation of natural selection. Trends in Ecology and Evolution 7: 274–277. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Lack AJ. 1998. Relationships between flowering phenology, plant size and reproductive success in Lotus corniculatus (Fabaceae). Plant Ecology 139: 35–47. [Google Scholar]
- O'Neil P. 1997. Natural selection on genetically correlated phenological characters in Lythrum salicaria L. (Lyrthraceae). Evolution 51: 267–274. [DOI] [PubMed] [Google Scholar]
- Ortegón I. 2000.Crecimiento, esfuerzo reproductivo y limitación por polinizadores en la producción de frutos de Myrmecophila christinae (Orchidaceae) en dos ambientes contrastantes de la costa del estado de Yucatán. BSc Thesis, UADY, México. [Google Scholar]
- Parra-Tabla V, Vargas MF, Magaña-Rueda S, Navarro J. 2000. Female and male pollination success of Oncidium ascendens (Orchidaceae) in two contrasting patches: forest vs. agricultural field. Biological Conservation 94: 335–340. [Google Scholar]
- Primack RB, Hall P. 1990. Cost of reproduction in the pink lady's slipper orchid: a four-year experimental study. American Naturalist 136: 638–656. [PubMed] [Google Scholar]
- Ratchke B. 1983. Competition and facilitation among plants for pollinators. In: Real L. ed. Pollination biology, New York: Academic Press, 309–329. [Google Scholar]
- Ratchke B, Lacey EP. 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. [Google Scholar]
- Rice WR. 1988. Analyzing tables of statistical tests. Evolution 43: 223–225. [DOI] [PubMed] [Google Scholar]
- Rico-Gray V, Thien LB. 1987. Some aspects of the reproductive biology of Schomburgkia tibicinis Batem. (Orchidaceae) in Yucatán, México. Brenesia 28: 13–24. [Google Scholar]
- Rico-Gray V, Thien LB. 1989. Effect of different ant species on reproductive fitness of Schomburgkia christinae Batem. (Orchidaceae). in Yucatán, México. Oecologia 81: 487–489. [DOI] [PubMed] [Google Scholar]
- Sabat A, Ackerman D. 1996. Fruit set in a deceptive orchid: the effect of flowering phenology, display size, and local floral abundance. American Journal of Botany 83: 1181–1186. [Google Scholar]
- Smithson A, Macnair MR. 1997. Negative frequency-dependent selection by pollinators on artificial flowers without rewards. Evolution 51: 715–723. [DOI] [PubMed] [Google Scholar]
- SYSTAT. 1997.Systat 7.0 for Windows: New Statistics. Chicago, Evanston, IL: SPSS. [Google Scholar]
- Thomson J D. 1980. Skewed flowering distributions and pollinator attraction. Ecology 6: 572–579. [Google Scholar]
- Widén B. 1991. Phenotypic selection on flowering phenology in Senecio integrifolius, a perennial herb. Oikos 61: 205–215. [Google Scholar]
- Zar JH. 1984.Biostatistical analysis. Englewood Clifs, NJ: Prentice Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


