Abstract
• Background and Aims The aim of this study was to investigate the effects of the interactions between the microbial symbionts, Rhizobium and arbuscular mycorrhizal fungi (AMF) on N and P accumulation by broad bean (Vicia faba) and how increased N and P content influence biomass production, leaf area and net photosynthetic rate.
• Methods A multi-factorial experiment consisting of four different legume–microbial symbiotic associations and two nitrogen treatments was used to investigate the influence of the different microbial symbiotic associations on P accumulation, total N accumulation, biomass, leaf area and net photosynthesis in broad bean grown under low P conditions.
• Key Results AMF promoted biomass production and photosynthetic rates by increasing the ratio of P to N accumulation. An increase in P was consistently associated with an increase in N accumulation and N productivity, expressed in terms of biomass and leaf area. Photosynthetic N use efficiency, irrespective of the inorganic source of N (e.g. NO3− or N2), was enhanced by increased P supply due to AMF. The presence of Rhizobium resulted in a significant decline in AMF colonization levels irrespective of N supply. Without Rhizobium, AMF colonization levels were higher in low N treatments. Presence or absence of AMF did not have a significant effect on nodule mass but high N with or without AMF led to a significant decline in nodule biomass. Plants with the Rhizobium and AMF symbiotic associations had higher photosynthetic rates per unit leaf area.
• Conclusions The results indicated that the synergistic or additive interactions among the components of the tripartite symbiotic association (Rhizobium–AMF–broad bean) increased plant productivity.
Key words: Arbuscular mycorrhizal fungi (AMF), nitrogen, phosphorus, Rhizobium, Vicia faba
INTRODUCTION
Under low soil P concentrations, most plant species are dependent on a symbiotic association with arbuscular mycorrhizal fungi (AMF) for the acquisition of P (Smith and Read, 1997). Under low N fertilizer inputs, soil P availability is usually the major factor limiting the rate of N2-fixation in legume crops (Toro et al., 1998) and, in the absence of AMF infection, supplementary P fertilization is generally necessary for the maintenance of N2-fixation rates by Rhizobium at the levels required for economically viable crop production (Andrade et al., 1998). In legumes the positive synergistic interactions among the members of the tripartite symbiotic association (Rhizobium–AMF–legume) result in improved rates of P uptake N2-fixation and crop biomass production under conditions of reduced N and P fertilizer inputs (Azcón et al., 1991; Xavier and Germida, 2002, 2003). However, there is little information on the influence of P on N productivity or photosynthetic N use efficiency. Nitrogen productivity has been defined as the rate of biomass production per unit biomass N content (Ågren, 1985), whereas the photosynthetic N use efficiency is the amount of CO2 fixed (mol CO2 m−2 s−1) per unit biomass N content. In general, photosynthetic and specific growth rates increase with increasing plant tissue N concentration or N supply in a curvilinear fashion (Hirose and Werger, 1987; Sinclair and Horie, 1989; Jia and Gray, 2004).
This study tests the hypothesis that the synergistic interactions among the members of the tripartite symbiotic association improve legume productivity through positive effects on the rates of photosynthetic CO2 assimilation, N2-fixation and P uptake. Furthermore, it is proposed that these three processes are interdependent or even tightly coupled. For example, the rate of photosynthetic CO2 assimilation is influenced by the rates of N and P supply, and the rate of N2-fixation is influenced by the rates of photosynthate and P supply to the nodules. Thus, the main objectives of this study were to investigate: (a) how the interactions between the microbial symbionts, Rhizobium and AMF affected the rate of N and P accumulation in broad bean; (b) how N productivity, irrespective of the inorganic source of N (e.g. NO3− or N2) was affected by increased P supply due to AMF; and (c) how photosynthetic N use efficiency, irrespective of the inorganic source of N (e.g. NO3− or N2), was affected by increased P supply due to AMF colonization.
MATERIALS AND METHODS
Plant material and growth conditions
The cold-hardy Vicia faba L. ‘Aquadulce Claudia’ (Straathof Seed Group), requiring 130–150 d for crop development, was used. Outdoor pot trials were carried out in spring from August to October (mean daily extraterrestrial global insolation 24·4–39·1 MJ m−2; mean day length 10·78–12·97 h; mean daily maximum temperature 19·3–23·8 °C; mean daily minimum temperature 6·4–11·3 °C and mean A-pan evaporation 8·0–6·4 mm d−1). Initially three seeds were planted per pot in sand in 1·6-L pots. The sand (2·5 mm sieve mesh) used in all the treatments was first thoroughly washed with running tap water and then autoclaved for 3 h at 121 °C and 103·4 kPa in metal buckets. The initial pH of the sand was 7·0. After germination and shoot emergence, one seedling of uniform size per pot was selected and the others removed.
Experimental design
The experimental design was a randomized complete block with four replications for each harvest interval, with combinations of treatment factors randomly assigned to pots in the block. The concentration of nitrate-N for the low N (LN) treatments was 0·71 mm KNO3 (10 ppm N) and 17·86 mm KNO3 (250 ppm N) for the high N treatments (HN). All treatments received a phosphorus supply of 0·05 mg P L−1 (1·61 µm NaH2PO4). The various treatments were: (a) two different N supply rates without any microbial symbiotic associations, LN and HN; (b) two different N supply rates plus AMF association, LNM and HNM; (c) two different N supply rates plus Rhizobium association, LNR and HNR; and (d) two different N supply rates plus both AMF and Rhizobium symbiotic associations: LNMR and HNMR. Plants were harvested at 54 d (4 October, T1) and 63 d (13 October, T2) after planting.
