Abstract
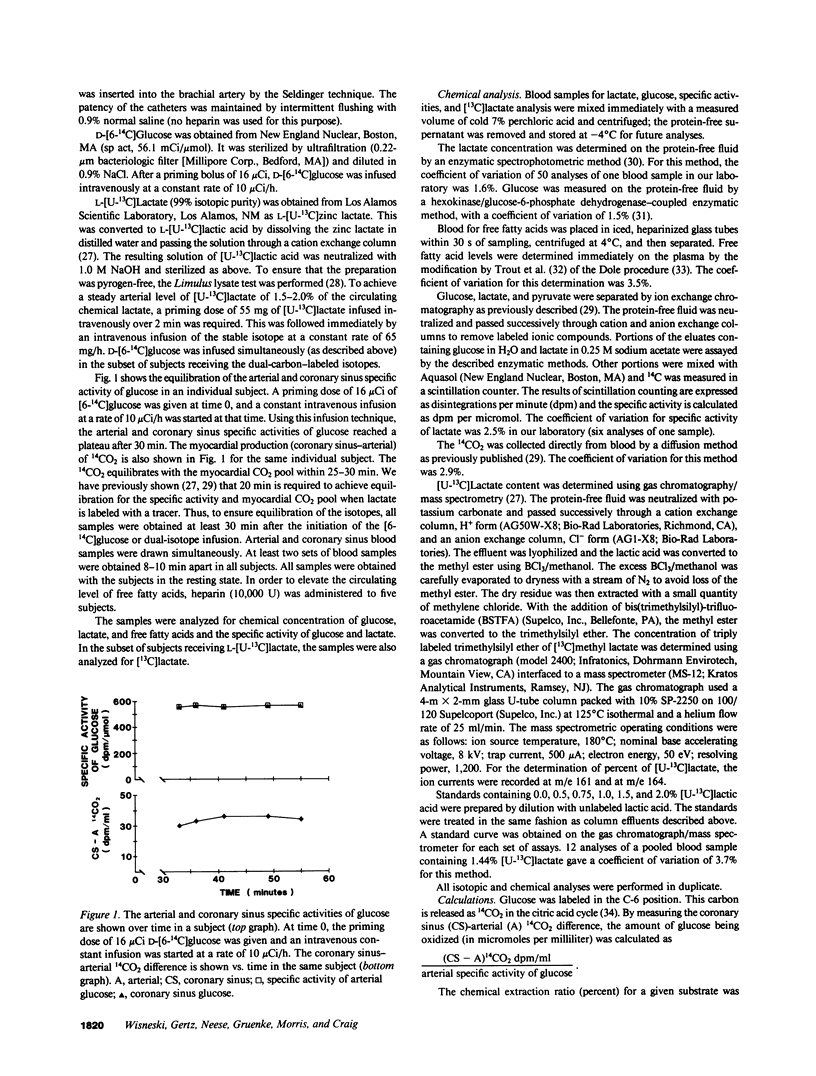
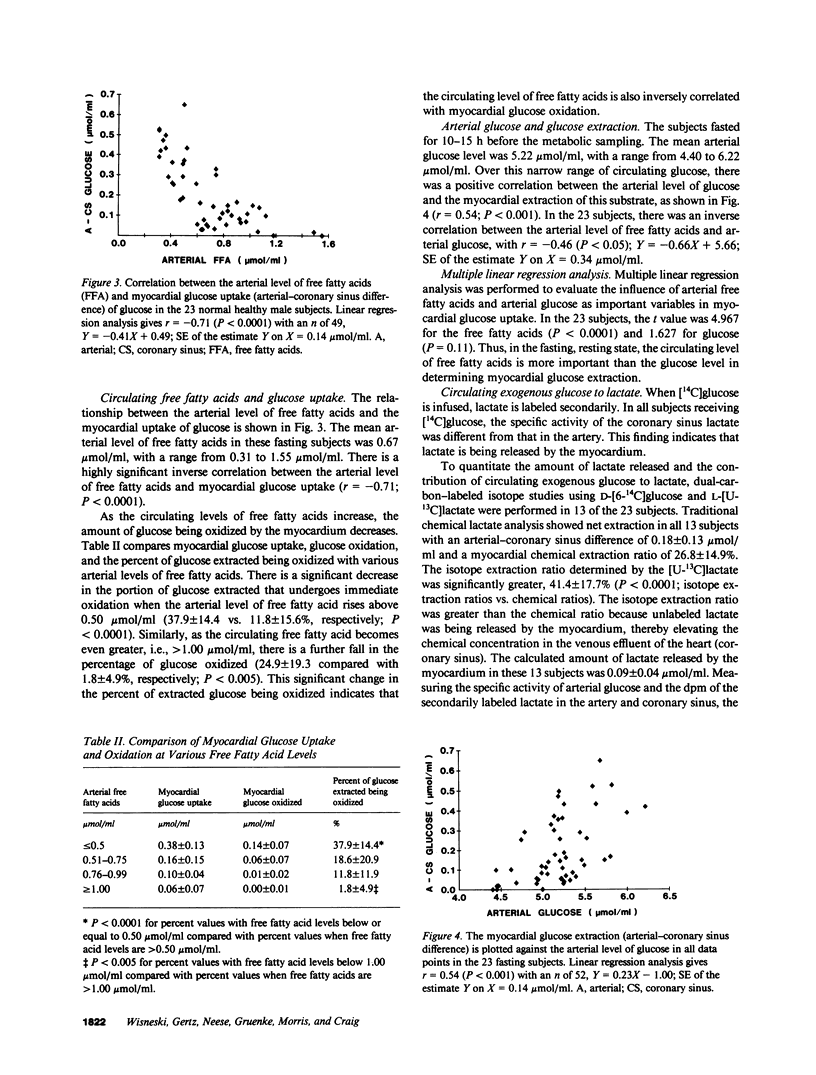
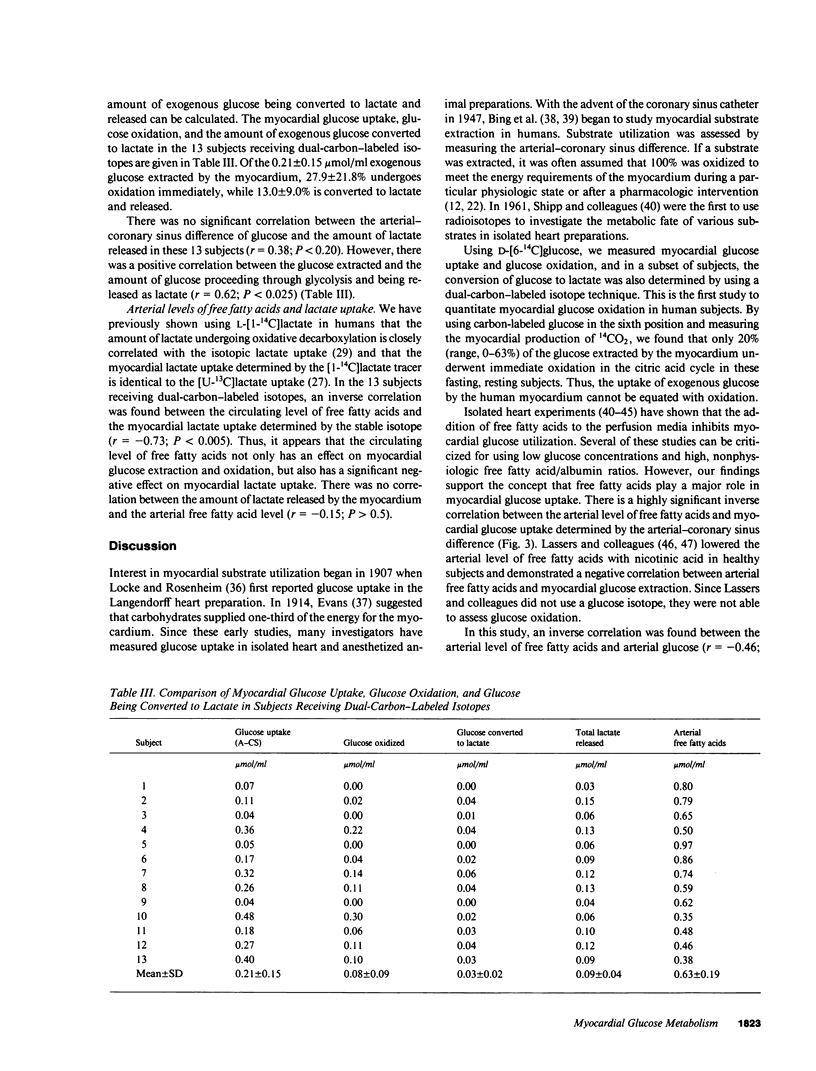
Glucose is an important substrate for myocardial metabolism. This study was designed to determine the effect of circulating metabolic substrates on myocardial glucose extraction and to determine the metabolic fate of glucose in normal human myocardium. Coronary sinus and arterial catheters were placed in 23 healthy male volunteers. [6-14C]Glucose was infused as a tracer in 10 subjects. [6-14C]Glucose and [U-13C]lactate were simultaneously infused in the other 13 subjects. Simultaneous blood samples were obtained for chemical analyses of glucose, lactate, and free fatty acids and for the the isotopic analyses of glucose and lactate. Glucose oxidation was assessed by measuring myocardial 14CO2 production. The amount of glucose extracted and oxidized by the myocardium was inversely correlated with the arterial level of free fatty acids (r = -0.71; P less than 0.0001). 20% (range, 0-63%) of the glucose extraction underwent immediate oxidation. Chemical lactate analysis showed a net extraction of 26.0 +/- 16.4%. However, isotopic analysis demonstrated that lactate was being released by the myocardium. In the 13 subjects receiving the dual-carbon-labeled isotopes, the lactate released was 0.09 +/- 0.04 mumol/ml and 49.5 +/- 29.5% of this lactate was from exogenous glucose. This study demonstrates that the circulating level of free fatty acids plays a major role in determining the amount of glucose extracted and oxidized by the normal human myocardium. Only 20.1 +/- 19.4% of the glucose extracted underwent oxidation, and 13.0 +/- 9.0% of the glucose extracted was metabolized to lactate and released by the myocardium. Thus, 60-70% of the glucose extracted by the normal myocardium is probably stored as glycogen in the fasting, resting state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. L., Morris R. G. Role of glycolysis in the relaxation process in mammalian cardiac muscle: comparison of the influence of glucose and 2-deoxyglucose on maintenance of resting tension. Life Sci. 1978 Jul 3;23(1):23–31. doi: 10.1016/0024-3205(78)90320-x. [DOI] [PubMed] [Google Scholar]
- Apstein C. S., Gravino F. N., Haudenschild C. C. Determinants of a protective effect of glucose and insulin on the ischemic myocardium. Effects on contractile function, diastolic compliance, metabolism, and ultrastructure during ischemia and reperfusion. Circ Res. 1983 May;52(5):515–526. doi: 10.1161/01.res.52.5.515. [DOI] [PubMed] [Google Scholar]
