Abstract
Members of the extended Fc receptor-like (FCRL) family in humans and mice are preferentially expressed by B cells and possess tyrosine-based immunoregulatory function. Although the majority of these proteins repress B cell receptor-mediated activation, there is emerging evidence for their bifunctionality and capacity to counter-regulate adaptive and innate signaling pathways. In light of these findings, the recent discovery of ligands for several of these molecules has begun to reveal exciting potential for them in normal lymphocyte biology and is launching a new phase of FCRL investigation. Importantly, these fundamental developments are also setting the stage for defining their altered roles in the pathogenesis of a growing number of immune-mediated diseases. Here we review recent advances in the FCRL field and highlight the significance of these intriguing receptors in normal and perturbed immunobiology.
1 Introduction
The identification of a family of Fc receptor-like (FCRL) molecules over 10 years ago revealed a much richer landscape of genes related to the conventional Fc receptors (FCR) for IgG and IgE than was previously anticipated. Although their existence escaped attention for decades, investigation of the FCRLs is uncovering unexpected phylogenetic and immunoregulatory complexity for this ancient molecular cluster. Despite syntenic chromosomal linkage, similar genetic organization, and shared Ig superfamily (IgSF) membership with the classical FCRs, their species-specificity as well as differences in their structural features and expression patterns imply a high degree of evolutionary plasticity for the FCRLs in adaptive immunity. As their ligands and complex tyrosine-based functions become clear, we are realizing that parallel studies in humans, mice, and perhaps other models with be required to better delineate their biologic and pathologic contributions. In this review we discuss exciting new developments in the FCRL field that are beginning to unearth the biological roles of these molecules in host protection and disease at the nexus of innate and adaptive immunity.
2 Discovery and Characteristics of FCRL Family Members
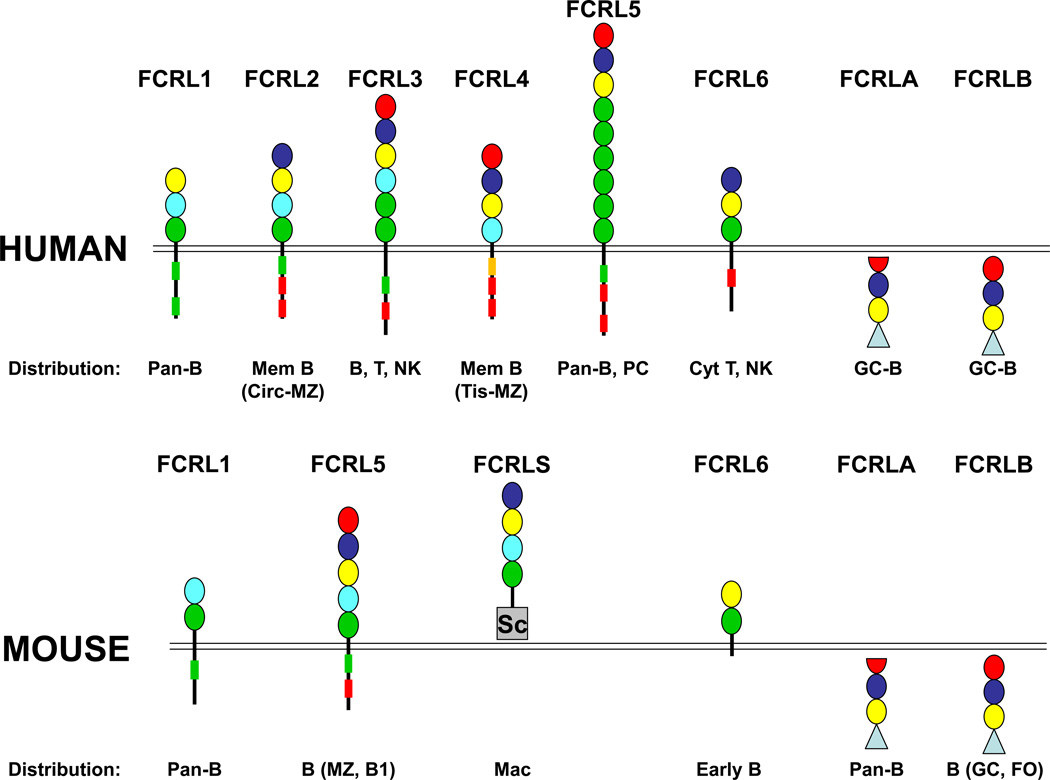
FCRL genes were discovered by several groups using different strategies and, as a result, a uniform nomenclature to designate them had to be established (Maltais et al., 2006). The first representative reported was a glycosyl-phosphatidylinositol (GPI)-anchored rat ortholog of FCRL6, initially termed gp42, that was identified in a search for markers of cytotoxic natural killer (NK) lymphocytes induced by IL-2 (Imboden et al., 1989, Seaman et al., 1991). However, it was not until meticulous work by the Dalla-Favera group nearly 10 years later that the breadth of this family became apparent. In an effort to define the genes joined at a t(1;14)(q21;q32) chromosomal translocation breakpoint in the FR4 multiple myeloma (MM) cell line, the second intron upstream of the exon encoding the C-terminal portion of the FCRL4 split signal peptide, originally named IgSF receptor translocation-associated gene 1 (IRTA1), was found fused to the intron proximal to the transmembrane encoding exon of IgA1 (Hatzivassiliou et al., 2001, Miller et al., 2002). Our bioinformatic approach of searching human genome sequences with a 32 amino acid consensus motif derived from the extracellular Ig-binding region of the classical FCRs yielded discovery of the FCR homolog (FCRH) family (Davis et al., 2001). In silico strategies were also employed by the Taranin group to identify molecules sharing features with the IgSF, FCR, and gp42 proteins (IFGP) (Guselnikov et al., 2002) and the Zhao laboratory to find novel Src homology (SH)-2 domain-containing phosphatase anchoring proteins (SPAP) (Xu et al., 2001). Additionally, using subtractive hybridization methodology, the B cell crosslinked by anti-IgM activation sequence (BXMAS) genes were found by Bothwell and colleagues (Nakayama et al., 2001). These studies collectively revealed that the human FCRL1-5 cluster spans a ~300 kB region of chromosome 1q21-22 at a locus telomeric of the high-affinity FcγRI/CD64 gene (FCGR1A) and encodes type I transmembrane glycoproteins with 3–9 extracellular Ig-like domains and cytoplasmic tails with immunoreceptor tyrosine-based activating (ITAM), switch (ITSM), and/or inhibitory (ITIM) motifs (Fig. 1). FCRL6, which also codes for a transmembrane receptor with similar features, was identified at a separate locus further telomeric and proximal to the high-affinity IgE (FCER1A) gene (Davis et al., 2002a). Finally, two additional relatives termed FCRLA and FCRLB were located proximal to the genes encoding the low affinity FcγRs (FCGR2-3) (Davis et al., 2002b, Facchetti et al., 2002, Mechetina et al., 2002, Masuda et al., 2005, Wilson and Colonna, 2005). Not surprisingly, the FCRL proteins encoded by this locus share significant sequence identity with the Ig-like domain subunits of the low affinity FcγRs and CD64/FcγRI. However, in contrast to other FCR/FCRL family members that reside at the cell surface, FCRLA and FCRLB lack transmembrane segments and are intracellular proteins. They also possess unique C-terminal mucin-like regions rich in serine/threonine, proline and leucine residues.
Figure 1.
Protein structure and distribution of human and mouse FCRL family members. Ig-like domains in the schematic diagram are color-coded to highlight their phylogenetic relationships. The first domain of FCRLA appears to be a degenerate Ig-like subunit and is thus truncated in the figure. The type-B cysteine rich scavenger receptor domain (Sc) of mouse FCRLS and mucin-like regions (triangles) of FCRLA and FCRLB are also specified. FCRL1-6 cytoplasmic tails possess potential consensus ITIM (L/V/I)-X-Y-X-X-(L/V/I) (red boxes), ITAM-like (E/D)-X-X-Y-X-X-(L/I)-X6–8-Y-X-X-(L/I) (green boxes), and ITSM (S/T)-X-Y-X-X-(V/I) (orange box) sequences. The expression patterns among B cells include memory (Mem), circulating (Circ) and tissue-based (Tis) marginal zone (MZ), plasma cell (PC), germinal center (GC), and follicular (FO) subsets. FCRL3 and FCRL6 are both expressed by cytotoxic (Cyt) T and NK cells, but FCRL3 is also found on CD4 T regulatory cells. Transcripts for FCRLS have been detected in macrophages (Mac).