Nutrients
A modified Long Ashton nutrient mixture was used to supply the non-nitrogen micro- and macro-nutrients. For the micronutrients 100× stock solutions were made up as follows: NaH2PO4 20·8 g L−1, MgSO4·7H2O 36·9 g L−1, MnSO4·H2O 0·223 g L−1, CuSO4·5H2O 0·024 g L−1, ZnSO4·7H2O 0·029 g L−1, H3BO3 0186 g L−1, (NH4)6Mo7·O244H2O 0·004 g L−1, CoSO4·7H2O 0·003 g L−1, NaCl 0·585 g L−1. For the macro-nutrients, 100× stock solutions were made up as follows: CaCl2 50·0 g L−1, K2SO4 21·75 g L−1, FeEDTA 3 g L−1. After adding the appropriate quantity of KNO3 (HN or LN), 10 mL of the stock solutions were added. The pH of the nutrient solutions was then adjusted to pH 7·0 and the solution was made up to 1000 mL with distilled water before being applied to the pots. Because of the low water-holding capacity of the sand-filled pots, it was necessary to water plants every 2 d with 150 mL of the modified Long Ashton nutrient solution. Inclusion of 0·218 g L−1 of K2SO4 in all treatments meant that potassium supply, while abundant for the high nitrogen treatments was not limiting in the low nitrogen treatments.
AMF inocula
For AMF infection, the mycorrhizal inoculum consisted of the roots of an Eragrostis sp. from the grassland close to the university campus. The pH of the grassland soil was 7·0. The inoculum was prepared by homogenizing and mixing surrounding soil with the Eragrostis sp. root systems, forming a mixed inoculum in terms of species and propagules. The inoculum was applied as a 1 cm layer to the surface of the soil, and covered with 1 cm layer of sand. Non-mycorrhizal treatments received inoculum that had been autoclaved at 121 °C for 20 min. No colonization of non-myorrhizal treatments by external sources of AMF spores was detected during the course of the experiment.
Rhizobium inocula
The original Rhizobium leguminosarum bv. viciae inoculum was obtained from the active nodules of plants of Vicia faba L. that had been infected previously with this strain (STIMUPLANT CC: Reg. No. CK89/04756/23; PO Box 11446, Brooklyn, South Africa 0011). Rhizobium colonies were maintained on solid culture medium consisting of 0·5 g K2HPO4, 0·2 g MgSO4·7H2O, 0·1 g NaCl, 10 g mannitol, 0·4 g yeast extract, 8 g agar and 0·25 % Congo Red, dissolved in 1000 mL distilled water and autoclaved at 121 °C for 20 min. Rhizobium inoculum was prepared by culturing selected colonies in liquid culture medium. The cultures were grown in 250 mL Erlenmeyer flasks and incubated at 25 °C until the bacterial cell optical density measured 0·6 at 600 nm (which corresponded to approx. 1·5 × 109 colony forming units per mL). Seven days after planting, each pot for the Rhizobium treatments was inoculated three times with 100 mL of a ten-fold dilution of the Rhizobium inoculum. To maintain uniformity of nutrient supply, all other non-Rhizobium treatments were inoculated with autoclaved Rhizobium inoculum (121 °C for 20 min). No nodules formed on plants inoculated with autoclaved inoculum.
Biomass measurements
At each harvest, four replicates of each treatment were selected randomly. The plants were separated into leaves, stems, roots and nodules, and oven-dried at 65 °C for 3 d. At each harvest, a root sub-sample from each treatment was taken before oven drying for estimation of AMF infection of the root tissue. The fresh mass of the sub-sample was recorded so that the dry mass of the sub-sample could be added to the total root dry mass.
N and P analysis
After the determination of dry mass, tissues were milled and analysed for total N and P concentrations. Sub-samples (0·1 ± 0·001 g) were digested in a hydrogen peroxide–sulfuric acid digestion mixture by the Kjeldahl procedure followed by standard colorimetric assays (Anderson and Ingram, 1993). All N and P measurements represent total elemental N and P (organic plus inorganic) present in plant tissues.
AMF detection and quantification
AMF colonization was evaluated from a random sub-sample of approx. 150 root segments per plant. Roots were cleared in 2·5 % KOH (90 °C) for 45 min, acidified in 1 % HCl for 15 min, stained with 0·05 % Trypan Blue in acid glycerol (90 °C) for 45 min, and then stored in acid glycerol according to the procedure of Koske and Gemma (1989). Randomly selected root fragments were mounted in a permanent mounting medium on slides (Omar et al., 1979) without squashing the root pieces. Percentage colonization by arbuscules, vesicles and total hyphae were recorded, at 250× magnification using the magnified intersections method of McGonigle et al. (1990).
Photosynthetic studies
Photosynthetic gas exchange rates were determined at each harvest on equal-aged cohorts. All plants were fully acclimatized to full sunlight (2000 µmol m−2 s−1). Net photosynthesis under light saturating conditions was measured with the portable CIRAS-1, PP systems, infrared gas analyser (Jia and Gray, 2004), using eight different youngest fully expanded leaves for each treatment.
Statistical analysis
AMF% infection data were transformed using arcsine square root to satisfy normal distribution and homogeneity of variance assumptions for three-way ANOVA. Dry mass and elemental (N and P) concentrations of leaves, stems, roots and nodules were analysed by three-way ANOVA for main effects and interactional effects (Zar, 1984). The significance of the differences in variables because of the interactions between factors A (N, NM, NR and NRM), B (N supply LN and HN) and C (harvest date T1 and T2) were tested. For the multifactorial experiment, the sources of variation were subdivided into three main effects: A (presence or absence of microbial symbiont(s); B (low and high nitrogen supplies); and C (harvest dates); three first-order interactions, A × B, A × C, and B × C and one second-order interaction, A × B × C. Multiple comparisons of means were performed by the Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation. The STATISTICA (version 6.0) package was used for statistical analysis.
