Abstract
• Background and aims The order Gnetales has been the central focus of controversy in seed plant phylogeny. Traditional treatment of morphology supports the anthophyte hypothesis with Gnetales sister to angiosperms but current molecular data reject this hypothesis. A new fossil gnetalean cone, Palaeognetaleana auspicia gen. et sp. nov., is reported from the Upper Permian in North China, and its phylogenic implications are considered.
• Methods Samples of cones from the upper part of the Upper Permian redbeds of Baode section, northwestern Shanxi Province, China, were examined.
• Key results The cone is characterized by its unusual nature of reproduction that combines features of post-Triassic gnetaleans and some of the Palaeozoic conifers. It is made up of a number of imbricate axillary units, each simply formed by an ovule and a subtending bract, which may be comparable with the axillary seed-scale complex of some of the Palaeozoic conifer cones. The cone exhibits at least a partially bisexual character that appears to have pollen sacs with monosulcate ribbed pollen grains and sessile, asymmetric, and radiospermic ovules. The ovule has an integument of three envelopes: an outer one of pointed scales; a middle sclerified one; and an inner cuticle that extends upward into a micropyle with an oblique tip.
• Conclusions The new Permian cone has unequivocal affinity with the Gnetales. The fossil has considerably extended the divergence time of the Gnetales from 140 (210?) back to 270 myr ago and, therefore, provides the first significant fossil evidence to support the current conclusion based on molecular data of seed plants, i.e. monophyletic gymnosperms, comprising the Gnetales are closely related to conifers.
Key words: Palaeognetaleana auspicia (gen. et sp. nov.), Gnetales, bisexual fossil cone, phylogeny of seed plants, radiation of gymnosperms, molecular data, Late Permian, North China
INTRODUCTION
The Gnetales is a small group of seed plants that has generated diverse controversies on phylogeny over many years. The order has attracted much attention from traditional botanists (Arber and Parkin, 1908; Crane, 1996; Endress, 2001), palaeobotanists (Cornet, 1996; Doyle, 1996; Krassilov, 1997) and molecular systematists (Frohlich, 1999; Hansen et al., 1999; Winter et al., 1999; Chaw et al., 2000; Rydin et al., 2002), because of its potential significance for understanding the phylogeny of seed plants. In past literature, the Gnetales was widely considered to be the closest sister group to the angiosperms (Arber and Parkin, 1908; Crane, 1985) as being a central concept of the anthophyte hypothesis (Doyle and Donoghue, 1986). Around the late 1990s, almost all results from multiple gene sequences claimed the anthophyte hypothesis to be incorrect (Bowe et al., 2000; Chaw et al., 1997, 2000; Hansen et al., 1999; Qiu et al., 1999; Winter et al., 1999). Meanwhile, many authors also recognized that most estimates of when extant seed plants diverged, based on molecular data, showed conflict either with each other or with fossil evidence (Axsmith et al., 1998; Donogue and Doyle, 2000; Sanderson et al., 2000; Sanderson and Doyle, 2001; Soltis et al., 2002b). Our understanding of the relationship among the main seed plant groups would be put into perspective by more attention to fossil taxa (Soltis et al., 2002a).
A total of five or six gnetalean megafossil genera have been described but these provide only limited information, mainly due to poor preservation of the fossils (Crane, 1996). The timing of divergence of the Gnetales from other seed plants has generally been estimated as Triassic and Jurassic (Crane et al., 1995; Doyle, 1996; Krassilov, 1997). Earlier than this, there was no record on fossil gnetalean plants but similar palynomorphs were reported in the Permian deposits (Wilson, 1959; Osborn et al., 1993).
MATERIALS
During the 1990s, the author collected about 30 what were thought to be ‘conifer’ cones from the upper part of the Upper Permian redbeds of Baode section, northwestern Shanxi (Fig. 1). The plant-bearing bed is a small yellow-greyish sandy or silty lens, 10 m long and no thicker than 0.4 m. Other associated fossil plants are mostly characteristic of the Late Permian flora in north China such as Lobatannularia ensifolia (Halle) Halle., L. heianensis (Kodaira) Kawasaki, Pecopteris hemitelioides Brongniart, Sphenopteris gothanii Halle, Emplectopteris triangularis Halle, Gigantonoclea crenata Wang, Ginkgophytopsis sp., Psygmophyllum sp., Nystroemia pectiniformis Halle, Chiropteris reniformis Kawasaki, Pterophyllum erratum Gu et Zhi, Taeniopteris densissima Halle and T. tingii Halle, among others. The stratigraphical horizon of the fossil-bearing bed corresponds to the uppermost plant-assemblage zone of the Tianlongsi Formation (=Upper Shihhotze Fr.) near Taiyuan, central Shanxi (Halle, in Norin, 1924). The new cone species, because of its superficial resemblance to Palaeozoic and Mesozoic conifer cones, was initially misidentified as Podocarpaceae (Wang and Zhang, 1998). The cone specimens are mostly compressed or adpressed in preservation but their carbonized remains contain ribbed pollen (or pollen masses) typical of the Gnetales, ovules and fragments of bract. Over a half of the cones have been investigated and seven of them were treated by bulk maceration. All have produced residues on the basis of which a new Permian cone with gnetalean affinity has been described.
Fig. 1.