- BARTHELMAI W., CZOK R. [Enzymatic determinations of glucose in the blood, cerebrospinal fluid and urine]. Klin Wochenschr. 1962 Jun 1;40:585–589. doi: 10.1007/BF01478633. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. REGULATION OF CORONARY BLOOD FLOW. Physiol Rev. 1964 Jan;44:1–29. doi: 10.1152/physrev.1964.44.1.1. [DOI] [PubMed] [Google Scholar]
- BING R. J., SIEGEL A., VITALE A., BALBONI F., SPARKS E., TAESCHLER M., KLAPPER M., EDWARDS S. Metabolic studies on the human heart in vivo. I. Studies on carbohydrate metabolism of the human heart. Am J Med. 1953 Sep;15(3):284–296. doi: 10.1016/0002-9343(53)90082-5. [DOI] [PubMed] [Google Scholar]
- Bruce R. A., Hornsten T. R. Exercise stress testing in evaluation of patients with ischemic heart disease. Prog Cardiovasc Dis. 1969 Mar;11(5):371–390. doi: 10.1016/0033-0620(69)90027-9. [DOI] [PubMed] [Google Scholar]
- Chiong M. A., West R., Parker J. O. The protective effect of glucose-insulin-potassium on the response to atrial pacing. Circulation. 1976 Jul;54(1):37–46. doi: 10.1161/01.cir.54.1.37. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entam M. L., Kanike K., Goldstein M. A., Nelson T. E., Bornet E. P., Futch T. W., Schwartz A. Association of gylcogenolysis with cardiac sarcoplasmic reticulum. J Biol Chem. 1976 May 25;251(10):3140–3146. [PubMed] [Google Scholar]
- Evans C. L. The effect of glucose on the gaseous metabolism of the isolated mammalian heart. J Physiol. 1914 Feb 27;47(6):407–418. doi: 10.1113/jphysiol.1914.sp001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti H. L., Carroll R. J., Marcus M. L. Temporal heterogeneity of myocardial blood flow in anesthetized dogs. Circulation. 1975 Nov;52(5):848–853. doi: 10.1161/01.cir.52.5.848. [DOI] [PubMed] [Google Scholar]
- Fanburg B. L. Experimental cardiac hypertrophy. N Engl J Med. 1970 Mar 26;282(13):723–732. doi: 10.1056/NEJM197003262821306. [DOI] [PubMed] [Google Scholar]
- Fletcher W. M. Lactic acid in amphibian muscle. J Physiol. 1907 Mar 27;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz E. W., Wisneski J. A., Neese R., Bristow J. D., Searle G. L., Hanlon J. T. Myocardial lactate metabolism: evidence of lactate release during net chemical extraction in man. Circulation. 1981 Jun;63(6):1273–1279. doi: 10.1161/01.cir.63.6.1273. [DOI] [PubMed] [Google Scholar]