RESULTS AND DISCUSSION
Effects of treatment factors on plant variables
Each of the treatment factors (A, B, C), when considered individually, had significant effects on all of the different plant variables listed Table 1. In relation to their combined effects, the three-way ANOVA results showed that the majority of first-order interactions had significant effects on all plant variables. The combined effects of the three factors on microbial colonization, plant biomass, rates of N and P accumulation, leaf area and photosynthetic rates are shown in Figs 1–5, and the combined effects of the three factors on N and P concentrations are shown in Table 2. In all instances, microbial colonization of plants was associated with significant increases in the magnitudes of plant variables such as biomass, leaf area, photosynthetic rate, N and P content. The effect of harvest date on the variables such as tissue N concentration and tissue P concentration is presented in Table 2. Except for leaf area, stem P content and root P content, there were no significant second-order interactions (Table 1).
Table 1.
The significance of the differences in plant parameters resulting from the interactions among factors A (N, NM, NR and NRM), B (N supplies LN and HN) and C (harvest dates T1 and T2)
| Source of variance | A | B | C | A × B | A × C | B × C | A × B × C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Degrees of freedom |
3 |
1 |
1 |
3 |
3 |
3 |
3 |
|||||||
| Variables: | ||||||||||||||
| Photosynthetic rate (µmol CO2 m−2 s−1) | *** | *** | *** | *** | ** | ** | * | |||||||
| AMF | ||||||||||||||
| AC (%) | *** | *** | *** | * | ns | ns | ns | |||||||
| VC (%) | *** | *** | * | *** | * | ns | ns | |||||||
| HC (%) | *** | *** | *** | *** | ns | ns | ns | |||||||
| N, P content | ||||||||||||||
| Leaf N (mg g−1) | *** | *** | *** | *** | *** | * | ns | |||||||
| Stem N (mg g−1) | *** | *** | *** | *** | *** | ns | ns | |||||||
| Root N (mg g−1) | *** | *** | *** | *** | ** | * | ns | |||||||
| Nodule N (mg g−1) | *** | *** | *** | ns | ns | * | ns | |||||||
| Leaf P (mg g−1) | *** | *** | *** | *** | ** | ** | ns | |||||||
| Stem P (mg g−1) | *** | *** | *** | *** | *** | *** | ** | |||||||
| Root P (mg g−1) | *** | *** | *** | *** | *** | *** | ** | |||||||
| Nodule P (mg g−1) | *** | *** | *** | ns | ns | ** | ns | |||||||
| Biomass of plant part | ||||||||||||||
| Leaf area (cm2 plant−1) | *** | *** | *** | *** | *** | ** | * | |||||||
| Leaf d. wt (g plant−1) | *** | *** | *** | ** | * | ** | ns | |||||||
| Stem d. wt (g plant−1) | *** | *** | *** | ns | * | * | ns | |||||||
| Root d. wt (g plant−1) | *** | *** | *** | * | * | ** | ns | |||||||
| Nodule d. wt (g plant−1) | * | *** | *** | ns | ns | *** | ns | |||||||
| Biomass (g plant−1) | *** | *** | *** | *** | *** | *** | ns | |||||||
Percentage AMF colonization, dry mass, leaf area, elemental N and P concentrations of leaves, stems, roots and nodules were analysed by three-way ANOVA with residual estimation, for main effects and interaction effects.
*, **, *** Effects that are significant at P < 0·05, 0·01 and 0·001, respectively; ns, effects that are not significant at P < 0·05.
Fig. 1.
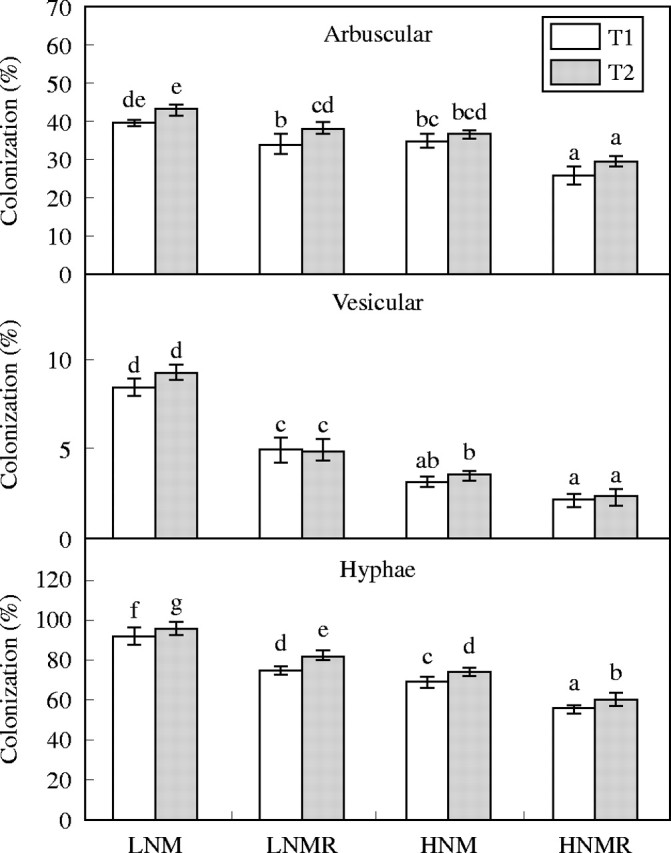
Percentage of arbuscular (AC%), vesicular (VC%) and hyphae colonization (HC%) in response to Rhizobium inoculation, low nitrogen (LN = 10 ppm) and high nitrogen (HN = 250 ppm N) supply at two harvests (T1 and T2). Vertical bars represent s.e. (n = 4) of the means. Different letters indicate significant differences assessed by the Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
Fig. 2.