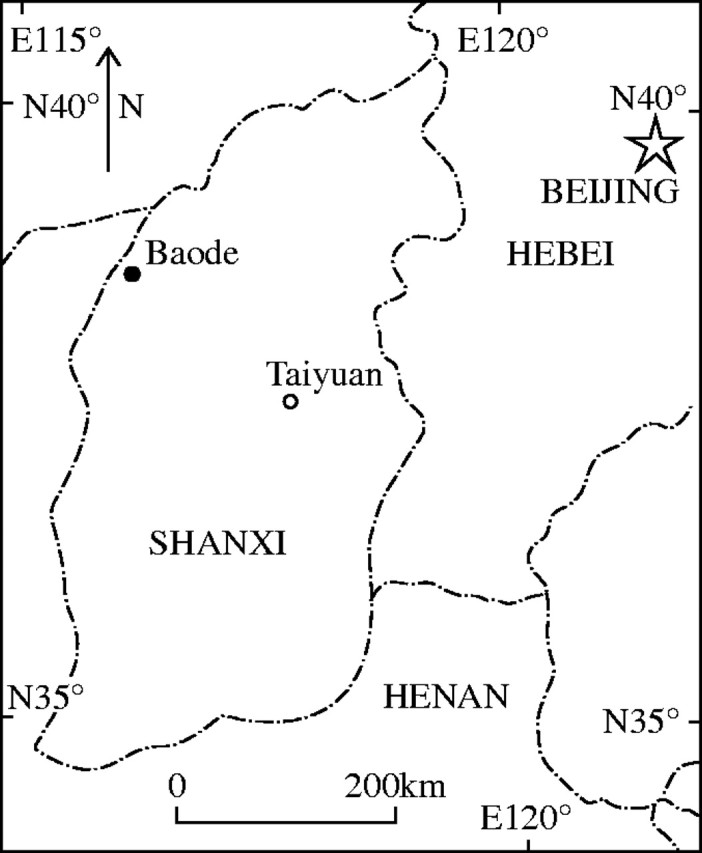
Map of the fossil locality.
SYSTEMATICS
Order Gnetales Engler 1892
Genus Palaeognetaleana gen. nov
Diagnosis: Cone at least partially bisexual, consisting of simple axillary units in imbricate and helical arrangement, each comprising a subtending bract, an asymmetric ovule, a number of fibres or scales surrounding the ovule, and pollen sacs appearing to be concealed beneath the ovule. Ovule enclosed by integument of three envelopes: an outer scale one, a middle sclerified one and an inner cuticle one. Pollen grains exhibiting slightly twisted ribs with monosulcate aperture.
Type species. Palaeognetaleana auspicia gen. et sp. nov
Etymology: The generic name refers to a Palaeozoic gnetalean plant.
Horizon and location: Lower Upper Permian Gigantonoclea-dominant assemblage (f10) from the Baode section in northwestern Shanxi, China (Wang, 2000).
Species Palaeognetaleana auspicia sp.nov. (Figs 2–6)
Diagnosis: Cones at least partially bisexual, asymmetrically ellipsoidal or ovoid in shape, 35–60 mm long and 25–45 mm wide, closely arranged by imbricate axillary units. Each unit simple, consisting of a single ovule lying on a subtending bract, fibres or pointed scales surrounding the ovule, and pollen sacs seemingly concealed beneath the ovule. Ovules sessile, radiospermic more or less asymmetric in outline, rounded, ovoid or ellipsoidal in shape, 0·6–7 mm long and 2–5 mm in diameter. The integument of the ovule comprises three envelopes: an outer pointed scales (or fibre); a middle sclerified envelope; and an inner cuticle that extends upward into a micropyle with an oblique tip. Bract lanceolate or wing-like with bilaterally asymmetric margins, 15–25 mm long and 2–5 mm wide at base, having acute apex and expanded base with denticles or spines developing along its anterior margin. Megaspore walls tough and thick. Pubescence of the tapetal tissue occurring either in wall of the ovule or in that of the pollen sac. Pollen grain Ephedripites-type, 40–50 µm long with monosulcate aperture and more or less twisted ribs.
Description: Figures 2 and 3A show the elliptical or obovate cones in longitudinal outline. The cone consists of a number of axillary fertile units in imbricate helical arrangement, and is terminally borne on a short or long peduncle that probably extends from a thick primary axis (Fig. 3A, lower right). The axis is possibly prostrate, about 10 mm thick, covered with fine longitudinal striae but no leaves. Bracts, asymmetrically lanceolate, 15–25 mm long and 3–5 mm wide at expanding base where the dentate and spinate anterior margin develops (Fig. 3D, arrow b), accompanied by numerous delicate fibres (Fig. 3D, arrow a). Each bract bears an ovule adnate to its expanding base (Fig. 3C). Part of a degraded pollen sac with pubescence of the tapetum tissue is seen in Fig. 4A and an in situ ribbed pollen mass was extracted from the sac (Fig. 4B). Another pollen mass (Fig. 4C and D) shows details of pollen grains with monosulcate aperture. Figure 5A (upper left) exhibits a small ovule enclosed by an outer envelope made up of fine fibres, and a much larger ovule surrounded by pointed scales (lower right). Figure 5B is another ovule or seed showing the rough surface of the solid middle envelope which is exposed after detachment of the scales and fibres. An ovule after strong maceration exhibits an inner cuticular envelope that extends upwards into the prominent micropyle (Fig. 5C) and there is a rare relict of pubescent tapetum tissue at the base. A long but unbifid micropylar tube can be sometimes visible (Fig. 6A) and some micropyles show an askew tip (Fig. 6B). All the ovules are generally 1–5 mm in diameter, sessile and more or less asymmetric in outline.
