Abstract
• Background and Aims Frost tolerance of wheat depends primarily upon a strong vernalization requirement, delaying the transition to the reproductive phase. The aim of the present study was to learn how saturation of the vernalization requirement and apical development stage are related to frost tolerance in wheat.
• Methods ‘Mironovskaya 808’, a winter variety with a long vernalization requirement, and ‘Leguan’, a spring variety without a vernalization requirement, were acclimated at 2 °C at different stages of development. Plant development (morphological stage of the shoot apex), vernalization requirement (days to heading) and frost tolerance (survival of the plants exposed to freezing conditions) were evaluated.
• Key Results ‘Mironovskaya 808’ increased its frost tolerance more rapidly; it reached a higher level of tolerance and after a longer duration of acclimation at 2 °C than was found in ‘Leguan’. The frost tolerance of ‘Mironovskaya 808’ decreased and its ability to re-acclimate a high tolerance was lost after saturation of its vernalization requirement, but before its shoot apex had reached the double-ridge stage. The frost tolerance of ‘Leguan’ decreased after the plants had reached the floret initiation stage.
• Conclusions The results support the hypothesis that genes for vernalization requirement act as a master switch regulating the duration of low temperature induced frost tolerance. In winter wheat, due to a longer vegetative phase, frost tolerance is maintained for a longer time and at a higher level than in spring wheat. After the saturation of vernalization requirement, winter wheat (as in spring wheat) established only a low level of frost tolerance.
Key words: Triticum aestivum, wheat, frost tolerance, vernalization, cold acclimation, apical development
INTRODUCTION
Winter wheat has to have sufficient frost tolerance to survive unfavourable winter temperatures, but the level is not constant and is dependent on both genotypic and environmental factors (Fowler et al., 1999; Mahfoozi et al., 2001b). In autumn, it is primarily low temperature that induces frost tolerance in wheat. A high level of tolerance must be maintained over winter, but wheat plants must also be able to re-establish sufficient frost tolerance following a drop in temperature after warm periods that have resulted in de-acclimation (Gusta and Fowler, 1979; Prášil and Zámečník, 1991). In spring, once temperatures increase, the plants resume growth and development and lose frost tolerance. The ability of wheat plants to maintain frost tolerance decreases after the vegetative/reproductive transition (Mahfoozi et al., 2001a, b), but mechanisms exist which slow down the rate of phenological development and extend the vegetative phase. These involve a requirement for vernalization (the necessity of going through a certain period of low temperatures) and responses to photoperiod (day-length sensitivity). Winter wheat varieties usually have a greater vernalization requirement and a higher sensitivity to short days than spring wheat varieties, which enable them to remain in the vegetative phase during winter. Spring wheat varieties have no, or a very limited, need for vernalization.
Saturation of the vernalization requirement has been suggested as the main factor responsible for the seasonal decline in frost tolerance of winter wheat (Roberts, 1979). Vernalization is determined by Vrn genes that are primarily located on group 5 chromosomes in wheat (Snape et al., 2001). Molecular studies have shown a very close genetic linkage between the vernalization and frost tolerance (Fr) genes (Galiba et al., 1995; Sutka, 2001). Fowler et al. (2001) and Danyluk et al. (2003) have concluded that the developmental genes (vernalization Vrn, photoperiod Ppd), which control the transition from the vegetative to the reproductive phase, also act to control genes affecting the expression of low temperature-induced genes associated with the acquisition of frost tolerance. A vernalization requirement enables winter wheat to maintain the expression of low-temperature genes at higher intensity and for a longer period of time than in spring wheat. According to this model, after the vernalization requirement of wheat plants has been satisfied, there is a gradual decrease not only in frost tolerance but also in the ability to re-establish a high level of frost tolerance. A decrease in tolerance after a long period of cold acclimation has been shown in several studies (Roberts, 1979; Veisz and Sutka, 1989; Fowler et al., 1996a; Mahfoozi et al., 2001a) but the decrease in the ability to re-acclimate has not been systematically studied under controlled conditions.
The development of wheat plants can be followed by observing the differentiation of the mainstem shoot apex. The transition from the vegetative to the reproductive phase is signalled by initiation of the collar (the first floral primordium) at the apex (Hay and Kirby, 1991). As the first spikelet primordia are visually indistinguishable from leaf primordia (Delécolle et al., 1989), in practice the double-ridge stage has normally been used as an index of the transition in relation to changes in the level of frost tolerance (Mahfoozi et al., 2001a, b). Recently Mahfoozi et al. (2001a) have shown that decrease in frost tolerance preceded the double-ridge stage in cold-acclimated winter wheat plants. If there is a developmental stage in spring wheat (without any vernalization requirement) after which plants gradually lose their frost tolerance this has not been observed.
The objective of the present project was to learn how the saturation of the vernalization requirement and the achieving of the double ridges are related to frost tolerance in wheat. Two main questions were studied: (1) What is the relationship between saturation of vernalization, developmental stages and loss of frost tolerance under cold acclimation? (2) What is the ability of plants of different stage of development to re-acclimate? These questions were studied using two different wheat varieties: ‘Mironovskaya 808’ (representative of winter varieties with a high frost tolerance and a long vernalization requirement) and ‘Leguan’ (spring wheat without a vernalization requirement, a faster rate of development and a lower ability to induce frost tolerance).