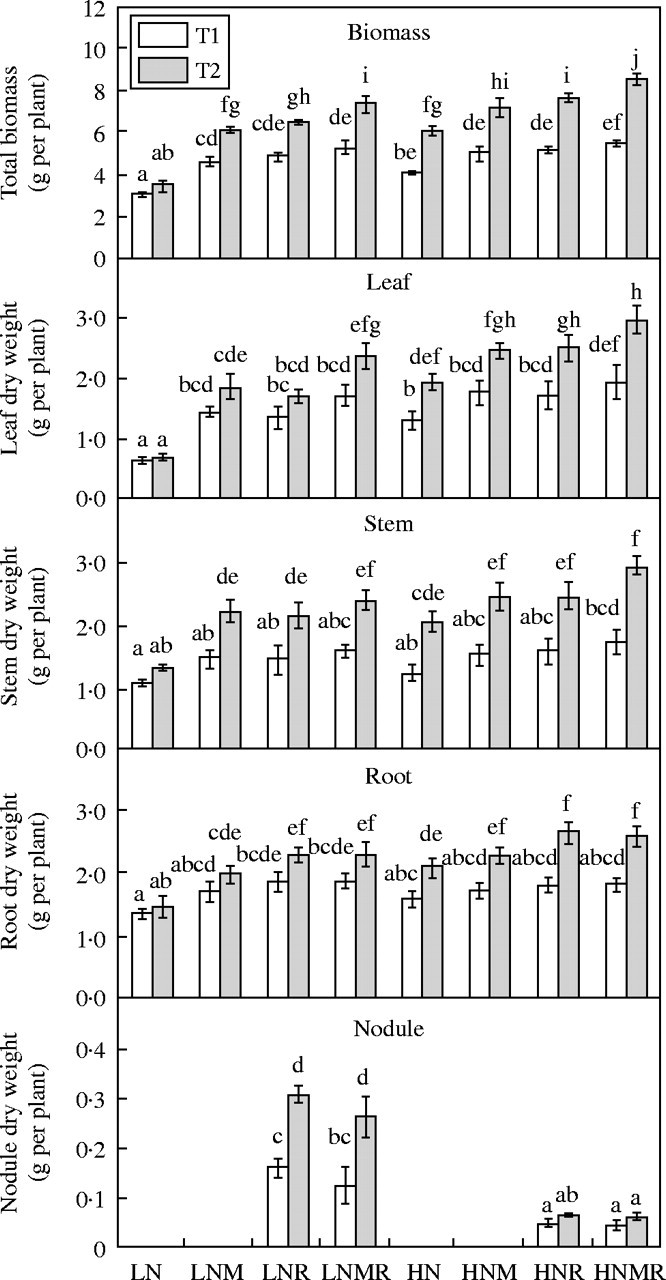
Leaf, stem, root, nodule dry weight production and total biomass as influenced by nitrogen supply (LN and HN) and microbial symbiotic association (N, NM, NR and NMR) at two harvests (T1 and T2). Vertical bars represent s.e. (n = 4) of the means. Different letters indicate significant differences assessed by Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
Fig. 3.
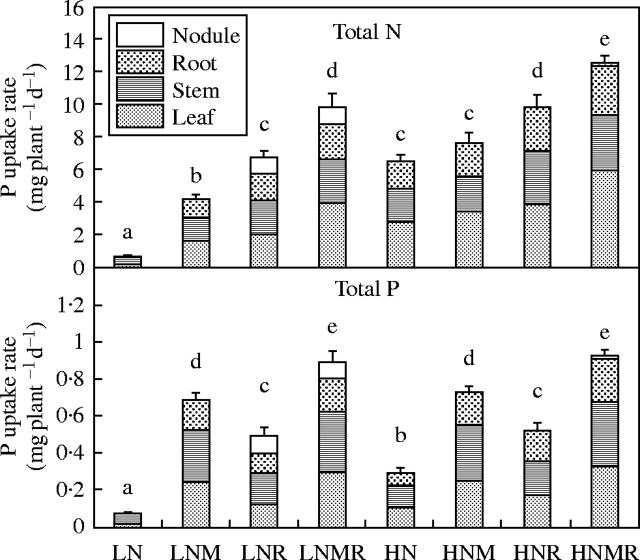
N and P accumulation rate and partitioning into leaves, stems, roots and nodules as influenced by nitrogen supply (LN and HN) and microbial symbiotic association (N, NM, NR and NMR). Vertical bars represent s.e. (n = 4) of the means. Different letters indicate significant differences assessed by the Tukey HSD test (P < 0·05) after performing two-way ANOVA with residual estimation. N and P accumulation rate (mg plant−1d−1) represents an average rate of accumulation calculated as (T2 amount − T1 amount)/9 d.
Fig. 4.
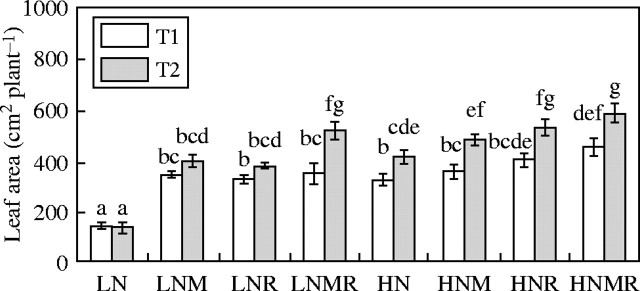
Leaf area (cm2 plant−1) production as influenced by nitrogen supply (LN and HN) and microbial symbiotic association (N, NM, NR and NMR) at two harvest intervals (T1 and T2). Vertical bars represent s.e. (n = 4) of the means. Different letters indicate significant differences assessed by Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
Fig. 5.