Fig. 2.
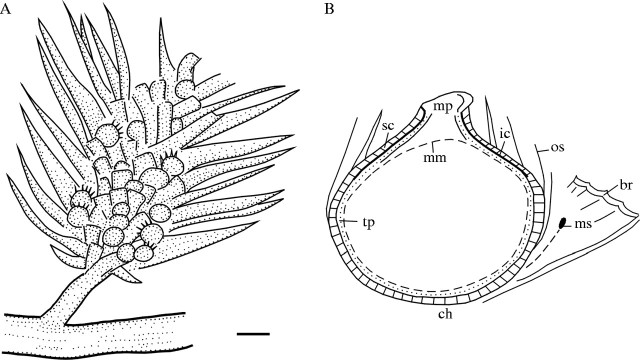
Drawings of Palaeognetaleana auspicia gen. and sp. nov.: (A) reconstruction of a Palaeognetaleana auspicia cone (holotype); (B) diagram of an axillary unit, showing structures of ovular integument: mm (dashed line), megaspore membrane (see Fig. 6A); tp, tapetal tissue (see Fig. 6A); ic, cuticle of inner envelope (see Fig. 5C, only developing at micropyle end); sc, sclerotic envelope (see Fig. 5B); os, outer scales or fibres (see Fig. 5A); ms, a presumed pollen sac; br, bract; ch, chalazal end (see suture of the ovule in Fig. 5B).
Fig. 3.
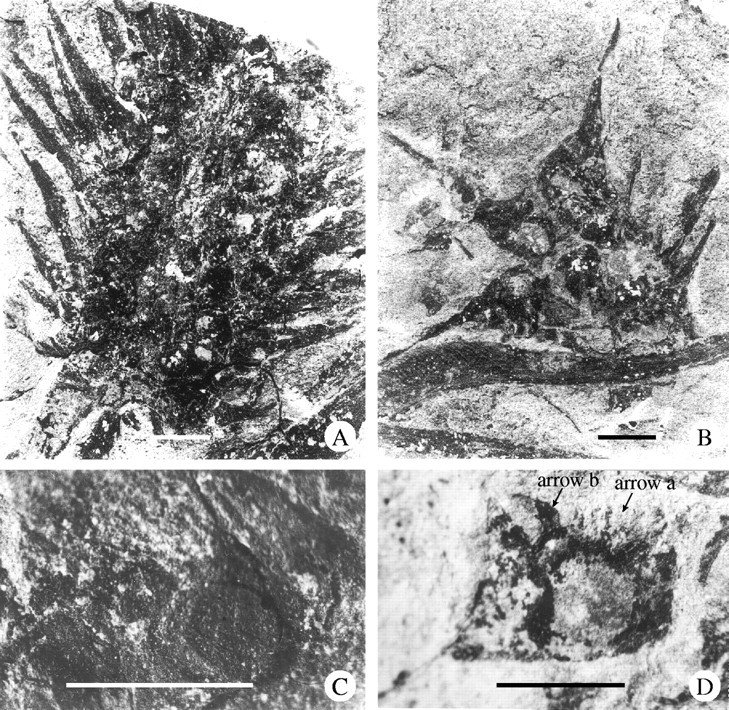
Palaeognetaleana auspicia gen. and sp. nov. cone and its bracts. (A) A cone borne terminally on a short peduncle that possibly extends from the primary axis (lower right), showing attached rounded ovules near to the cone axis and dentate margin of the bract (lower left), 9107–1, ×2. (B) Some isolated bracts preserved in a transverse plan, each showing a long lateral ridge and an expansive base on which one ovule overlies, 9705–45, ×2. (C) Basal part of a bract showing one ovule lying on it, 9705–41, ×8. (D) An isolated bract showing the long lateral ridge of its margin and dentate anterior margin (arrow b) at expansive base. Note the ovule having become detached but having left delicate fibres (arrow a), 9805–048, ×5. All scale bars = 5 mm.
Fig. 4.