- Griggs D. M., Jr, Nagano S., Lipana J. G., Novack P. Myocardial lactate oxidation in situ and the effect thereon of reduced coronary flow. Am J Physiol. 1966 Aug;211(2):335–340. doi: 10.1152/ajplegacy.1966.211.2.335. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Abiko Y. Difference between endocardial and epicardial utilization of glycogen in the ischemic heart. Am J Physiol. 1975 Dec;229(6):1585–1589. doi: 10.1152/ajplegacy.1975.229.6.1585. [DOI] [PubMed] [Google Scholar]
- JEDEIKIN L. A. REGIONAL DISTRIBUTION OF GLYCOGEN AND PHOSPHORYLASE IN THE VENTRICLES OF THE HEART. Circ Res. 1964 Mar;14:202–211. doi: 10.1161/01.res.14.3.202. [DOI] [PubMed] [Google Scholar]
- JOLLEY R. L., CHELDELIN V. H., NEWBURGH R. W. Glucose catabolism in fetal and adult heart. J Biol Chem. 1958 Dec;233(6):1289–1294. [PubMed] [Google Scholar]
- Klocke F. J. Coronary blood flow in man. Prog Cardiovasc Dis. 1976 Sep-Oct;19(2):117–166. doi: 10.1016/0033-0620(76)90020-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Neely J. R. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res. 1979 Feb;44(2):166–175. doi: 10.1161/01.res.44.2.166. [DOI] [PubMed] [Google Scholar]
- Lassers B. W., Kaijser L., Carlson L. A. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3 H-palmitate. Eur J Clin Invest. 1972 Aug;2(5):348–358. doi: 10.1111/j.1365-2362.1972.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Lassers B. W., Kaijser L., Wahlqvist M. L., Carlson L. A. Relationship in man between plasma free fatty acids and myocardial metabolism of carbohydrate substrates. Lancet. 1971 Aug 28;2(7722):448–450. doi: 10.1016/s0140-6736(71)92624-9. [DOI] [PubMed] [Google Scholar]
- Lesch M., Teichholz L. E., Soeldner J. S., Gorlin R. Ineffectiveness of glucose, potassium, and insulin infusion during pacing stress in chronic ischemic heart disease. Circulation. 1974 Jun;49(6):1028–1037. doi: 10.1161/01.cir.49.6.1028. [DOI] [PubMed] [Google Scholar]
- Leunissen R. L., Piatnek-Leunissen D. A. Myocardial lactate oxidation and release in the dog in vivo. Pflugers Arch. 1973 Nov 28;344(3):261–270. doi: 10.1007/BF00588465. [DOI] [PubMed] [Google Scholar]
- Locke F. S., Rosenheim O. Contributions to the physiology of the isolated heart: The consumption of dextrose by mammalian cardiac muscle. J Physiol. 1907 Dec 31;36(4-5):205–220. doi: 10.1113/jphysiol.1907.sp001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundsgaard-hansen P., Meyer C., Riedwyl H. Transmural gradients of glycolytic enzyme activities in left ventricular myocardium. I. The normal state. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;297(2):89–106. doi: 10.1007/BF00363632. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Sobel B. E., Bloor C. M., Sybers H. D., Shell W. E., Covell J. W., Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972 Jun;45(6):1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- Marshall R. C., Huang S. C., Nash W. W., Phelps M. E. Assessment of the [18F]fluorodeoxyglucose kinetic model in calculations of myocardial glucose metabolism during ischemia. J Nucl Med. 1983 Nov;24(11):1060–1064. [PubMed] [Google Scholar]
- Marshall R. C., Tillisch J. H., Phelps M. E., Huang S. C., Carson R., Henze E., Schelbert H. R. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation. 1983 Apr;67(4):766–778. doi: 10.1161/01.cir.67.4.766. [DOI] [PubMed] [Google Scholar]
- Mathey D. G., Chatterjee K., Tyberg J. V., Lekven J., Brundage B., Parmley W. W. Coronary sinus reflux. A source of error in the measurement of thermodilution coronary sinus flow. Circulation. 1978 Apr;57(4):778–786. doi: 10.1161/01.cir.57.4.778. [DOI] [PubMed] [Google Scholar]
- Mudge G. H., Jr, Goldberg S., Gunther S., Mann T., Grossman W. Comparison of metabolic and vasoconstrictor stimuli on coronary vascular resistance in man. Circulation. 1979 Mar;59(3):544–550. doi: 10.1161/01.cir.59.3.544. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Oram J. F. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972 Nov-Dec;15(3):289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- Neese R. A., Gertz E. W., Wisneski J. A., Gruenke L. D., Craig J. C. A stable isotope technique for investigating lactate metabolism in humans. Biomed Mass Spectrom. 1983 Aug;10(8):458–462. doi: 10.1002/bms.1200100804. [DOI] [PubMed] [Google Scholar]
- OPIE L. H., EVANS J. R., SHIPP J. C. EFFECT OF FASTING ON GLUCOSE AND PALMITATE METABOLISM OF PERFUSED RAT HEART. Am J Physiol. 1963 Dec;205:1203–1208. doi: 10.1152/ajplegacy.1963.205.6.1203. [DOI] [PubMed] [Google Scholar]
- OPIE L. H., SHIPP J. C., EVANS J. R., LEBOEUF B. Metabolism of glucose-U-C14 in perfused rat heart. Am J Physiol. 1962 Nov;203:839–843. doi: 10.1152/ajplegacy.1962.203.5.839. [DOI] [PubMed] [Google Scholar]
- Oliver M. F. Metabolic response during impending myocardial infarction. II. Clinical implications. Circulation. 1972 Feb;45(2):491–500. doi: 10.1161/01.cir.45.2.491. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Effects of regional ischemia on metabolism of glucose and fatty acids. Relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circ Res. 1976 May;38(5 Suppl 1):I52–I74. [PubMed] [Google Scholar]