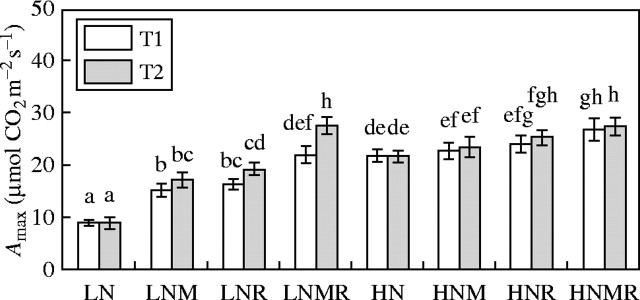
Light saturated (2000 µmol quanta m−2 s−1) photosynthetic rate as influenced by nitrogen supply (LN and HN) and microbial symbiotic association (N, NM, NR and NMR) at two harvest intervals (T1 and T2). Vertical bars represent s.e. (n = 8) of the means. Different letters indicate significant differences assessed by Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
Table 2.
(a) N and (b) P content in leaf, stem, root and nodule of Vicia faba L. as influenced by the factors A (N, NM, NR and NRM), B (N supplies LN and HN) and C (harvest intervals T1 and T2)
| (a) Variable value = N concentration (mg g−1 dry mass) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors |
Variable |
Variable |
Variable |
Variable |
||||||||||||||||
| A |
B |
C |
Leaf N |
Tukey-t |
Stem N |
Tukey-t |
Root N |
Tukey-t |
Nodule N |
Tukey-t |
||||||||||
| N | LN | T1 | 25·61 ± 1·07 | a | 11·79 ± 0·46 | a | 20·48 ± 0·98 | a | ||||||||||||
| N | LN | T2 | 25·64 ± 0·78 | a | 11·89 ± 0·54 | a | 20·15 ± 0·53 | a | ||||||||||||
| N | HN | T1 | 39·51 ± 0·61 | cd | 19·61 ± 0·59 | cd | 23·67 ± 1·34 | bc | ||||||||||||
| N | HN | T2 | 40·05 ± 0·65 | de | 19·69 ± 0·66 | cd | 23·84 ± 0·49 | bcd | ||||||||||||
| NM | LN | T1 | 27·14 ± 0·85 | a | 13·10 ± 0·65 | ab | 22·62 ± 0·94 | ab | ||||||||||||
| NM | LN | T2 | 29·47 ± 0·55 | b | 14·99 ± 0·53 | b | 25·01 ± 0·56 | bcde | ||||||||||||
| NM | HN | T1 | 40·61 ± 0·70 | de | 20·23 ± 0·68 | d | 24·08 ± 0·48 | bcd | ||||||||||||
| NM | HN | T2 | 41·80 ± 0·95 | ef | 21·37 ± 0·41 | def | 24·89 ± 0·81 | bcde | ||||||||||||
| NR | LN | T1 | 37·62 ± 1·10 | c | 17·81 ± 0·45 | c | 25·67 ± 0·95 | cdef | 51·53 ± 0·75 | abc | ||||||||||
| NR | LN | T2 | 41·44 ± 1·13 | def | 20·31 ± 0·40 | de | 28·60 ± 1·17 | gh | 54·40 ± 1·61 | c | ||||||||||
| NR | HN | T1 | 41·70 ± 0·64 | ef | 20·98 ± 0·74 | def | 26·68 ± 1·01 | defg | 49·99 ± 0·64 | a | ||||||||||
| NR | HN | T2 | 43·31 ± 0·92 | fg | 22·58 ± 0·94 | f | 28·40 ± 1·22 | fgh | 50·83 ± 1·49 | ab | ||||||||||
| NMR | LN | T1 | 41·97 ± 0·73 | ef | 22·50 ± 0·93 | f | 27·45 ± 1·13 | efg | 53·89 ± 0·93 | bc | ||||||||||
| NMR | LN | T2 | 45·28 ± 1·26 | g | 25·20 ± 0·91 | g | 31·14 ± 0·89 | h | 57·88 ± 1·40 | d | ||||||||||
| NMR | HN | T1 | 45·05 ± 0·95 | g | 22·24 ± 0·90 | ef | 28·22 ± 0·70 | fg | 52·61 ± 1·54 | abc | ||||||||||
| NMR | HN | T2 | 47·82 ± 0·87 | h | 25·06 ± 0·75 | g | 29·46 ± 1·36 | gh | 53·91 ± 1·45 | bc | ||||||||||
| (b) Variable value = P concentration (mg g−1 dry matter) |
||||||||||||||||||||
| Factors |
Variable |
Variable |
Variable |
Variable |
||||||||||||||||
| A |
B |
C |
Leaf P |
Tukey-t |
Stem P |
Tukey-t |
Root P |
Tukey-t |
Nodule P |
Tukey-t |
||||||||||
| N | LN | T1 | 1·59 ± 0·06 | a | 1·42 ± 0·07 | a | 1·69 ± 0·04 | a | ||||||||||||
| N | LN | T2 | 1·60 ± 0·04 | a | 1·41 ± 0·04 | a | 1·68 ± 0·07 | a | ||||||||||||
| N | HN | T1 | 1·71 ± 0·04 | ab | 1·55 ± 0·08 | abc | 1·73 ± 0·04 | a | ||||||||||||
| N | HN | T2 | 1·72 ± 0·05 | ab | 1·54 ± 0·12 | abc | 1·73 ± 0·08 | a | ||||||||||||
| NM | LN | T1 | 2·23 ± 0·05 | de | 2·11 ± 0·09 | gh | 2·20 ± 0·09 | cd | ||||||||||||
| NM | LN | T2 | 2·65 ± 0·09 | gh | 2·67 ± 0·10 | i | 2·79 ± 0·10 | f | ||||||||||||
| NM | HN | T1 | 2·05 ± 0·12 | cd | 1·84 ± 0·06 | def | 2·17 ± 0·09 | cd | ||||||||||||
| NM | HN | T2 | 2·33 ± 0·10 | ef | 1·97 ± 0·05 | efg | 2·24 ± 0·10 | d | ||||||||||||
| NR | LN | T1 | 1·79 ± 0·05 | ab | 1·54 ± 0·08 | abc | 1·81 ± 0·04 | ab | 3·84 ± 0·09 | b | ||||||||||
| NR | LN | T2 | 2·08 ± 0·09 | d | 1·66 ± 0·06 | bcd | 1·99 ± 0·05 | bc | 4·49 ± 0·23 | c | ||||||||||
| NR | HN | T1 | 1·77 ± 0·08 | ab | 1·67 ± 0·05 | bcd | 1·78 ± 0·07 | a | 3·04 ± 0·15 | a | ||||||||||
| NR | HN | T2 | 1·86 ± 0·06 | bc | 1·77 ± 0·03 | cde | 1·79 ± 0·05 | ab | 3·85 ± 0·21 | b | ||||||||||
| NMR | LN | T1 | 2·45 ± 0·13 | efg | 2·35 ± 0·12 | h | 2·45 ± 0·09 | e | 4·91 ± 0·09 | cd | ||||||||||
| NMR | LN | T2 | 2·87 ± 0·07 | h | 2·93 ± 0·14 | j | 2·93 ± 0·05 | f | 5·25 ± 0·09 | d | ||||||||||
| NMR | HN | T1 | 2·28 ± 0·06 | de | 2·06 ± 0·11 | fg | 2·24 ± 0·60 | d | 3·68 ± 0·15 | b | ||||||||||
| NMR | HN | T2 | 2·51 ± 0·07 | fg | 2·15 ± 0·08 | gh | 2·34 ± 0·10 | de | 4·73 ± 0·26 | c | ||||||||||
Values are means ± s.e, n = 8.