Significant disparity in gene number as well as genetic and primary amino acid structure is evident for the murine relatives of human FCRLs. Three FCRL genes are located in tandem at a syntenic position of mouse chromosome 3 (Davis et al., 2002a, Guselnikov et al., 2002, Davis et al., 2004). Mouse Fcrl1 and Fcrl5 encode type I transmembrane proteins with moderately different features from their human cousins. Notably, mouse FCRL5 shares greater structural similarity to human FCRL2 and FCRL3 than its designated name suggests. Its closer relatedness to these receptors may also be supported by the expression patterns and ligands of these proteins (see below). By contrast, Fcrls, which is not present in the human genome, encodes a soluble chimeric protein with four Ig-like domains resembling human FCRL2, and a C-terminal type B scavenger receptor cysteine-rich domain. Three other FCRL relatives, Fcrl6, Fcrla, and Fcrlb are located in syntenic locations on mouse chromosome 1. Although mouse FCRL6 shares greater identity to rat gp42 than human FCRL6, FCRLA and FCRLB possess the highest interspecies orthology of the family.
3 Cellular Distribution of the FCRLs
FCRL gene expression is almost entirely restricted to lymphocytes and is preferentially concentrated within the B lineage. Transcript analyses from tissues or sorted cells by Northern blot, PCR, and in situ hybridization showed that FCRL1-5 expression increases as a function of B cell differentiation and peaks among circulating cells and those localized in secondary lymphoid tissues (Davis et al., 2001, Miller et al., 2002). The development of receptor-specific monoclonal antibodies (mAbs) confirmed these findings and enabled refined examination of their individual and sometimes overlapping expression patterns (see Fig. 1 summary). FCRL1 emerges at the pre B cell stage and increases with B cell maturation, peaking on naïve and memory subpopulations (Leu et al., 2005, Polson et al., 2006). Although this general distribution implies its practical utility as a pan B cell marker, FCRL1, as well as its other four relatives, are downregulated by activated germinal center (GC) B cells. Evidence that the FCRL1 mouse ortholog exhibits similar broad expression among B cells (Davis et al., 2004)(W.J. Won and R.S. Davis, unpublished results), suggests that despite their interspecies structural variation, their regulation is strongly conserved. By contrast, FCRL2-5 exhibit subset-specific differences in their expression by B cells. FCRL2 and FCRL3 both peak on memory B cells in the periphery and mark a circulating innate-like marginal zone (MZ) B cell equivalent (Weller et al., 2004, Li et al., 2013). Their presence on this latter subset is noteworthy given the discrete basal regulation of the mouse FCRL5 protein by innate-like MZ and B1 B cells in mice (Won et al., 2006). These specialized B cells are distinguished by their germline-biased Ig repertoires, potential to secrete broadly-reactive natural Abs, ability to respond to T cell-independent (TI) antigens, and involvement in primary humoral responses (Martin and Kearney, 2000, Cerutti et al., 2013). Consistent with the sensitivity of these cells to innate stimulation, both human FCRL2 and FCRL3 as well as FCRL5 in mice are strongly induced by Toll-like receptor (TLR) agonists (Li et al., 2013)(W.J. Won and R.S. Davis, unpublished results). However, FCRL3 is also individually expressed outside the B lineage by subpopulations of cytotoxic NK and CD8+ T cells as well as a dysfunctional population of CD4+ regulatory T cells (Polson et al., 2006, Nagata et al., 2009, Swainson et al., 2010). FCRL4 defines a subpopulation of tissue-based memory B cells with an activated phenotype and a discriminating transcript profile. These cells occupy sites in mucosa-associated lymphoid tissues (MALT) that correspond to an anatomical equivalent of the MZ (Falini et al., 2003, Ehrhardt et al., 2005, Ehrhardt et al., 2008); hence, FCRL4 is typically scarce among circulating B cell populations in healthy donors. FCRL5 has a broader B cell distribution that extends to, and reaches highest surface density on, terminally differentiated plasma cells (PC) derived from the bone marrow, tonsils, or spleen (Polson et al., 2006). By contrast, human FCRL6 is not expressed by B cells, but is rather a distinguishing surface glycoprotein of perforin-expressing cytotoxic NK and CD8+ T cells as well as a rare CD4+ T lineage subset with similar lytic features (Wilson et al., 2007, Schreeder et al., 2008, Kulemzin et al., 2011). Thus, FCRL3 and FCRL6 are expressed by non-B cells and share overlapping expression on lymphocytes with cytolytic potential. In mice, FCRL6 can be induced in T cells by IL-2, but is constitutively produced by B cell precursors (W.J. Won and R.S. Davis, unpublished results). Human FCRLA is predominantly found in subsets of GC B cells, mainly the proliferating centroblasts, but is expressed at some level by all B cell subsets in the tonsil, with the notable exception of PCs where it is very low/absent, (Davis et al., 2002b, Facchetti et al., 2002, Mechetina et al., 2002, Masir et al., 2004) and in freshly isolated blood B cells (Santiago et al., 2011). In mice, FCRLA is broadly expressed among peripheral B cells, being highest in PCs, but is downregulated by GC B cells (Wilson et al., 2010, Reshetnikova et al., 2012). FCRLB has been difficult to study because its low level transcripts are undetectable by Northern blot and even difficult to resolve by RT-PCR; with a single round of amplification, human FCRLB is found in placenta, kidney and spleen (Wilson and Colonna, 2005). Based on analysis of cell lines, FCRLB appears to be restricted among hematopoietic cells to the B lineage. To the extent that it has been examined in primary human tissue, FCRLB is restricted to the GC; however, FCRLB+ cells are very rare, small, non-proliferating (Ki-67−) B cells that do not co-express FCRLA (Wilson and Colonna, 2005). Thus, despite many shared features, these two proteins appear to have mutually exclusive expression patterns among B cells. Essentially nothing is known about mouse FCRLB, except that the gene knockout has no obvious phenotype (Masuda et al., 2010). Outside of the B lineage, both FCRLA and FCRLB have been reported to be expressed in human melanocytes and melanoma cells (Chikaev et al., 2005, Inozume et al., 2005). The function of FCRLA/B in melanocytes has not been examined, but the expression of these receptors in non-B cells may suggest a more general role for them as ER chaperones.
4 Emerging FCRL Ligands
A major hurdle for understanding the immunologic function of these receptors has been the enigmatic nature of their counterpart ligands. While most FCRLs still remain orphan receptors, ligands for several family members have recently been discovered (see summary in Table 1). There was early anecdotal evidence that FCRL4 and FCRL5 could bind heat-aggregated IgA and IgG (Hatzivassiliou et al., 2001), an anticipated finding given their homology to the classical FCRs. This unpublished data was unconfirmed until Polson et al. also detected interactions between FCRL5 and IgG when staining with a preparation of mixed isotypes, but reactivity was lost when individual subclasses were used (Polson et al., 2006). However, recent work by the Colonna group has confirmed and extended these early findings. By generating FCRL1-6 transient transfectants for flow cytometry-based Ig binding studies, Wilson et al. confirmed that FCRL4 and FCRL5, but not other FCRLs, can bind heat-aggregated IgA and IgG (Wilson et al., 2012). FCRL5 demonstrated relatively stronger binding to IgG1 and IgG2 aggregates than to IgG4, and reactivity required the three N-terminal Ig-like FCRL domains. The specificity of FCRL4 and FCRL5 interactions with Ig observed in these studies was further supported by blockade studies using receptor-specific mAbs.
Table 1.