- Opie L. H., Mansford K. R., Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971 Sep;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H. Metabolic response during impending myocardial infarction. I. Relevance of studies of glucose and fatty acid metabolism in animals. Circulation. 1972 Feb;45(2):483–490. doi: 10.1161/01.cir.45.2.483. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Metabolism of the heart in health and disease. I. Am Heart J. 1968 Nov;76(5):685–698. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Owen P., Riemersma R. A. Relative rates of oxidation of glucose and free fatty acids by ischaemic and non-ischaemic myocardium after coronary artery ligation in the dog. Eur J Clin Invest. 1973 Sep;3(5):419–435. doi: 10.1111/j.1365-2362.1973.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Orlick A. E., Ricci D. R., Alderman E. L., Stinson E. B., Harrison D. C. Effects of alpha adrenergic blockade upon coronary hemodynamics. J Clin Invest. 1978 Aug;62(2):459–467. doi: 10.1172/JCI109147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps M. E., Hoffman E. J., Selin C., Huang S. C., Robinson G., MacDonald N., Schelbert H., Kuhl D. E. Investigation of [18F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med. 1978 Dec;19(12):1311–1319. [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rackley C. E., Russell R. O., Jr, Rogers W. J., Mantle J. A., McDaniel H. G., Papapietro S. E. Clinical experience with glucose-insulin-potassium therapy in acute myocardial infarction. Am Heart J. 1981 Dec;102(6 Pt 1):1038–1049. doi: 10.1016/0002-8703(81)90488-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold R. B., Fine J. A technique for quantitative measurement of endotoxin in human plasma. Proc Soc Exp Biol Med. 1971 May;137(1):334–340. doi: 10.3181/00379727-137-35572. [DOI] [PubMed] [Google Scholar]
- Rogers W. J., Russell R. O., Jr, McDaniel H. G., Rackley C. E. Acute effects of glucose-insulin-potassium infusion on myocardial substrates, coronary blood flow and oxygen consumption in man. Am J Cardiol. 1977 Sep;40(3):421–428. doi: 10.1016/0002-9149(77)90166-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. D., Warshaw J. B. Interaction of fatty acid and glucose oxidation by cultured heart cells. J Cell Biol. 1973 Aug;58(2):332–339. doi: 10.1083/jcb.58.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau J., Boerboom L. E., Surjadhana A., Hoffman J. I. The role of autoregulation and tissue diastolic pressures in the transmural distribution of left ventricular blood flow in anesthetized dogs. Circ Res. 1979 Dec;45(6):804–815. doi: 10.1161/01.res.45.6.804. [DOI] [PubMed] [Google Scholar]