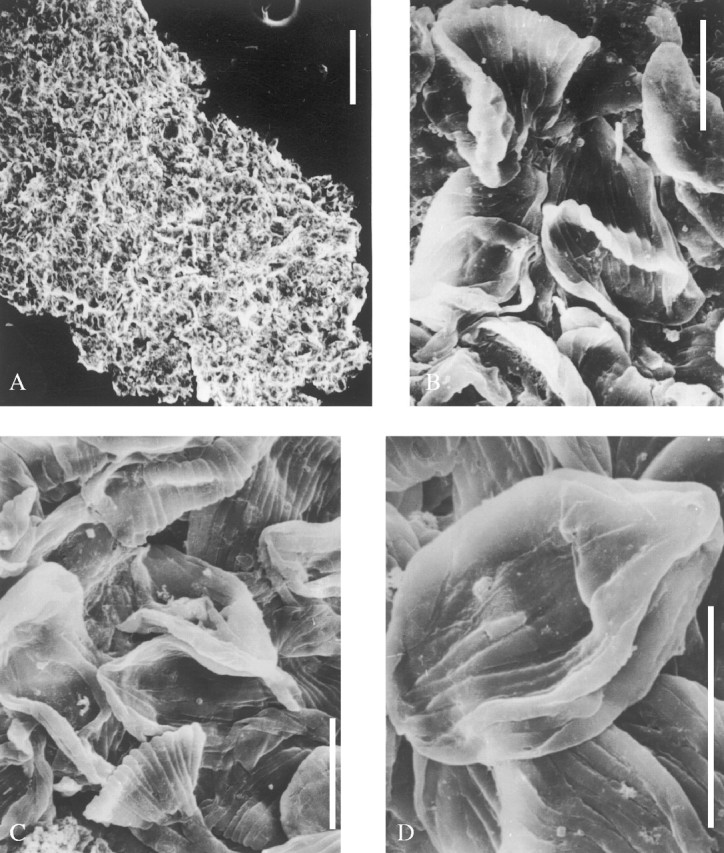
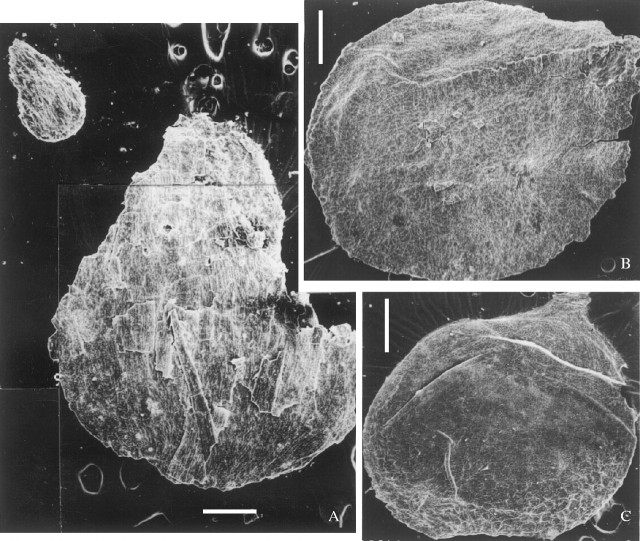
Pollen sacs and in situ pollen grains. (A) Part of a degraded pollen sac enclosed by pubescence of tapetal tissue, 9705–37, ×42; scale bar = 240 µm. (B) Local magnification of A showing ribbed pollen mass, ×600. (C) Another pollen mass, 9705–39, ×600. (D) Showing an entire ribbed grain of C, ×1200. Scale bars in B and C = 25 µm.
Fig. 5.
Ovules without or with little maceration. (A) A small ovule (upper left) enclosed by fine fibres, and a large ovule (lower right) enclosed by narrow and pointed scales, both from same cone, 9805–045, ×20. (B) An ovule after incomplete maceration, showing the solid middle layer of the integument and a suture ridge, 9705–39a, ×20. (C) An ovule after complete maceration, exhibiting the thin cuticle of the inner integument that extends upwards into an asymmetric micropyle and rare remnants of fibres at the base, 9107–8 (counterpart of 9107–1), ×8. All scale bars = 0·5 mm.
Fig. 6.
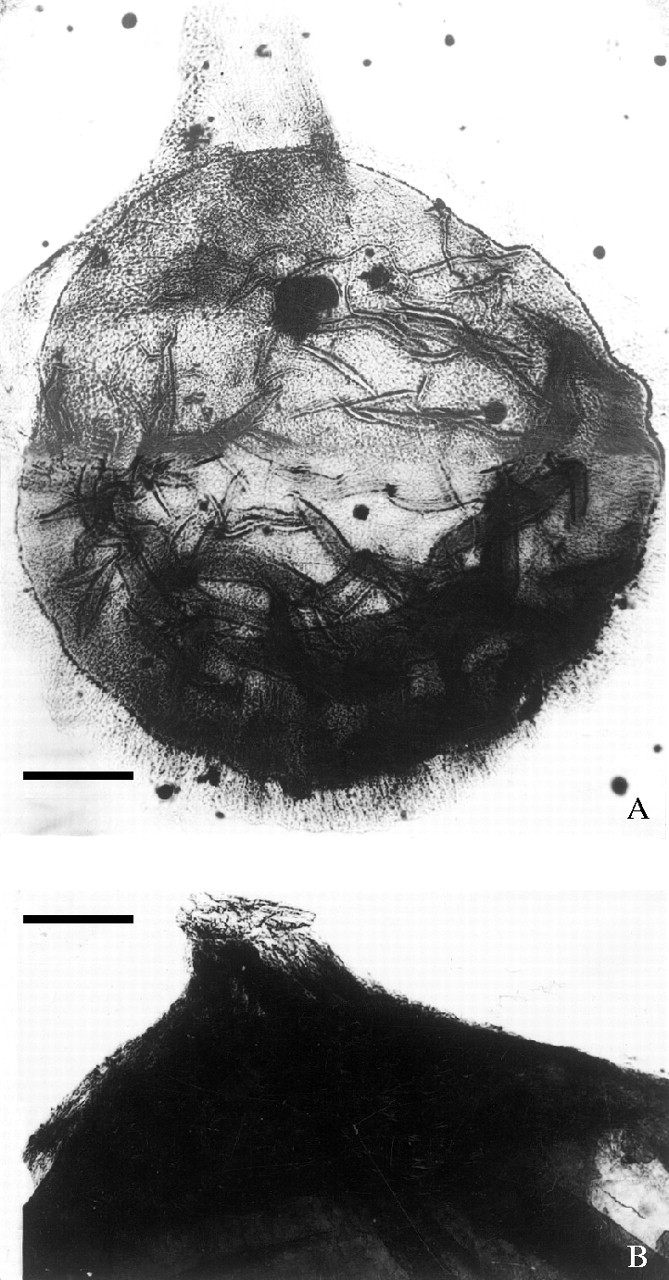
Ovules through maceration: (A) an ovule showing a long, unbifid micropylar tube at apex, 9705–0; ×40; (B) upper part of an ovule showing a prominent micropyle with an oblique tip, 9107–8, ×40. Both scale bars = 0·25 mm.