Different letters indicate significant differences assessed by Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
Effects of N supply and Rhizobium on mycorrhizal colonization
Table 1 shows that first-order interactions (A × B) had significant effects on AMF colonization. Colonization levels of AMF were higher in low N treatments (Fig. 1). With regard to arbuscular colonization, two kinds of mycorrhizal root colonization response were evident (Fig. 1). The presence of Rhizobium infection resulted in a significant decline in %AC, %VC and %HC at low and high concentrations of N supply. Low concentrations of N in the presence of Rhizobium infection (LNMR) resulted in %AC values similar to those associated with high rates of N supply in the absence of Rhizobium infection (HNM). At low N supply, the presence of Rhizobium infection (LNMR) resulted in about a 50 % reduction in %VC (Fig. 1). However, at high concentrations of N there was no effect of Rhizobium infection on %VC. The presence of Rhizobium infection and/or high concentrations of N supply were each associated with 50 % or lower %VC compared with the roots of LNM plants. In the case of hyphal colonization (%HC), the presence of Rhizobium infection was associated with a significant decline in %HC (Fig. 1). Whenever N supply was not limiting a significant reduction in %HC occurred. For example, under high N Rhizobium infection resulted in further significant decline in %HC (Fig. 1). A similar pattern was observed for %AC.
In all treatments, the presence of a mycorrhizal association had a positive impact on nutrient uptake and biomass production. For example, when the treatments for HN, HNM and HNMR are compared, the plants had similar total root biomass (Fig. 2), but, irrespective of the level of percentage AMF colonization (Fig. 1), the presence of an AMF association always resulted in enhanced nutrient uptake and total biomass production (Figs 2 and 3).
Effects of N supply and AMF on Rhizobium infection
Table 1 shows that first order interactions (A × B) had significant effects on nodule dry mass. Figure 2 shows the impact of AMF and N supply on nodule mass. Unlike the Rhizobium effects on AMF colonization, AMF infection did not have a significant effect on nodule mass. However, high N supply was associated with a large reduction in nodule dry mass relative to low N plants (Fig. 2). At low N there was a doubling in nodule dry mass between T1 and T2 but only a slight increase over the same period at high N, indicating that high concentrations of nitrate inhibited nodule growth. A significantly larger quantity of P accumulated in LNR and LNMR nodules between harvests compared with the HNR and HNMR nodules (Table 2 and Fig. 3). The concentration of P in the LNR and LNMR nodules was also significantly higher than in the HNR and HNMR nodules (Table 2). In the case of low N treatments, P concentration was significantly higher in LNMR nodules than in LNR nodules (Table 2).
The effect of the N:P ratio on biomass
The level of P supply had a significant effect on plant biomass (Fig. 2), root nodulation (Fig. 2), N accumulation rate (Fig. 3), leaf area (Fig. 4), net photosynthetic rate (Fig. 5) and tissue N concentration (Table 2). Increase in P supply as a consequence of AMF colonization was consistently associated with a significant increase in N accumulation and biomass production (Figs 2 and 3). For the LNMR plants the N : P ratio was 11 mg N d−1 : 1 mg P d−1, and 14 mg N d−1 : 1 mg P d−1 for the HNMR plants. For HNM and HN the N : P ratios were 10·8 mg N : 1 mg P d−1 and 21·7 mg N d−1 : 1 mg P d−1, respectively. These results indicate that the rate of biomass production increased as the proportion of total P accumulated increased relative to the total N accumulated. AMF contributed to an increase in N productivity in terms of biomass production by increasing the supply of P.
Effect of P on total N accumulation
The amounts of external hyphae in the pots were not determined, nor the metabolically active proportion of the internal colonization. In LNM the %HC was almost 100 %, whereas for HNMR it was approx. 60 %, although the HNMR plants accumulated significantly more P than did the LNM plants. Plants with both Rhizobium and AMF associations (LNMR and HNMR) accumulated the most P. In low N treatments AMF colonization had a significant positive impact on the rate of nitrate-N accumulation (Fig. 3), and in high N treatments, non-AMF plants such as the HNR plants accumulated significantly less N than did HNMR plants. These results are consistent with the finding of Tobar et al. (1994a, b) that AMF colonization increased plant nitrate-N uptake.