- Rovetto M. J., Whitmer J. T., Neely J. R. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ Res. 1973 Jun;32(6):699–711. doi: 10.1161/01.res.32.6.699. [DOI] [PubMed] [Google Scholar]
- SHIPP J. C., DELCHER H. K., CREVASSE L. E. GLUCOSE METABOLISM BY THE HEXOSE MONOPHOSPHATE PATHWAY IN THE PERFUSED RAT HEART. Biochim Biophys Acta. 1964 May 11;86:399–402. doi: 10.1016/0304-4165(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Schelbert H. R., Henze E., Phelps M. E., Kuhl D. E. Assessment of regional myocardial ischemia by positron-emission computed tomography. Am Heart J. 1982 Apr;103(4 Pt 2):588–597. doi: 10.1016/0002-8703(82)90462-8. [DOI] [PubMed] [Google Scholar]
- Semple P. F., Morton J. J. Angiotensin II and angiotensin III in rat blood. Circ Res. 1976 Jun;38(6 Suppl 2):122–126. doi: 10.1161/01.res.38.6.122. [DOI] [PubMed] [Google Scholar]
- Sheffield L. T., Roitman D. Stress testing methodology. Prog Cardiovasc Dis. 1976 Jul-Aug;19(1):33–49. doi: 10.1016/0033-0620(76)90007-4. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- Wahlqvist M. L., Kaijser L., Lassers B. W., Löw H., Carlson L. A. The role of fatty acid and of hormones in the determination of myocardial carbohydrate metabolism in healthy fasting men. Eur J Clin Invest. 1973 Jan;3(1):57–65. doi: 10.1111/j.1365-2362.1973.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Weiss J., Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985 Feb;75(2):436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow P. L., Rogers W. J., Smith L. R., McDaniel H. G., Papapietro S. E., Mantle J. A., Logic J. R., Russell R. O., Jr, Rackley C. E. Enhancement of left ventricular function by glucose-insulin-potassium infusion in acute myocardial infarction. Am J Cardiol. 1982 Mar;49(4):811–820. doi: 10.1016/0002-9149(82)91963-4. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]
- Wisneski J. A., Gertz E. W., Neese R., Soo W. J., Bristow J. D., Adams J. R., Beaudry J. P. Myocardial metabolic alterations after contrast angiography. Am J Cardiol. 1982 Aug;50(2):239–245. doi: 10.1016/0002-9149(82)90172-2. [DOI] [PubMed] [Google Scholar]