Holotype: Specimen No. 9107–1 (Figs 2–3)
Etymology: Specific name prefers to this auspicious discovery of the so far earliest record of this group.
Specimen deposition: All specimens described here are deposited in Tianjin Institute of Geology and Mineral Resources, Chinese Academy of Geological Sciences.
Comparisons: For Palaeognetaleana auspicia gen. et sp. nov., the most impressive feature is its reproductive nature that combines gnetalean and conifer-like features.
In terms of current male theory (Frohlich and Parker, 2000), a critical feature for determining affinity of the Palaeognetaleana cone is the ribbed pollen with monosulcate aperture and slightly twisted ribs, which is so far known only from living Welwitschia of the Gnetales (Crane, 1996; Osborn, 2000). Vitattina polynomorph, which was partially attributed to some of the upper Permian peltasperms in Angara (Meyen, 1984), shows little superficial similarity to the ribbed grain of the Gnetales but is different from the latter in a faint area longitudinally across the distal surface of the pollen without sulcate aperture.
In the ovulate structure, on the other hand, a significant correlation for Palaeognetaleana with living Gnetales is the presence of a micropylar tube of ovule that has an askew tip (Endress, 1996). The three envelopes of the Palaeognetaleana integument also correspond to those of living Gnetales (Rodin and Kapil, 1969) or ‘initiate (bitegmic) integument’ interpreted by Endress (2001: 107). The sex distribution of the new gnetalean cone has been cautiously interpreted as at least partially bisexual because more than half the cones collected produce both ovulate and polleniferous organs. This is the main character of a clade referred to as anthophyte group (Crane et al., 1995; Doyle, 1996).
On the whole, there seems to be little doubt about the gnetalean affinity of the new fossil cone.
The most remarkable distinction of Palaeognetaleana from living Gnetales is the imbricate cone with upward and outward bracts extending in a helical arrangement. This is clearly different from the opposite-decussate arrangement of paired bracts in all extant Gnetales and most known fossil ones (Crane and Upchurch, 1987; Krassilov, 1997). Another critical feature in which the new cone differs from extant Gnetales is in its reproductive organization: the Palaeognetaleana cone has a number of axillary fertile units, each consisting of one subtending bract, one ovule plus pollen sacs that appear to be concealed beneath the ovule, and fibres or scales filling the gap between the two. This is much simpler than those reproductive organs of living Gnetales with a cone-like inflorescence that is organized by paired or decussate bracts (collar), each containing two or more ovuliferous organs plus pollen sacs (Endress, 1996). In these features, the Palaeognetaleana cone exhibits obvious similarities to those of some Palaeozoic conifers with an axillary dwarf-shoot system (Florin, 1951). This is an old issue argued by many authors who paid special attention to the anthophyte hypothesis (Crane, 1985; Doyle and Donogue, 1986, 1992; Taylor and Taylor, 1993; Rothwell and Serbert, 1994; Doyle, 1996, 1998). The wing-like bract and subtended ovule of the Palaeognetaleana cone could be correlated with the axillary fertile system borne in the axils of scales in most Paleozoic conifer female cones (Rothwell and Mapes, 2001), if considering those pointed scales surrounding the ovule as being homologous to the axillary dwarf shoot of the conifer. The dentate or spinate margin at the bract base may also be observed in some of the Palaeozoic conifers (Hernandez-Castillo et al., 2001). However, the specific ovule of Palaeognetaleana is characterized by its sessile base, radiospermic–asymmetric shape, and prominent but unbifid apex of the micropyle. These can all exclude the possibility for Palaeognetaleana to be attributed to Coniferophytes that have stalked, platyspermic and flattened, bilaterally symmetric ovules with a bifid micropyle at the apex such as that of the Permian Otovicia hypnoides (Brongniart) in West Europe (Kerp et al., 1990).
DISCUSSION
The Palaeognetaleana cone is important for its unusual nature that links it to the Palaeozoic conifers in some critical aspects, implying that a certain relationship between gnetaleans and conifers existed early in the Permian. This is congruent with current conclusion drawn by molecular analyses to extant main seed plants. Therefore, Palaeognetaleana provides the first fossil evidence to support the gnetaleans–conifers relationship of current molecular data.