Ligands identified for human and mouse FCRL family members
| Receptor | FCRL Ligands |
|---|---|
| Human | |
| FCRL1 | N.D. |
| FCRL2 | N.D. |
| FCRL3 | N.D. |
| FCRL4 | Heat-aggregated IgA - flow cytometry (Wilson 2012). |
| FCRL5 | Heat-aggregated IgG: IgG1, IgG2>IgG3>IgG4-flow cytometry (Wilson et al. 2012). Intact IgG; IgG1, IgG4 (~1µM) >IgG3 (18µM) >IgG2 (0.03–205µM) - SPR (Franco et al. 2013). |
| FCRL6 | MHC class II/HLA-DR (Schreeder et al. 2010). |
| FCRLA | Intracellular IgM and IgG (Wilson et al. 2010). Intracellular IgM, IgG, and IgA (Santiago et al. 2010). |
| FCRLB | N.D. |
| Mouse | |
| FCRL1 | N.D. |
| FCRL5 | OMCP (Orthopox MHC class I-like protein) (Campbell et al. 2010). |
| FCRL6 | N.D. |
| FCRLS | N.D. |
| FCRLA | N.D. |
| FCRLB | N.D. |
No data (N.D.); surface plasmon resonance (SPR).
The binding specificity and kinetics of FCRL5/IgG associations have now been independently confirmed by surface plasmon resonance (SPR) analyses. Using FCRL5 recombinant protein, the Tolnay laboratory validated interactions with IgG and similarly localized the binding interface with a panel of mAbs reactive with the three membrane distal domains of FCRL5 (Franco et al., 2013). Moreover, these studies also revealed several novel and unexpected features of the FCRL5-Ig interaction. First, although binding affinities were estimated overall in the micromolar (µM) range, variation was evident among monoclonal and polyclonal IgG1-4 subclass preparations. IgG1 and IgG4 bound with a KD of ~1 µM, but the affinity of IgG3 was about a log lower at ~10 µM. However, IgG2 bound over a range of affinities from 35 nM (nanomolar) to 205 µM that varied according to the Ig sample. Secondly, IgG bound FCRL5 with unusual heterogeneous two-state kinetics that differed among the subclasses. In general SPR sensorgrams demonstrated a fast initial on rate (Ka1) followed by a slow secondary association (Ka2), whereas dissociation at the end of the injection was initially fast (Kd1) followed by a slower secondary phase (Kd2). Notably, these parameters differ from the 1:1 kinetics that typify classical FCR/Ig interactions (Bruhns et al., 2009) and indicate that other properties, beyond the isotype, influence FCRL5’s recognition of IgG. Third, in contrast to the FCRs for IgG, IgM and IgE, which interact strictly with the Fc region, high-affinity binding to FCRL5 required intact Ig molecules. Enzymatic digestion and biochemical strategies to correlate structure-function contributions of IgG1 anatomy with the two-state kinetics evident by SPR confined a primary interaction with the Fc portion and a secondary interaction with the F(ab’)2 region. Finally, binding affinities were also strongly dependent on Ig glycosylation status. Sialic acid enrichment of IVIg preparations promoted higher affinity kinetics, whereas deglycosylation abrogated nearly all binding activity. These intriguing findings introduce a second immunoregulatory IgG-binding receptor on B cells that possesses complex binding properties and potentially higher differential affinity for some intact IgG isotypes than CD32/FcγRIIB, which has a KA of ~ 2.5 × 104 – 2 × 105 M−1, depending on the IgG isotype (Bruhns et al., 2009).
Evidence for unconventional Ig binding has also been shown for the FCRLA intracellular protein, whose two Ig domains resemble two of the three Ig-like subunits present in the high affinity CD64/FcγRI. Early studies using chimeric proteins artificially expressed on the cell surface failed to demonstrate interactions with Igs (Facchetti et al., 2002); however, immunoprecipitation of endogenous FCRLA disclosed its co-association with intracellular IgM, IgG, and IgA in cell lines as well as in primary B cells (Wilson et al., 2010, Santiago et al., 2011). The elevated expression of FCRLA in GC B cells, together with its ability to bind multiple isotypes of intracellular Ig, suggests a possible role for FCRLA in Ig retention during affinity maturation. Indeed, FCRLA has been shown to preferentially associate in the ER with the secretory versus membrane form of IgM in the GC-like human B cell line Ramos (Santiago et al., 2011). Although FCRLB has even greater sequence identity with CD64, no evidence of Ig binding has been established for it yet and, like the Fcrla knockout mouse (Wilson et al., 2010), the Fcrlb knockout mouse had no discernible phenotype (Masuda et al., 2010).
Aside from Ig, MHC-related proteins have been identified as ligands for two other FCRLs, human FCRL6 and mouse FCRL5. FCRL6 is not expressed on B cells but on cytotoxic T cells and NK cells. To search for its ligand(s), we employed a cell line engineered with an NFAT driven GFP reporter that was co-transduced with a construct encoding the human FCRL6 extracellular region fused to the ITAM-bearing mouse CD3ζ cytoplasmic tail (Schreeder et al., 2010). GFP induction was triggered when cells from different sources expressing MHC class II were used for co-culture assays. These studies defined HLA class II as an FCRL6 ligand. Importantly, variability in FCRL6 staining of transductants expressing MHCII heterodimers with different beta subunits indicated that FCRL6 binding affinities may differ according to the MHCII haplotype. These studies thus introduce a novel interaction between FCRL6 expressing cytotoxic NK and T lymphocytes that are critical for maintaining cell-mediated immunity and antigen presenting cells or other cells that upregulate MHCII. Another MHC-related protein was discovered as a mouse FCRL5 ligand. Using a hidden Markov model to identify MHC-like viral proteins that might function as immune decoys, Campbell et al. found a immunoevasin encoded by a cowpox virus termed orthopox MHC class I protein (OMCP). OMCP had first been identified as a ligand for the NK cell activation receptor NKG2D and could suppress its role in cytotoxicity (Campbell et al., 2007). However, beyond NK cells, OMCP also bound innate-like MZ and B1 B cells. An expression cloning approach defined mouse FCRL5 as a second OMCP receptor and the use of blocking mAbs and receptor mutants narrowed the binding interface to FCRL5’s three N-terminal domains (Campbell et al., 2010). Although the functional impact of these associations is not yet clear, the tyrosine-based regulatory potential of these receptors, their interactions with fundamental elements of adaptive immunity, and exploitation as targets of manipulation by pathogens underscore their critical role in lymphocyte biology.
5 Functional and Regulatory Properties
Roles in adaptive B cell signaling
In addition to their divergent extracellular Ig-like domain configurations, the cytoplasmic properties of FCRLs are also more complex than those of the classical FCRs. A common theme among IgSF protein families such as the FCR, leukocyte Ig-like receptors (LILR), and paired Ig-like receptors (PIR) is to balance cellular responses by expressing representatives with either activating or inhibitory capacity. These tyrosine-based signals may be transmitted directly via motifs in their cytoplasmic tails or indirectly through non-covalent transmembrane interactions with adaptor proteins harboring cytoplasmic ITAMs. In contrast to this form of bimodal regulation using separate paired receptors, most FCRL cytoplasmic tails possess both ITAM-like and ITIM elements. The possession of these tandem intracellular sequences indicates that the majority of these molecules may be capable of exerting dual-modulation in an autonomous fashion. FCRL1 appears to be an exception to this. It has two ITAM-like sequences and serves as a co-activation receptor. Its ligation by receptor-specific mAbs results in its tyrosine phosphorylation (pTyr) and stimulates human B cell proliferation (Leu et al., 2005). Moreover, cross-linking FCRL1 with the B cell receptor (BCR) augments activation as indicated by enhanced calcium flux and B cell proliferation. Our unpublished observations of the mouse FCRL1 protein show that it has similar activating properties (W.J. Won and R.S. Davis, unpublished results). FCRL1 is also unique among FCRL in humans and mice by virtue of a charged glutamic acid residue in its transmembrane region. This feature indicates that FCRL1 likely co-associates with another partner in cis, but what effector proteins are recruited to its intracellular tyrosine-based sequences or its acidic transmembrane region remain under investigation.