P-limitation of N2-fixation rates appears to be the reason for the lower rates of N accumulation in LNR relative to LNMR plants (Fig. 3). Nitrate was the major N source for HNMR plants mainly because of their lower nodule mass compared with the LNR and LNMR plants (Figs 2 and 3). The N2-fixing LNMR plants differed only slightly from the nitrate assimilating HNMR plants in rate of total elemental N accumulation. For example, at T1, HNMR had accumulated 7·0 % more N than LNMR and, at T2, HNMR had accumulated 5·3 % more N.
It is not clear whether the differences in nitrate accumulation rates among the LN, HN, LNM, HNM and HNMR plants were a direct consequence of increased uptake and mobilization of nitrate-N by the external hyphae, or a indirect consequence of an increase in the plant P content. If the latter held, then plant N productivity in terms of leaf area production would have been enhanced by an increase in P accumulation. Increase in the plant's total transpirational surface area would tend to increase the flux of water through the soil–plant–atmosphere continuum and increase the flux of nitrate to the plant root system. This could be a possible explanation for indirect effects of AMF colonization on plant nitrate uptake.
Effect of N on total P accumulation
Root biomass did not appear to be a major factor in either P or N uptake. Since, apart from the LN plants, root biomass did not differ significantly in the other treatments (Fig. 2) it alone could not account for the variation in the quantity of P accumulated in the different treatments (Fig. 3). Plants with roots colonized by AMF had P accumulation rates that were 9·3–12·2 times the rates for the non-microbial low N treatments (LN versus LNM and LN versus LNMR in Fig. 3). At high N, all plants with AMF colonized roots had P accumulation rates that were 2·5–3·2 times the rates for the non-microbial high N treatments; see HN versus HNM and HN versus HNMR in Fig. 3. However, roots infected only with Rhizobium also showed an increase in P accumulation relative to the low N or high N non-mycorrhizal control plants. For example, LNR plants had a 6·6-fold increase in the level of P accumulation compared with LN plants, and there was a 1·8-fold increase in the level of P accumulation when HN plants were infected with Rhizobium (Fig. 3). Here the increase in P accumulation appeared to be influenced indirectly by the association with N2-fixing Rhizobium in the low N plants, or by the increased nitrate N supply in the high N treatment plants.
Increased N accumulation due to N2-fixation in LNR and HNR plants (Fig. 3) was associated with an increase in leaf area expansion (Fig. 4). Thus, it was possible that the AMF-independent enhanced rates of P accumulation in LNR and HNR plants were due to the effects that N accumulation had on leaf area production.
The influence of N, P and microbial symbionts on photosynthesis
Figures 3 and 5 show that photosynthetic rates increased as the ratio of P to N supply increased (note also the relationship between the N : P trend in Table 2 and the trend in photosynthetic rates in Fig. 5). The impact that the microbial symbionts had on photosynthetic rates appeared to be mediated by their effects on the plant N:P ratio. Separate experiments to test the effect of P supply on the photosynthetic N use efficiency and the quantum yield efficiency of leaf N showed that an increase in the supply of P increases the photosynthetic N use efficiency and the quantum yield efficiency of leaf N (Table 3). The results in Table 2 and Fig. 5 are consistent with the hypothesis that increasing P supply enhances photosynthetic N use efficiency.
Table 3.
The influence of N and P supply rates on leaf N and P concentration, total P accumulated, leaf area production, light saturated photosynthetic rate (Amax), and quantum yield efficiency (QYE)
| Factors |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
SLN (g N m−2) |
SLP (g P m−2) |
Total P (g plant−1 × 10−3) |
Leaf area (cm2 plant−1) |
Amax (µmol CO2 m−2 s−1) |
QYE (µmol CO2 µmol Q−1 × 10−2) |
||||||||||||
| 10 ppm N | LP | 0·721 ± 0·067 | a | 0·045 ± 0·004 | c | 3·171 ± 0·368 | a | 117·7 ± 21·6 | a | 2·180 ± 0·253 | a | 0·230 ± 0·016 | a | ||||||
| 25 ppm N | LP | 0·812 ± 0·070 | ab | 0·042 ± 0·005 | bc | 3·412 ± 0·377 | a | 157·9 ± 24·3 | ab | 7·562 ± 0·620 | b | 1·001 ± 0·116 | b | ||||||
| 50 ppm N | LP | 0·871 ± 0·074 | abc | 0·038 ± 0·003 | abc | 3·571 ± 0·366 | a | 197·6 ± 19·5 | bcd | 12·110 ± 0·792 | c | 1·541 ± 0·144 | c | ||||||
| 100 ppm N | LP | 0·969 ± 0·099 | bcd | 0·034 ± 0·004 | abc | 3·850 ± 0·346 | a | 250·3 ± 20·3 | e | 15·039 ± 0·994 | d | 1·830 ± 0·180 | cd | ||||||
| 250 ppm N | LP | 1·036 ± 0·098 | cde | 0·031 ± 0·004 | ab | 4·128 ± 0·628 | a | 307·1 ± 25·6 | f | 17·166 ± 1·248 | ef | 2·140 ± 0·123 | d | ||||||
| 500 ppm N | LP | 1·111 ± 0·137 | de | 0·029 ± 0·003 | a | 4·309 ± 0·522 | a | 355·8 ± 30·8 | f | 18·374 ± 1·076 | fg | 2·320 ± 0·119 | de | ||||||
| 10 ppm N | HP | 1·098 ± 0·097 | de | 0·124 ± 0·010 | f | 9·903 ± 1·171 | b | 177·1 ± 21·7 | bc | 6·398 ± 0·516 | b | 2·739 ± 0·144 | e | ||||||
| 25 ppm N | HP | 1·230 ± 0·087 | ef | 0·120 ± 0·009 | f | 10·525 ± 0·972 | b | 210·8 ± 21·8 | cde | 12·391 ± 0·822 | c | 3·861 ± 0·176 | f | ||||||
| 50 ppm N | HP | 1·349 ± 0·122 | fg | 0·114 ± 0·008 | ef | 11·298 ± 1·129 | bc | 248·3 ± 27·2 | de | 16·145 ± 0·675 | de | 6·879 ± 0·379 | g | ||||||
| 100 ppm N | HP | 1·521 ± 0·095 | g | 0·105 ± 0·006 | e | 12·178 ± 1·157 | cd | 319·8 ± 30·1 | f | 19·108 ± 0·914 | g | 7·988 ± 0·610 | h | ||||||
| 250 ppm N | HP | 1·849 ± 0·126 | h | 0·093 ± 0·007 | d | 13·620 ± 1·012 | de | 433·2 ± 31·2 | g | 20·875 ± 1·246 | h | 8·742 ± 0·496 | i | ||||||
| 500 ppm N | HP | 2·227 ± 0·142 | i | 0·083 ± 0·008 | d | 14·929 ± 0·996 | e | 584·3 ± 38·6 | h | 21·692 ± 1·152 | h | 9·151 ± 0·498 | i | ||||||
| n | 6 | 6 | 6 | 6 | 8 | 8 | |||||||||||||
Low P (LP) plants were supplied with 0·5 mm P and high P (HP) plants were supplied with 1·6 mm P.