Among those molecular analyses during 1999–2000, however, an agreed phylogenetic tree for seed plants has not yet been produced, except for one consistent point that the Gnetales is sister to conifers rather than to angiosperms, as previously thought (Donogue and Doyle, 2000). Nevertheless, through calibration to bias or error in age estimates by using molecular clock (Sanderson et al., 2000; Sanderson and Doyle, 2001) and analyses of more gene sets (Bowe et al., 2000; Soltis et al., 2002b), a congruent conclusion has been reached to the effect that extant gymnosperms are monophyletic and extant Gnetales are closely related to conifers, although the precise relationship of the Gnetales to conifers is unclear (Soltis et al., 2002a).
The discovery of Palaeognetaleana is an important contribution to our understanding of the Permian radiation of gymnosperms. In macroevolution, the great radiation of the angiosperms in the Cretaceous has attracted much attention, as has the faunal radiation in the Cambrian (Stanley, 1990); but the Permian gymnosperm radiation has been the subject of less interest (DiMichele et al., 1987; Kerp et al., 2001). The radiation of gymnosperms in the Permian floras took place somewhat diachronously in the Northern Hemisphere, including the conifers (Kerp et al., 1990; Kerp, 1996; Rothwell and Mapes, 2001), ginkgophytes (Yao, 1989; DiMichele et al., 2001), cycads (Mamay, 1976; Zhu and Du, 1981; Wang, 2000) and peltasperms (Poort and Kerp, 1990; Wang and Zhang, 1998; Kerp et al., 2001).
The appearance of the Permian Palaeognetaleana auspicia (gen. et sp. nov.), so far the earliest known fossil record of the Gnetales, demonstrates that the divergent time of the group from other seed plants was in the Late Palaeozoic, much earlier than the previously known Late Triassic (Ash, 1972) or even Late Jurassic–Cretaceous (Hughes, 1994; Krassilov, 1997). It may represent an archetype of the crown lineage of Gnetales. It is now clear that all four extant gymnosperm orders (Cycadales, Ginkgoales, Coniferales and Gnetales) have their own ancestors, which roughly simultaneously appeared during the Late Paleozoic, and became dominant members of the Latest Permian and Mesozoic floras. This means that extant gymnosperms all have their own crown clades evolved early in the Permian (Soltis et al., 2002b), and therefore has satisfied a precondition for establishing monophyletic gymnosperms that could have diverged about 274·5 myr ago based on molecular data by using the calibration point approach (Soltis et al., 2002b). Recently, a significant magnetostratigraphic marker, the late Permian Illawarra Reversal, was identified in the typical Late Palaeozoic Section near Taiyuan, Shanxi (Embleton et al., 1996) and is delimited a little above the bottom of the Tianlongsi Formation spanning 268 myr or so in the geological timescale. This has ascertained the age of the Late Permian floras containing Palaeognetaleana in Shanxi to about 270–250 myr ago, which may be near to the divergent time of gymnosperms based on the molecular estimate (Soltis et al., 2002b), or about 300 myr ago based on the estimation from MADS-box genes (Becker et al., 2000).
It is notable that some of the current results of molecular data have not entirely ruled out the possibility of an anthophyte clade sister to angiosperms (Sanderson and Doyle, 2001; Rydin et al., 2002; Soltis et al., 2002a). This opens up the potential to look deep within the phylogeny of seed plants to examine the sources of other characters such as sex distribution (bisexual), seed structure and others.
Acknowledgments
The project was supported by National Natural Sciences (No. 39970052) and funded by the Major Basic Research Projects of Ministry of Science and Technology, China (G2000077700). My great thanks go to Prof. J. A. Doyle (University of California) and Prof. P. K. Endress (University of Zurich, Switzerland) for their help in the initial stages of work on the fossil. I sincerely thank Dr Christopher J. Cleal (National Museum of Wales, Cardiff, UK) for his comments and encouragement. Many thanks also go to P. R. Crane (Royal Botanic Garden of Kew, UK), E. M. Friis (Swedish Museum, Sweden), J. Hilton (Natural Museum of Scotland, UK) and H. Kerp (Westfaelische Wilhelms-Universitet, Germany) for comments on a previous draft and to D. Watson (University of Manchester, UK) and an anonymous referee for their comments on the present version. I must also thank Dr D. R. Causton (University of Wales, UK) for his precise revision of the English in the paper; Mr Chen An-shu of our Institute for his help in preparing the manuscript; and Mrs Zhang Zhi-ping of Tianjin Center of Physical and Chemical Techniques for the SEM photographs.