Work exploring the contributions of the FCRL2-5 cytoplasmic tyrosine-based motifs on BCR-mediated activation has been carried out by several groups (Ehrhardt et al., 2003, Haga et al., 2007, Kochi et al., 2009, Jackson et al., 2010). Details of these mutagenesis and chimeric receptor analyses have been carefully summarized in a recent review (Ehrhardt and Cooper, 2011). Despite the binary potential implied by their composite intracellular regions, in general BCR co-ligation studies have identified a suppressive function for them. While their engagement alone does not appear to impact basal B cell function, cross-linkage with the BCR induces pTyr of FCRL2-5 and coincident docking of the SHP-1 and/or SHP-2 SH-2 domain-containing phosphatases at consensus ITIMs (Fig. 2). Accordingly, these repressive components attenuate antigen receptor-mediated calcium mobilization and MAPK activation. However, these studies have also uncovered subtle hints of possible dual functionality. This was initially suggested by the potential of FCRL3 to recruit Syk and ZAP-70 as well as SHP-1 and SHP-2 (Xu et al., 2002), a finding that was recently confirmed (Kochi et al., 2009). Moreover, experiments employing B cell lines transfected with disabled FCRL2-5 ITIM mutants frequently result in enhanced calcium flux compared to BCR engagement alone.
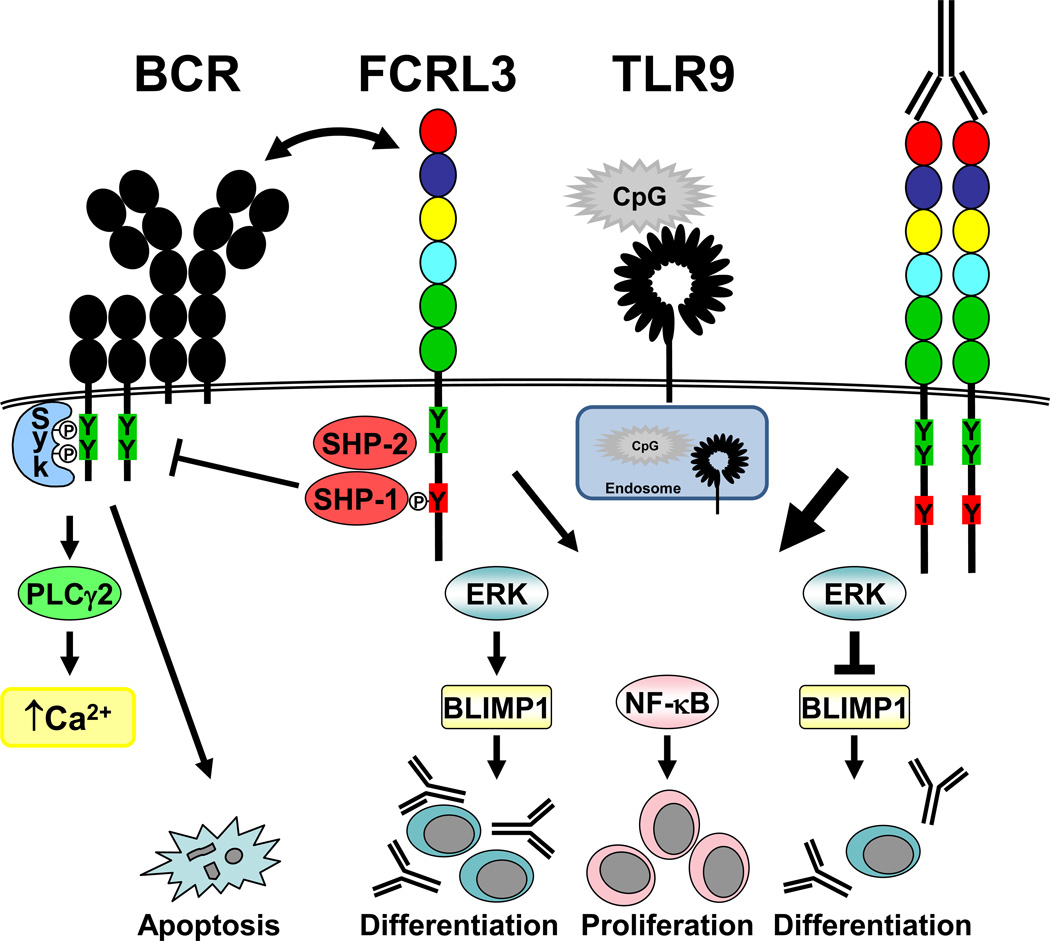
Figure 2.
Differential FCRL3 regulation of adaptive and innate signaling pathways. Co-ligation of FCRL3 with the BCR induces pTyr of the receptor, facilitates the recruitment of SHP-1 and SHP-2 that inhibit Syk and PLCγ2 phosphorylation, and suppresses downstream calcium signaling and apoptosis. Following exposure to the CpG DNA TLR9 agonist, FCRL3 expressing B cells are globally pTyr and activate the NF-κB/p-p65 and MAPK/pERK and p-p38 pathways that drive proliferation. pERK additionally induces expression of the BLIMP1 plasma cell (PC) commitment factor that stimulates B cell differentiation and Ab production. By contrast, simultaneous cross-linking of FCRL3 with receptor-specific mAbs in TLR9 activated B cells significantly elevates whole pTyr, p-p65, pERK, and p38 to promote proliferation and survival. However, augmented pERK activation in TLR9/FCRL3 co-stimulated B cells represses BLIMP1 induction and abrogates PC differentiation and Ab production.
To more carefully investigate their suspected dualistic function, we dissected the tyrosine-based regulation of the mouse FCRL5 ortholog that like human FCRL2-5 similarly possesses both cytoplasmic ITAM-like and ITIM consensus sequences. Initial work in WEHI231 and primary MZ B cells showed that FCRL5 also inhibits BCR-mediated calcium signaling (Won et al., 2006), but surprisingly had little impact on activation in innate-like B1 B cells that also discretely express it. As in earlier work (Ehrhardt et al., 2003), we engineered a panel of chimeric receptor mutant constructs comprised of the extracellular and transmembrane portions of mouse FcγRIIB fused to different FCRL5 Y>F tail variants for transduction into the FcγR deficient IgG2a class-switched A20IIA1.6 B cell line (Zhu et al., 2013). This system permits a comparative analysis of downstream signaling pathways engendered by chimeric receptor/BCR co-engagement by using intact anti-mouse IgG versus BCR-only triggering using F(ab’)2 fragments (Okazaki et al., 2001). These studies indeed disclosed inverse contributions for the FCRL5 intracellular motifs on BCR driven calcium flux and MAPK activation. Effector recruitment experiments identified binding of SHP-1 at the ITIM and the Lyn Src family kinase at the N-terminal ITAM-like tyrosine residue, but the C-terminal tyrosine was dispensable. These relationships were also confirmed in sorted primary MZ and B1 B cells. To further validate these findings in primary cells and to investigate the apparent impotent effects of FCRL5 on BCR activation in B1 B cells, we utilized mice deficient in Lyn or SHP-1 activity (motheaten/Mev) (Shultz et al., 1993, Chan et al., 1997). Both of these models have expanded B1 B cells, but they express FCRL5 at levels comparable to C57BL/6 wild-type mice. Using B1 B cells from these mutant strains allowed us to directly deconstruct the impact of these opposing signaling proteins on calcium flux and whole-cell pTyr signaling and correlate observations made in A20IIA1.6 cells. These findings revealed a critical role for the SHP-1/Lyn signaling circuit in balancing FCRL5 function. These binary regulatory properties were unique compared to other well-studied inhibitory receptors including CD5, CD22, CD32, and CD70, which failed to acquire enhancing function in SHP-1 deficient B cells. Moreover, the relative activity of SHP-1/Lyn differed in MZ versus B1 B cells. The dominant inhibitory function for FCRL5 directly correlated with a two-fold higher level of SHP-1 in MZ B cells, whereas the lack of FCRL5 influence in B1 B cells was ascribed to more balanced SHP-1/Lyn activity in this subset. These data provide robust molecular and functional evidence for novel dual-regulatory features of FCRL molecules and uncover subset-specific differences in their activity in innate-like B cells.