Values are means ± s.e.
Different letters indicate significant differences assessed by Tukey HSD test (P < 0·05) after performing three-way ANOVA with residual estimation.
CONCLUSIONS
The findings of this experiment are consistent with other observations (Xavier and Germida, 2002, 2003) of the positive impact of the synergistic interactions between AMF and Rhizobium on legumes. The magnitude of the increases in both leaf area and biomass production with each increment in N supply was dependent on the level of P supply. Increasing P supply as a direct consequence of AMF colonization or as an indirect consequence of Rhizobium infection had positive effects on N accumulation, leaf area production and biomass production. Increasing P accumulation had a positive influence on photosynthetic N use efficiency. Thus, it is reasonable to conclude that, with respect to the legume tripartite symbiotic association examined here, the upper limits of nitrogen productivity or photosynthetic N use efficiency can depend on the level of P supply.
Supplementary Material
Acknowledgments
The authors thank Prof. M. Scholes for assisting with N and P analysis and Prof. J. S. Galpin for helping with the statistical analysis. The research was funded by the University Senate Research Grant.
LITERATURE CITED
- Ågren GI. 1985. Theory for growth of plants derived from the nitrogen productivity concept. Physiologia Plantarum 64: 17–28. [Google Scholar]
- Anderson JM, Ingram JSI. 1993.Tropical soil biology and fertility—a handbook of methods, 2nd edn. Wallingford, UK: CAB International, 70–89. [Google Scholar]
- Andrade G, De Leij F, Lynch JM. 1998. Plant mediated interactions between Pseudomonas fluorescens, Rhizobium leguminosarum and arbuscular mycorrhizae on pea. Letters in Applied Microbiology 26: 311–316. [Google Scholar]
- Azcón R, Rubio R, Barea JM. 1991. Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytologist 117: 339–404. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987. Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiologia Plantarum 70: 215–222. [Google Scholar]
- Jia YS, Gray VM. 2004. Interrelationships between nitrogen supply and photosynthetic parameters in Vicia faba L. Photosynthetica 41: 605–610. [Google Scholar]
- Koske RE, Gemma NJ. 1989. A modified procedure for staining roots to detect VA myckorrhiza. Mycological Research 92: 486–505. [Google Scholar]
- McGonigle TP, Miller ML, Evans DG. 1990. A new method which gives an objective measure of colonization of roots by vesicular-Mycorrhizal fungi. New Phytologist 115: 495–501. [DOI] [PubMed] [Google Scholar]
- Omar MB, Bolland L, Heather WA. 1979. A permanent mounting medium for fungi. Bulletin of the British Mycological Society 13: 31–32. [Google Scholar]
- Sinclair TR, Horie T. 1989. Leaf nitrogen, photosynthesis and crop radiation use efficiency: a review. Crop Science 29: 90–98. [Google Scholar]
- Smith SE, Read DJ. 1997.Mycorrhizal symbiosis, 2nd edn. San Diego, CA: Academic Press. [Google Scholar]
- Tobar RM, Azcón R, Barea JM. 1994. Improved nitrogen uptake and transport from15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytologist 126: 119–122. [Google Scholar]
- Tobar RM, Azcón R, Barea JM. 1994. The improvement of plant N acquisitionfrom an ammonium-treated, drought-stressed soil by the fungal symbiont in arbuscular mycorrhizae. Mycorrhiza, 4: 105–108. [Google Scholar]
- Toro M, Azcón R, Barea JM. 1998. The use of isotopic dilution techniques to evaluate the interactive effects of Rhizobium genotype, mycorrhiza fungi, phosphate-solubilizing Rhizobacteria and rock phosphate on nitrogen and phosphorus acquisition by Medicago sativa New Phytologist 138: 265–273. [DOI] [PubMed] [Google Scholar]
- Xavier LJC, Germida JJ. 2002. Response of lentil under controlled conditions to co-inoculation with arbuscular mycorrhizal fungi and rhizobia varying in efficacy. Soil Biology & Biochemistry 34: 181–188. [Google Scholar]
- Xavier LJC, Germida JJ. 2003. Selective interactions between arbuscular myocrrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biology and Fertility of Soils 37: 262–267. [Google Scholar]
- Zar JH. 1984.Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.