LITERATURE CITED
- Arber EAN, Parkin J. 1908. The relationship of the angiosperms to the Gnetales. Annals of Botany 22: 489–515. [Google Scholar]
- Ash SR. 1972. Late Triassic plants from the Chinle Formation in north-eastern Arizona. Palaeontology 15: 598–618. [Google Scholar]
- Axsmith BJ, Taylor EL, Taylor TN. 1998. The limitations of molecular systematics: a palaeobotanical perspective. Taxon 47: 105–108. [Google Scholar]
- Becker A, Winter K-U, Meyer B, Saedler H, Theissen G. 2000. MADS-box gene diversity in seed plants 300 million years ago. Molecular Biology and Evolution 17: 1425–1434. [DOI] [PubMed] [Google Scholar]
- Bowe LM, Coat G, dePamphilis CW. 2000. Phylogeny of seed plants based on all three genomic compartments: Extant gymnosperms are monophyletic and Gnetales' closest relatives are conifers. Proceedings of the National Academy of Sciences of the USA 97: 4092–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw SM, Parkinson CL, Cheng YC, Vincent TM, Palmer JD. 2000. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proceedings of the National Academy of Sciences, USA 97: 4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw SM, Zharkikh A, Sung HM, Lau TC, Li WH. 1997. Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18s rRNA sequences. Molecular Biology and Evolution 14: 56–68. [DOI] [PubMed] [Google Scholar]
- Cornet B. 1996. A new gnetophyte from the Late Carnian (Late Triassic) of Texas and its bearing on the origin of the angiosperm carpel and stamen. In: Taylor DW, Hickey LJ, eds. Flowering plant origin, evolution, and phylogeny. New York: Chapman and Hall, 32–67. [Google Scholar]
- Crane PR. 1985. Phylogenetic analysis of seed plants and the origin of angiosperms. Annals of the Missouri Botanical Garden 72: 716–793. [Google Scholar]
- Crane PR. 1996. The fossil history of the Gnetales. International Journal of Plant Sciences 157 (6 Suppl.): S50–S57. [Google Scholar]
- Crane PR, Upchurch GR. 1987.Drewria potomacensis gen. et sp. nov. an early Cretaceous member of Gnetales from the Potomac Group of Virginia. American Journal of Botany 74: 1722–1736. [Google Scholar]
- Crane PR, Friis EM, Pederson, KR. 1995. The origin and early diversification of angiosperms. Nature 374: 27–33. [Google Scholar]
- DiMichele WA, Mamay SH, Chaney DS, Hook RW, Nelson WJ. 2001. An Early Permian flora with Late Permian and Mesozoic affinities from North-Central Texas. Journal of Paleontology 75: 449–460. [Google Scholar]
- DiMichele WA, Phillips TL, Olmstead RG. 1987. Opportunistic evolution: abiotic environmental stress and the fossil record of plants. Review of Palaeobotany and Palynology 50: 151–178. [Google Scholar]
- Donoghue MJ, Doyle JA. 2000. Seed plant phylogeny: demise of the anthophyte hypothesis? Current Biology 10: R106–R109. [DOI] [PubMed] [Google Scholar]
- Doyle JA. 1996. Seed plant phylogeny and the relationships of Gnetales. International Journal of Plant Sciences 157 (6 Suppl.): S3–S39. [Google Scholar]
- Doyle JA. 1998. Molecules, morphology, fossils, and the relationships of angiosperms and Gnetales. Molecular Phylogenetics and Evolution 9: 448–462. [DOI] [PubMed] [Google Scholar]
- Doyle JA, Donoghue MJ. 1986. Seed plant phylogeny and the origin of the angiosperms: an experimental cladistic approach. Botanical Review 52: 321–431. [Google Scholar]
- Doyle JA, Donoghue MJ. 1992. Fossils and seed plant phylogeny reanalyzed. Brittonia 44: 89–106. [Google Scholar]
- Embleton BJJ, McElhinny MW, Ma X, Zhang Z, Li ZX. 1996. Permo-Triassic magnetostratigraphy in China: the type section near Taiyuan, Shanxi Province, North China. Geophysical Journal International 126: 382–388. [Google Scholar]
- Endress PK. 1996. Structure and function of female and bisexual organ complexes in Gnetales. International Journal of Plant Sciences 157 (6 Suppl.): S113–S125. [Google Scholar]
- Endress PK. 2001. Origins of flower morphology. Journal of Experimental Zoology (Molecular Development and Evolution) 291: 105–115. [DOI] [PubMed] [Google Scholar]
- Florin R. 1951. Evolution in cordaites and conifers. Acta Horti Bergiani 15: 285–388. [Google Scholar]
- Frohlich MW. 1999. MADS about Gnetales. Proceedings of the National Academy Sciences of the USA 96: 8816–8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich MW, Parker DS. 2000. The mostly male theory of flower evolutionary origin: from genes to fossils. Systematic Botany 25: 155–170. [Google Scholar]
- Hansen A, Hansmann S, Samigullin T, Antonov A, Martin W. 1999.Gnetum and the angiosperms: molecular evidence that their shared morphological characters are convergent, rather than homologous. Molecular Biology and Evolution 16: 1006–1009. [Google Scholar]
- Hernandez-Castillo GR, Rothwell GW, Mapes G. 2001. Thucydiaceae fam. nov., with a review and reevaluation of Paleozoic walchian conifers. International Journal Plant Sciences 162: 1155–1185. [Google Scholar]
- Hughes NF. 1994.The enigma of angiosperm origin. Cambridge: Cambridge University Press. [Google Scholar]
- Kerp H. 1996. Post-Variscan late Paleozoic Northern Hemisphere gymnosperms: the onset to the Mesozoic. Review of Palaeobotany and Palynology 90: 263–285. [Google Scholar]