Influence on innate-like B cell responses
Although the majority of FCRL signaling work has been focused on the modulation of antigen-receptor activation pathways in B cells, more recent studies have begun to explore the effects of human FCRLs on innate-driven cascades. Extending work on FCRL4 signaling, Sohn et al. detected constitutive pTyr and SHP-1 and SHP-2 binding to FCRL4 in transfected unstimulated Ramos B cells (Sohn et al., 2011). BCR crosslinking did not appear to influence these relationships, but all three tyrosine residues were required for proximal inhibition of Syk activation and the downstream PLCγ2, Vav, and calcium signaling pathways by FCRL4. Consequently, FCRL4 also halted CD69 induction following BCR co-ligation, but its expression alone independently impaired immune synapse formation as determined by time-lapse imaging using TIRF microscopy. However, in addition to adaptive stimulation, the Pierce group also examined the impact of FCRL4 on TLR responses. Remarkably, exposure of FCRL4 expressing B cells to the TLR9 agonist CpG prompted co-localization of these receptors in endosomes and the upregulation of CD23. These results strongly indicate a differential regulatory role for FCRL4 in adaptive versus innate signaling.
Additional evidence for these proteins promoting TLR-mediated signaling has also been observed for FCRL3. We recently examined the impact of this disease-associated receptor (see below) on TLR9-mediated B cell responses. FCRL3 engagement with receptor-specific mAbs augmented TLR9 triggered blood B cell proliferation, survival, and induction of the CD25, CD86, and HLA-DR activation markers (Li et al., 2013) (see Fig. 2). Remarkably though, FCRL3 had inverse effects on Ig production. To examine its role in TI PC generation, we adapted a cord blood differentiation model (Capolunghi et al., 2008). Culturing transitional B cells with CpG 2006 and FCRL3 mAbs promoted B cell proliferation, but halted the differentiation of Ab secreting cells. Flow-based analyses revealed that FCRL3 enhances CpG-mediated NF-κB p65 and MAPK pERK and p38 activation. Because ERK signaling can modulate the expression and regulation of the PC commitment factor BLIMP1 (Rui et al., 2003, Yasuda et al., 2011), we considered this pathway as a mechanistic link for these surprising observations. Exposure of the FCRL3-expressing SUDHL5 B cell line to CpG indeed upregulated BLIMP1 expression, but coincident FCRL3 ligation substantially blocked induction of this repressor protein. Consequently, ERK-dependent BLIMP1 suppression transmitted by FCRL3 could be restored by treatment with a MEK inhibitor. These data provide additional support for counter-regulatory functions of FCRL proteins in adaptive and innate B cell responses. Furthermore, the finding that FCRL3 modulates the differentiation of Ab secreting cells may be important in its implicated role in the pathogenesis of autoimmune (AI) disorders discussed below.
Stimulatory properties for FCRL5 in the interplay of adaptive and innate pathways have also been found by the Tolnay group. Their earlier studies had shown that the viral Notch analog Epstein-Barr virus nuclear antigen 2 (EBNA2) could induce FCRL5 expression via interactions with the CBF1/RBP-Jκ DNA-binding protein in its promoter (Mohan et al., 2006). In a follow up analysis they investigated FCRL5 modulation following exposure to different stimuli and explored the consequences of its ligation on B cell responses. Although naïve B cells isolated from blood modestly upregulated protein levels when exposed to CpG, FCRL5 was markedly induced by anti-Ig co-stimulation (Dement-Brown et al., 2012). By contrast, the addition of T cell-dependent (TD) stimuli in the form of anti-CD40 and IL-2 had little effect. However, this combination of stimuli along with mAb-directed co-ligation of FCRL5 and the BCR enhanced B cell proliferation during TLR9 stimulation. This approach yielded other positive effects including the generation of IgG and IgA isotype switched cells as well as unusual cells that co-expressed several Ig isotypes. This phenomenon has been previously seen in patients with hairy cell leukemia (HCL) (Forconi et al., 2001). It is important to note that, despite similar effects on proliferation, these results concerning a positive role for FCRL5 in driving isotype switched cells may conflict with our recent findings that FCRL3 inhibits PC generation and Ig secretion. Given the alternate experimental strategies, including the use of T cell help that can relieve ERK-mediated BLIMP1 repression (Rui et al., 2006), follow up studies will be required to carefully dissect the nature of these divergent outcomes.
Insight from in vivo models
Finally, little is known about the in vivo roles of these molecules as only a few genetically deficient mice have been generated. To this point, two transgenic models have now been published describing mice with targeted disruption of Fcrla and Fcrlb. The Colonna group developed Fcrla−/− mice by cre-mediated ablation of the third and fourth exons that encode the two Ig-like domains in 129 ES cells (Wilson et al., 2010). Lymphocyte development was grossly normal including CD4 and CD8 T cell populations in the thymus and B cells in the bone marrow and spleen. Lymphoid architecture of splenic GCs that formed following challenge with SRBCs was also unremarkable in these mice and the absence of FCRLA protein was confirmed with rabbit polyclonal antisera. However, the availability of pAbs did clarify that intracellular FCRLA expression is restricted to and evident throughout B cell development in wild-type mice. Although primary humoral responses to SRBCs were unperturbed, secondary challenge indicated significantly higher anti-SRBC IgG1 levels in Fcrla−/− animals. However, immunization with another TD antigen NP-KLH did not show alterations in primary or secondary NP-specific IgM or IgG responses as a function of the dose or memory recall time out to 6 months. TI type II responses were also intact. Furthermore, despite the ability of human FCRLA to bind intracellular Ig, the quality and functional activity of Ig produced by Fcrla−/− mice was also normal. Aside from potentially disadvantageous strain-specific regulatory differences (see below), alternative immune challenge strategies or crosses with disease-susceptible models maybe required to unveil the subtle phenotype of these mice. However, it is also possible that FCRLA possesses redundant properties to other Ig-binding chaperones. Because FCRLB has similar features it could certainly serve as a candidate, although the evidence to date suggests that these to molecules are not co-expressed, at least in humans (Wilson and Colonna, 2005).
The Fcrlb gene was targeted by the Burrows group with a construct designed to replace ~1.5 kb of its 5’ upstream sequence including the promoter as well as exons 1, 2, and the 5’ end of exon 3 with neomycin cassette in 129 ES cells (Masuda et al., 2010). Despite the lack of FCRLB-specific Abs, a PCR-based analysis validated loss of Fcrlb expression at the transcript level. Mice were viable and backcrossed with C57BL/6. Similar to Fcrla deficiency, there were no global differences in lymphocyte development either from bone marrow and spleen-derived B lineage cells or CD4 and CD8 T cell populations from the thymus or spleen. Although in vitro proliferation studies were unrevealing, TD challenge with NP-CGG indicated enhanced NP-specific IgG1 Ab responses that were more pronounced for those with high-affinity. Moreover, ELISPOT assays showed increased numbers of Ab secreting cells in the spleen and bone marrow. Unfortunately, these results were confounded by a 13 bp deletion in the Fcgr2b promoter common to AI mouse strains including 129. This promoter variation disturbs two putative transcription factor binding sites for AP4 and an S box resulting in reduced FcγRIIB expression and inhibitory function (Pritchard et al., 2000). Thus, strains harboring this anomaly have relatively more exuberant TD Ab responses and a greater propensity to develop auto-Abs than mice with intact regulatory regions that are governed by higher FcγRIIB expression and consequent SHIP1 repression (Xiu et al., 2002). Unfortunately, PCR amplification clarified that Fcrlb deficient mice indeed possess this deletion making the independent impact of FCRLB difficult to discern. Furthermore, the use of 129 background ES cells for the generation of Fcrla knockout mice may also provide rationale for the enhanced SRBC production seen in these mice (Wilson et al., 2010). Investigators will need to be attentive to this issue and craft suitable strategies for accurately assessing humoral responses in FCRL-related transgenic mice.
6 FCRL Involvement with Disease
Given their preferential expression by B cells, it comes as no surprise that since their initial discovery, associations for FCRL family members with immune-mediated disorders have been steadily growing. Not only are they candidate biomarkers for clinical diagnosis and prognosis as well as logical therapeutic targets, but roles for them in disease pathogenesis are also becoming clear. As nearly 75% of leukemias and lymphomas are B cell-derived, multiple groups have detected their expression and dysregulation in various lymphoproliferative disorders. In fact, their involvement in B cell malignancies first led to the Dalla-Favera laboratory’s unearthing of FCRL4/IRTA1 as a partner joined at a t(1;14)(q21;32) translocation breakpoint in a MM cell line (Hatzivassiliou et al., 2001, Miller et al., 2002). Northern blot analyses of Burkitt lymphoma (BL) cell lines harboring 1q21 abnormalities revealed upregulation of FCRL5/IRTA2 in the majority of samples. Early searches of FCRL expressed sequence tags (ESTs) in the Lymphochip microarray database also showed differential upregulation for the FCRLs among diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia (CLL) samples (Alizadeh et al., 2000). The availability of mAbs confirmed FCRL1-5 protein surface expression on DLBCL, FL, CLL, BL, HCL, and mantle cell lymphoma (MCL) samples (Ise et al., 2005, Polson et al., 2006, Du et al., 2008). However, studies by Ise et al. also detected soluble FCRL5 in the sera of patients with various B lineage malignancies (Ise et al., 2007). Given the lack of diagnostic markers for MZ lymphomas (MZL) and evidence that FCRL4 distinguishes a subset of B cells positioned near the epithelium in MALT corresponding to the MZ, Falini et al. recently investigated its use as a novel histopathologic marker. FCRL4 was identified in the majority of nodal (154/210 73%) and extranodal (307/329 93%) MZLs, but was not present in the subtype derived from the spleen (Falini et al., 2012).
Apart from their applicability in diagnosis, the use of FCRLs as prognostic biomarkers has also been under investigation. CLL, the most common leukemia in Western countries, can be segregated into two subtypes that differ in clinical aggressiveness according to the degree of somatic hypermutation in the heavy chain variable region (IGHV) gene expressed by the clonally expanded B cells (Damle et al., 1999, Hamblin et al., 1999). To assess whether FCRL1-5 might be useful surrogates for predicting IGHV mutation status, we analyzed 107 CLL samples, including 55 mutated-indolent and 52 unmutated-aggressive patients, with a panel of FCRL-specific reagents by flow cytometry (Li et al., 2008). While FCRL1-3 and FCRL5 were all significantly upregulated by the mutated-indolent subtype, FCRL4 was not detected. Remarkably, FCRL2 emerged as 94% concordant with IGHV status and was superior to two established markers of aggression, CD38 and ZAP-70 (Rassenti et al., 2008), in predicting this hallmark feature, and by multivariate analysis was more robust at forecasting first time to progression. Current validation of these initial findings using optimized reagents and expanded samples shows growing promise for FCRL2 as a novel biomarker in CLL.
Strategies to immunotherapeutically target these molecules has also been explored. Because FCRL1 is broadly expressed by B cells it may be a useful candidate. Work by Du et al., who have retained an interest in immunotoxin treatment approaches, found FCRL1 on the majority of CLL, FL, HCL, and MCL samples analyzed and explored the cytotoxicity of anti-FCRL1 toxin conjugated mAbs (Du et al., 2008). Additionally, since FCRL5 is present on PCs, it is being pursued as a tool for MM immunotherapy. An analysis of bone marrow aspirates from MM, monoclonal gammopathy of unknown significance (MGUS), and healthy donors confirmed FCRL5 expression on PCs and the development of antibody-drug conjugates has shown promising preclinical efficacy for targeting it in xenograft models (Elkins et al., 2012).
Several FCRLs have also drawn interest by virtue of their upregulation among lymphocyte populations in individuals with infectious diseases. Perhaps the best investigated representative in this context is the appearance of FCRL4+ cells in the circulation of patients afflicted with chronic viral diseases including HIV and Hepatitis C. However, a similar innate-like B cell population has also been found in patients with combined variable immunodeficiency (CVID) (Rakhmanov et al., 2009). Moir et al. identified a subset of CD19+CD20+CD27−CD21−CD10− B cells in the blood of viremic HIV patients that surprisingly co-expressed FCRL4 (Moir et al., 2008). This subpopulation exhibited features of ‘exhaustion’ akin to T cells in persistent LCMV infections (Zajac et al., 1998, Wherry et al., 2007), which are characterized by the upregulation of inhibitory receptors as well as diminished proliferative potential and replication history. Despite limited Ig diversity, this distinct tissue-like FCRL4+ subset possessed an Ab repertoire enriched for HIV-specific antigens, implying a key role for it in effector humoral responses. To clarify its contribution to their arrested function, a siRNA approach found that knockdown of FCRL4 and several other inhibitory receptors could restore BCR-mediated proliferation, HIV-specific Ab responses, as well as cytokine and chemokine production (Kardava et al., 2011). These findings suggested that lingering tonic signaling by the HIV pathogen might lead to reciprocal dampening mechanisms in these B cells. However, exciting new findings by the Fauci laboratory have provided a novel perspective on the ability of HIV to directly handicap B cell responsiveness. Their earlier work had demonstrated that the gp120 HIV envelope protein can directly bind the α4β7 integrin on NK and T cells (Arthos et al., 2008). In a recent study, gp120 was similarly found to interact with this ligand on B cells and in turn inhibit proliferation and cell cycle progression (Jelicic et al., 2013). Microarray profiling disclosed the upregulation of TGFβ1 together with related elements of this cascade and FCRL4. Because TGFβ has repressive effects on B cell function (Kehrl et al., 1991), the investigators established that gp120/α4β7 integrin binding initiated an axis of suppression by triggering TGFβ secretion and the autocrine induction of FCRL4, as well as downmodulation of the CD80 co-stimulatory protein. These results have important implications for understanding the humoral dysfunction in HIV patients and could be informative for clarifying why a similar FCRL4+ population materializes in the circulation of individuals infected with malaria and Hepatitis C (Charles et al., 2008, Weiss et al., 2009). How FCRL4 contributes to B cell impairment in these chronic infections and in what way its newfound IgA ligand is integrated will require further study (Wilson et al., 2012).
With regard to tolerance and AI, disease risk associations for single nucleotide polymorphisms (SNP) located in the intergenic non-coding and coding regions of FCRL genes have been mounting in a variety of disorders and syndromes. Pioneering work by Kochi et al. surveyed a ~2 Mb region around the FCRL1-5 locus and identified 41 SNPs in the gene cluster including one that had a peak association among 658 Japanese controls and 830 individuals afflicted with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Grave’s disease, and other types of AI (Kochi et al., 2005). The principal variant (rs7528684; P=8.5 × 10−7; OR=2.15; 95% confidence interval=1.58–2.93) was located in a potential NF-κB consensus binding motif within the FCRL3 promoter region, 169 bp upstream of the transcription initiation site. Intriguingly, the C susceptibility allele of this −169T→C SNP generated a more orthodox NF-κB binding sequence, fostered higher promoter activity via p50, p65, and c-Rel binding, and exerted a dose dependent regulatory effect on FCRL3 transcript and protein expression as well as on auto-Ab production (Kochi et al., 2005, Gibson et al., 2009). This report has stimulated over 80 publications that have focused on this functional SNP as well as others among the FCRLs in a multitude of AI disorders. A recent genome-wide association study of Grave’s disease in a Chinese-Han population has verified the FCRL3 association and refined the analysis of FCRL SNPs in this 1q21 region (Zhao et al., 2013). Accordingly, there is now also evidence that FCRL3 is modulated by this SNP in T cells and is associated with clinical progression in RA (Maehlen et al., 2011, Bajpai et al., 2012). Confirmation that the −169 SNP confers risk or protection in different AI conditions has led to the candidature of FCRL3 as a general AI susceptibility gene (Chistiakov and Chistiakov, 2007). However, there have also been conflicting results for many analyses that find no link with AI disease susceptibility. These incompatible outcomes may reflect differences in racial and ethnic backgrounds. An updated meta-analysis was recently performed to assess the growing number of FCRL3 case-control association studies and highlight its heterogeneous pathogenic potential in AI (Yang et al., 2013). These intriguing genetic relationships, along with our growing understanding of FCRL3’s complex influence on lymphocyte biology in innate versus adaptive responses, have the potential to provide exciting new insight into AI disease pathogenesis.