- Kerp H, Broutin J, Lausberg S, Aassoumi, H, 2001. Discovery of Latest Carboniferous-Early Permian radially symmetrical peltaspermaceous megasporophylls from Europe and North Africa. Comptes-Rendus de l'Academie des Sciences. Paris, Sciences de la Terre et des Planétes 332: 513–519. [Google Scholar]
- Kerp JHF, Poort RJ, Swinkels HAJM, Verwer R. 1990. Aspects of Permian palaeobotany and palynology. IX. Conifer-dominanted Rotliegend floras from the Saar-Nahe Basin (?Late Carboniferous-Early Permian; SW-Germany) with special reference to the reproductive biology of early conifers. Review of Palaeobotany and Palynology 62: 205–248. [Google Scholar]
- Krassilov VA. 1997.Angiosperm origin. Sofia: Academis PublishingHouse. [Google Scholar]
- Mamay SH. 1976. Paleozoic origin of the cycads. United States Geological Survey Professional Paper 934: 1–48. [Google Scholar]
- Meyen SV. 1984. Basic features of gymnosperm systematics and phylogeny as evidenced by the fossil record. Botanical Review 50: 1–111. [Google Scholar]
- Norin E. 1924. The lithological character of the Permian sediments of the Angara Series in Central Shanxi, N. China. Geologiska Föreningens i Stockholm Förhandlingar 46, 19–55. [Google Scholar]
- Osborn JM. 2000. Pollen morphology and ultrastructure of gymnospermous anthophytes. In: Harley MM, Morton CM, Blackmore S, eds. Pollen and spores: morphology and biology. Kew: Royal Botanic Gardens, 163–185. [Google Scholar]
- Osborn JM, Taylor TE, de Lima MR. 1993. The ultrastructure of fossil ephedroid pollen with gnetalean affinities from the Lower Cretaceous of Brazil. Review of Palaeobotany and Palynology 77: 171–184. [Google Scholar]
- Poort RJ, Kerp JHF. 1990. Aspects of Permian palaeobotany and palynology. XI. On the recognition of true peltasperms in the Upper Permian of Western and Central Europe and a reclassification of species formerly included in Peltaspermum Harris. Review of Palaeobotany and Palynology 63: 197–225. [Google Scholar]
- Qiu YL, Lee JH, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen ZD, Savolainen V, Chase MW. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and unclear genomes. Nature 402: 404–407. [DOI] [PubMed] [Google Scholar]
- Rodin RJ, Kapil RN. 1969. Comparative anatomy of the seed coats of Gnetum and their probable evolution. American Journal Botany 56: 420–431. [Google Scholar]
- Rothwell GW, Mapes G. 2001.Barthelia furcata gen. et sp. nov. with a review of Paleozoic coniferophytes and a discussion of coniferophyte systematics. International Journal of Plant Sciences 162: 637–667. [Google Scholar]
- Rothwell GW, Serbert R. 1994. Lignophyte phylogeny and the evolution of spermatophytes: a numerical cladistic analysis. Systematic Botany 19: 443–482. [Google Scholar]
- Rydin C, Källersjö M, Friis EM. 2002. Seed plant relationships and the systematic position of Gnetales based on nuclear and chloroplast DNA: conflicting data, rooting problems, and the monophyly of conifers. International Journal Plant Sciences 163: 197–214. [Google Scholar]
- Sanderson MJ, Doyle JA. 2001. Sources of error and confidence intervals in estimating the age of angiosperms from rbcL American Journal Botany 88: 1499–1516. [PubMed] [Google Scholar]
- Sanderson MJ, Wojciechowski MF, Hu J-M, Khan TS, Brady SG. 2000. Error, bias, and long-branch attraction in data for two chloroplast photosystem genes in seed plants. Molecular Biology and Evolution 17: 782–797. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Zanis MJ. 2002. Phylogeny of seed plants based on evidence from eight genes. American Journal of Botany 89: 1670–1681. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE, Savolainen V, Crane PR, Barraclough TG. 2002. Rate heterogeneity among lineages of tracheophytes: integration of molecular and fossil data and evidence for molecular living fossils. Proceedings of the National Academy of Sciences, USA 99: 4430–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SM. 1990. Adaptive radiation and macroevolution. In: Taylor PD, Larwood GP, eds. Major evolutionary radiations. Systematics Association, Special Volume 42. Oxford: Clarendon Press, 1–15. [Google Scholar]
- Taylor TN, Taylor EL. 1993.The biology and evolution of fossil plants. Englewood Cliffs, New Jersey: Prentice-Hall. [Google Scholar]
- Winter KU, Becker A, Muenster T, Kim JT, Saedler H, Theissen G. 1999. MADS-box genes reveal that gnetophytes are more closely related to conifers than to flowering plants. Proceedings of the National Academy of Science of the USA 96: 7342–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Q. 2000. Vegetation declination on the eve of the P-T event in North China and plant survival strategies: an example of Upper Permian refugium in northwestern Shanxi, China. Acta Palaeontologica Sinica 39 (Suppl.): 127–153. [Google Scholar]
- Wang Z-Q, Zhang Z-P. 1998. Gymnosperms on the eve of the terminal Permian mass extinction in North China and their survival strategies. Chinese Science Bulletin 43: 889–897. [Google Scholar]
- Wilson LR. 1959. Geological history of the Gnetales. Oklahoman Geological Notes 19: 35–40 [Google Scholar]
- Yao Z-Q. 1989. Psygmophylloids of the Cathaysia flora. Acta Palaeontologica Sinica 28: 171–191 [in Chinese and English]. [Google Scholar]
- Zhu J-N, Du X-M. 1981. A new cycad-Primocycas chinensis gen.et sp.nov. from the Lower Permian in Shanxi, China, and its significance. Acta Botanica Sinica 23: 401–404 [in Chinese]. [Google Scholar]