7 Conclusions
Substantial recent progress has been made in the FCRL field and these discoveries are beginning to unravel fundamental roles for this extended family in the immune system. The recent identification of ligands for several FCRLs presents a new gateway for realizing their biology, but further work will be required to understand the functional consequences of these interactions. How their complex dual-regulation is integrated during these encounters and how they are impacted by innate and adaptive responses will also need to be explored. Another major hurdle has been their evident interspecies differences and a lack of fruitful genetic deficiency models for study in mice. However, several trends are beginning to take shape. For example, the preferential expression of human FCRL2-FCRL3 as well as mouse FCRL5 by innate-like MZ B cells and their capacity to promote TI responses suggests that the regulation of these genes is to a certain extent conserved. Thus, investigating these relatives in parallel will be important. Furthermore, FCRL4’s unique distribution among innate-like tissue-based B cells and ability to enhance TI signaling indicates that several FCRLs serve as facilitators of innate stimulation. Determining how their tyrosine-based signaling features modulate TLR versus BCR activation is another area ripe for investigation. Finally, their significance as pathologic, diagnostic, prognostic, and therapeutic agents is showing great promise in a large number of lymphoid malignancies and immune-mediated disorders. In conclusion, recent advances have launched a new phase of exploration for FCRL family members in lymphocyte biology. These intriguing developments portend key roles for these receptor-genes in normal and perturbed immunity and we expect this momentum will accelerate our basic and therapeutic understanding of the FCRLs in the coming years.
Acknowledgments
P.D.B. was supported by NIH grant AI100076. R.S.D. was supported by funding from the Lupus Research Institute, the UAB Center for AIDS Research (AI027767), and NIH grants AI097729 and CA161731.
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- Bajpai UD, Swainson LA, Mold JE, Graf JD, Imboden JB, McCune JM. A functional variant in FCRL3 is associated with higher Fc receptor-like 3 expression on T cell subsets and rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64:2451–2459. doi: 10.1002/art.34457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Davis RS, Lilly LM, Fremont DH, French AR, Carayannopoulos LN. Cutting edge: FcR-like 5 on innate B cells is targeted by a poxvirus MHC class I-like immunoevasin. J Immunol. 2010;185:28–32. doi: 10.4049/jimmunol.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J Exp Med. 2007;204:1311–1317. doi: 10.1084/jem.20062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, et al. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–1356. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikaev NA, Bykova EA, Najakshin AM, Mechetina LV, Volkova OY, Peklo MM, et al. Cloning and characterization of the human FCRL2 gene. Genomics. 2005;85:264–272. doi: 10.1016/j.ygeno.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Chistiakov AP. Is FCRL3 a new general autoimmunity gene? Hum Immunol. 2007;68:375–383. doi: 10.1016/j.humimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- Davis RS, Dennis G, Jr., Odom MR, Gibson AW, Kimberly RP, Burrows PD, et al. Fc receptor homologs: newest members of a remarkably diverse Fc receptor gene family. Immunol.Rev. 2002a;190:123–136. doi: 10.1034/j.1600-065x.2002.19009.x. [DOI] [PubMed] [Google Scholar]
- Davis RS, Li H, Chen CC, Wang YH, Cooper MD, Burrows PD. Definition of an Fc receptor-related gene (FcRX) expressed in human and mouse B cells. Int.Immunol. 2002b;14:1075–1083. doi: 10.1093/intimm/dxf074. [DOI] [PubMed] [Google Scholar]
- Davis RS, Stephan RP, Chen CC, Dennis G, Jr., Cooper MD. Differential B cell expression of mouse Fc receptor homologs. Int Immunol. 2004;16:1343–1353. doi: 10.1093/intimm/dxh137. [DOI] [PubMed] [Google Scholar]
- Davis RS, Wang YH, Kubagawa H, Cooper MD. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc.Natl.Acad.Sci.U.S.A. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol. 2012;91:59–67. doi: 10.1189/jlb.0211096. [DOI] [PubMed] [Google Scholar]
- Du X, Nagata S, Ise T, Stetler-Stevenson M, Pastan I. FCRL1 on chronic lymphocytic leukemia, hairy cell leukemia, and B-cell non-Hodgkin lymphoma as a target of immunotoxins. Blood. 2008;111:338–343. doi: 10.1182/blood-2007-07-102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR, Cooper MD. Immunoregulatory roles for fc receptor-like molecules. Curr Top Microbiol Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci U S A. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR, Hijikata A, Kitamura H, Ohara O, Wang JY, Cooper MD. Discriminating gene expression profiles of memory B cell subpopulations. J.Exp.Med. 2008;205:1807–1817. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp.Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K, Zheng B, Go M, Slaga D, Du C, Scales SJ, et al. FcRL5 as a target of antibody-drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther. 2012;11:2222–2232. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- Facchetti F, Cella M, Festa S, Fremont DH, Colonna M. An unusual Fc receptor-related protein expressed in human centroblasts. Proc.Natl.Acad.Sci.U.S.A. 2002;99:3776–3781. doi: 10.1073/pnas.022042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B, Agostinelli C, Bigerna B, Pucciarini A, Pacini R, Tabarrini A, et al. IRTA1 is selectively expressed in nodal and extranodal marginal zone lymphomas. Histopathology. 2012;61:930–941. doi: 10.1111/j.1365-2559.2012.04289.x. [DOI] [PubMed] [Google Scholar]
- Falini B, Tiacci E, Pucciarini A, Bigerna B, Kurth J, Hatzivassiliou G, et al. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT) Blood. 2003;102:3684–3692. doi: 10.1182/blood-2003-03-0750. [DOI] [PubMed] [Google Scholar]
- Forconi F, Sahota SS, Raspadori D, Mockridge CI, Lauria F, Stevenson FK. Tumor cells of hairy cell leukemia express multiple clonally related immunoglobulin isotypes via RNA splicing. Blood. 2001;98:1174–1181. doi: 10.1182/blood.v98.4.1174. [DOI] [PubMed] [Google Scholar]
- Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, et al. Human Fc Receptor-Like 5 Binds Intact IgG via Mechanisms Distinct from Those of Fc Receptors. J Immunol. 2013;190:5739–5746. doi: 10.4049/jimmunol.1202860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AW, Li FJ, Wu JM, Edberg JC, Su KH, Cafardi J, et al. The FCRL3-169CT promoter single-nucleotide polymorphism, which is associated with systemic lupus erythematosus in a Japanese population, predicts expression of receptor protein on CD19+B cells. Arthritis Rheum. 2009;60:3510–3512. doi: 10.1002/art.24915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guselnikov SV, Ershova SA, Mechetina LV, Najakshin AM, Volkova OY, Alabyev BY, et al. A family of highly diverse human and mouse genes structurally links leukocyte FcR, gp42 and PECAM-1. Immunogenetics. 2002;54:87–95. doi: 10.1007/s00251-002-0436-x. [DOI] [PubMed] [Google Scholar]
- Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptorlike 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci U S A. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- Imboden JB, Eriksson EC, McCutcheon M, Reynolds CW, Seaman WE. Identification and characterization of a cell-surface molecule that is selectively induced on rat lymphokine-activated killer cells. J Immunol. 1989;143:3100–3103. [PubMed] [Google Scholar]
- Inozume T, Matsuzaki Y, Kurihara S, Fujita T, Yamamoto A, Aburatani H, et al. Novel melanoma antigen, FCRL/FREB, identified by cDNA profile comparison using DNA chip are immunogenic in multiple melanoma patients. Int.J Cancer. 2005;114:283–290. doi: 10.1002/ijc.20735. [DOI] [PubMed] [Google Scholar]
- Ise T, Maeda H, Santora K, Xiang L, Kreitman RJ, Pastan I, et al. Immunoglobulin superfamily receptor translocation associated 2 protein on lymphoma cell lines and hairy cell leukemia cells detected by novel monoclonal antibodies. Clin.Cancer Res. 2005;11:87–96. [PubMed] [Google Scholar]
- Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007;21:169–174. doi: 10.1038/sj.leu.2404445. [DOI] [PubMed] [Google Scholar]
- Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-Like 2 Inhibition of B Cell Receptor-Mediated Activation of B Cells. J.Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic K, Cimbro R, Nawaz F, Huang DW, Zheng X, Yang J, et al. The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-beta1 production and FcRL4 expression. Nat Immunol. 2013 doi: 10.1038/ni.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121:2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl JH, Thevenin C, Rieckmann P, Fauci AS. Transforming growth factor-beta suppresses human B lymphocyte Ig production by inhibiting synthesis and the switch from the membrane form to the secreted form of Ig mRNA. J Immunol. 1991;146:4016–4023. [PubMed] [Google Scholar]
- Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, et al. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- Kochi Y, Yamada R, Suzuki A, Harley JB, Shirasawa S, Sawada T, et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005;37:478–485. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulemzin SV, Zamoshnikova AY, Yurchenko MY, Vitak NY, Najakshin AM, Fayngerts SA, et al. FCRL6 receptor: expression and associated proteins. Immunol Lett. 2011;134:174–182. doi: 10.1016/j.imlet.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Leu CM, Davis RS, Gartland LA, Fine WD, Cooper MD. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–1126. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- Li FJ, Ding S, Pan J, Shakhmatov MA, Kashentseva E, Wu J, et al. FCRL2 expression predicts IGHV mutation status and clinical progression in chronic lymphocytic leukemia. Blood. 2008;112:179–187. doi: 10.1182/blood-2008-01-131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FJ, Schreeder DM, Li R, Wu J, Davis RS. FCRL3 promotes TLR9-induced B-cell activation and suppresses plasma cell differentiation. Eur J Immunol. 2013;43:2980–2992. doi: 10.1002/eji.201243068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlen MT, Nordang GB, Syversen SW, van der Heijde DM, Kvien TK, Uhlig T, et al. FCRL3 −169C/C genotype is associated with anti-citrullinated protein antibody-positive rheumatoid arthritis and with radiographic progression. J Rheumatol. 2011;38:2329–2335. doi: 10.3899/jrheum.110489. [DOI] [PubMed] [Google Scholar]
- Maltais LJ, Lovering RC, Taranin AV, Colonna M, Ravetch JV, Dalla-Favera R, et al. New nomenclature for Fc receptor-like molecules. Nat.Immunol. 2006;7:431–432. doi: 10.1038/ni0506-431. [DOI] [PubMed] [Google Scholar]
- Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- Masir N, Jones M, Pozzobon M, Marafioti T, Volkova OY, Mechetina LV, et al. Expression pattern of FCRL (FREB, FcRX) in normal and neoplastic human B cells. Br.J Haematol. 2004;127:335–343. doi: 10.1111/j.1365-2141.2004.05193.x. [DOI] [PubMed] [Google Scholar]
- Masuda K, Davis RS, Maruyama T, Zhang J, He T, Cooper MD, et al. FcRY, an Fc receptor related gene differentially expressed during B lymphocyte development and activation. Gene. 2005;363:32–40. doi: 10.1016/j.gene.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Masuda K, Mori H, Ohara O, Nakayama M, Wang JY, Burrows PD. Defining the immunological phenotype of Fc receptor-like B (FCRLB) deficient mice: Confounding role of the inhibitory FcgammaRIIb. Cell Immunol. 2010;266:24–31. doi: 10.1016/j.cellimm.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechetina LV, Najakshin AM, Volkova OY, Guselnikov SV, Faizulin RZ, Alabyev BY, et al. FCRL, a novel member of the leukocyte Fc receptor family possesses unique structural features. Eur.J.Immunol. 2002;32:87–96. doi: 10.1002/1521-4141(200201)32:1<87::AID-IMMU87>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Miller I, Hatzivassiliou G, Cattoretti G, Mendelsohn C, Dalla-Favera R. IRTAs: a new family of immunoglobulinlike receptors differentially expressed in B cells. Blood. 2002;99:2662–2669. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- Mohan J, Dement-Brown J, Maier S, Ise T, Kempkes B, Tolnay M. Epstein-Barr virus nuclear antigen 2 induces FcRH5 expression through CBF1. Blood. 2006;107:4433–4439. doi: 10.1182/blood-2005-09-3815. [DOI] [PubMed] [Google Scholar]
- Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J.Immunol. 2009;182:7518–7526. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Weissman SM, Bothwell AL. BXMAS1 identifies a cluster of homologous genes differentially expressed in B cells. Biochem.Biophys.Res.Commun. 2001;285:830–837. doi: 10.1006/bbrc.2001.5231. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int.Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KG. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnikova ES, Mechetina LV, Volkova OY, Guselnikov SV, Chikaev NA, Kovesdi D, et al. Differential expression of FCRLA in naive and activated mouse B cells. Cell Immunol. 2012;272:182–192. doi: 10.1016/j.cellimm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- Santiago T, Kulemzin SV, Reshetnikova ES, Chikaev NA, Volkova OY, Mechetina LV, et al. FCRLA is a resident endoplasmic reticulum protein that associates with intracellular Igs, IgM, IgG and IgA. Int Immunol. 2011;23:43–53. doi: 10.1093/intimm/dxq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreeder DM, Cannon JP, Wu J, Li R, Shakhmatov MA, Davis RS. Cutting edge: FcR-like 6 is an MHC class II receptor. J Immunol. 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreeder DM, Pan J, Li FJ, Vivier E, Davis RS. FCRL6 distinguishes mature cytotoxic lymphocytes and is upregulated in patients with B-cell chronic lymphocytic leukemia. Eur.J.Immunol. 2008;38:3159–3166. doi: 10.1002/eji.200838516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman WE, Niemi EC, Stark MR, Goldfien RD, Pollock AS, Imboden JB. Molecular cloning of gp42, a cell-surface molecule that is selectively induced on rat natural killer cells by interleukin 2: glycolipid membrane anchoring and capacity for transmembrane signaling. J Exp.Med. 1991;173:251–260. doi: 10.1084/jem.173.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Sohn HW, Krueger PD, Davis RS, Pierce SK. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118:6332–6341. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson LA, Mold JE, Bajpai UD, McCune JM. Expression of the autoimmune susceptibility gene FcRL3 on human regulatory T cells is associated with dysfunction and high levels of programmed cell death-1. J.Immunol. 2010;184:3639–3647. doi: 10.4049/jimmunol.0903943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J.Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Colonna M. A new Fc receptor homolog, FREB2, found in germinal center B cells. Genes Immun. 2005;6:341–346. doi: 10.1038/sj.gene.6364185. [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Fuchs A, Colonna M. Cutting Edge: Human FcRL4 and FcRL5 Are Receptors for IgA and IgG. J Immunol. 2012;188:4741–4745. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Gilfillan S, Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. J Immunol. 2010;185:2960–2967. doi: 10.4049/jimmunol.1001428. [DOI] [PubMed] [Google Scholar]
- Wilson TJ, Presti RM, Tassi I, Overton ET, Cella M, Colonna M. FcRL6, a new ITIM-bearing receptor on cytolytic cells, is broadly expressed by lymphocytes following HIV-1 infection. Blood. 2007;109:3786–3793. doi: 10.1182/blood-2006-06-030023. [DOI] [PubMed] [Google Scholar]
- Won WJ, Foote JB, Odom MR, Pan J, Kearney JF, Davis RS. Fc receptor homolog 3 is a novel immunoregulatory marker of marginal zone and B1 B cells. J Immunol. 2006;177:6815–6823. doi: 10.4049/jimmunol.177.10.6815. [DOI] [PubMed] [Google Scholar]
- Xiu Y, Nakamura K, Abe M, Li N, Wen XS, Jiang Y, et al. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J Immunol. 2002;169:4340–4346. doi: 10.4049/jimmunol.169.8.4340. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Zhao R, Cao H, Zhao ZJ. SPAP2, an Ig family receptor containing both ITIMs and ITAMs. Biochem.Biophys.Res.Commun. 2002;293:1037–1046. doi: 10.1016/S0006-291X(02)00332-7. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Zhao R, Zhao ZJ. Molecular cloning and characterization of SPAP1, an inhibitory receptor. Biochem.Biophys.Res.Commun. 2001;280:768–775. doi: 10.1006/bbrc.2000.4213. [DOI] [PubMed] [Google Scholar]
- Yang Y, Su X, Zhang K, Zhou R. The Fc receptor-like 3 gene polymorphisms and susceptibility to autoimmune diseases: An updated meta-analysis. Autoimmunity. 2013;46:547–558. doi: 10.3109/08916934.2013.835804. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci Signal. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J.Exp.Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SX, Liu W, Zhan M, Song ZY, Yang SY, Xue LQ, et al. A refined study of FCRL genes from a genome-wide association study for Graves’ disease. PLoS One. 2013;8:e57758. doi: 10.1371/journal.pone.0057758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Li R, Li H, Zhou T, Davis RS. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Natl Acad Sci U S A. 2013;110:E1282–E1290. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]