MATERIALS AND METHODS
Genotypes
Seeds of Triticum aestivum L., ‘Mironovskaya 808’ winter wheat, and ‘Leguan’ spring wheat were obtained from the breeders, Selgen a.s, Prague.
Growth conditions
After rinsing with warm water (50 °C) the seeds were spread on filter papers and held in a germination box at 20 °C for 4 d. Germinating seedlings were fixed in ten-unit holders and were grown in a Hoagland 3 nutrient solution (including microelements) at a constant temperature of 17 °C under a 12-h photoperiod and an irradiance 400 µmol m−2 s−1 provided by a combination of vapour lamps and high intensity discharge lamps (LU/400/T/40, Tungsram, Hungary) in a growth cabinet (Tyler, Hungary). The nutrient solution was aerated and changed twice a week. Two experimental procedures were used:
Plants were grown at 17 °C to the three-leaf stage, and then acclimated at 2 ± 1 °C and two photoperiods, 16 h (long day) or 8 h (short day) at the same level of irradiance (400 µmol m−2 s−1) for 12 weeks.
Plants were sown at 2-week intervals and grown up to the first-leaf stage under the environmentally controlled conditions described above; they were then acclimated at 2±1 °C and an 8-h photoperiod for varying periods of time (0, 2, 4, 6 or 8 weeks) to obtain plants at different levels of saturation of vernalization at one time. Subsequently, the plants were transferred to 17 °C and a 12-h photoperiod. Once they reached the three-leaf stage (about 2 weeks), they were exposed to 2 ± 1 °C and a 12-h photoperiod for 5 weeks to assess their re-acclimation.
In both arrangements, frost tolerance, vernalization requirement and apical development were analysed in plants sampled before and during the cold acclimation.
Frost tolerance
Frost tolerance was determined by direct freezing of plants in freezing boxes. Plants taken from the holders were divided into bundles of 10–12 units and exposed to −4 °C for 20 h, followed by five or six different freezing temperatures in freezing boxes for 24 h. Temperatures in the boxes differed by 2 °C and were chosen according to the predicted frost tolerance of the plants. The rate of cooling and thawing was 2 °C h−1. After thawing, the plants were cut at 2·5 cm from the crown, the roots were submerged in a dish filled with fresh water and the plants placed in a glasshouse at 20 °C. After 5–6 d, the numbers of living and regenerating plants were determined for each freezing treatment. Frost tolerance was expressed in LT50 values, calculated according to the model of Janáček and Prášil (1991).
Vernalization requirement
Days to heading, indicating the time of vernalization saturation, were determined for ten plants taken from each treatment after the predetermined time (0, 2, 4, 6 or 8 weeks) of exposure to cold. The plants were grown for up to 100 d in a field soil in a glasshouse at 20 ± 2 °C and a 16-h photoperiod provided by supplemental lighting (high intensity discharge lamps LU/400/T/40, Tungsram, Hungary). The vernalization requirement was defined as the minimum number of weeks required for full vernalization (i.e. when time of heading was not significantly decreased by additional weeks of cold exposure).
Apical development
The stage of phenological development was determined from changes in the morphology of the shoot apex and was expressed by decimal code (DC) according to Nátrová and Jokeš (1993). Shoot apices from three plants were dissected. If they were not at the same stage, another three plants were taken and the mean code was calculated. The decimal code of the plants was scored as follows:
- Vegetative development
- 11 Early vegetative development of the shot apex; apex is short, of hemispherical shape with one or two initiated leaves
- 13 Beginning of the shoot apex elongation; at its base, the number of leaf primordia increases
- 16 Beginning of single ridges, i.e. leaf primordia initiation on elongating shoot apex
- 19 Single ridges, i.e. leaf primordia initiated along the whole shoot apex
- Spikelet initiation and differentiation
- 20 Formation of double ridges DR 1 – the size of leaf primordia is bigger than that of the spikelets
- 22 Formation of double ridges DR 2 – the spikelet and leaf primordia are of similar size
- 24 Formation of double ridges DR 3 – spikelet primordia increase in size, and the growth of leaf primordia is inhibited
- 26 Spikelet primordia are elongating; on the shoot apex only spikelet primordia are apparent
- 27 Glume initiation
- 29 Lemma initiation
- Floret initiation and differentiation
- 30 Initiation of first florets in spikelets; hemispherical meristematic ridges above initiated glumes and lemmas on both sides of spikelet
- 31 Initiation of the other florets on the spikelets
Statistical evaluation
Statistical evaluation of the results was carried out using an ANOVA design with multiple comparisons (LSD test at the 5 % level) using the statistical package UNISTAT 5.0 (Unistat Ltd, UK). Means were calculated from three replicates.
RESULTS
Vernalization, shoot apex development and frost tolerance during cold acclimation
The heading time of ‘Leguan’ plants treated according to experimental procedure I was unaffected by cold acclimation under SD (Fig. 1), and it was only slightly earlier under LD over 12 weeks, confirming that the variety did not have a vernalization requirement. ‘Mironovskaya’ plants did not head in the glasshouse within 100 d unless they were cold acclimated for at least 4 weeks. Their heading time decreased progressively between 4 and 8 weeks of acclimation, particularly under LD, and from 8 weeks onwards it did not change, indicating that the vernalization requirement of the variety had been saturated by 8 weeks of cold treatment.
Fig. 1.
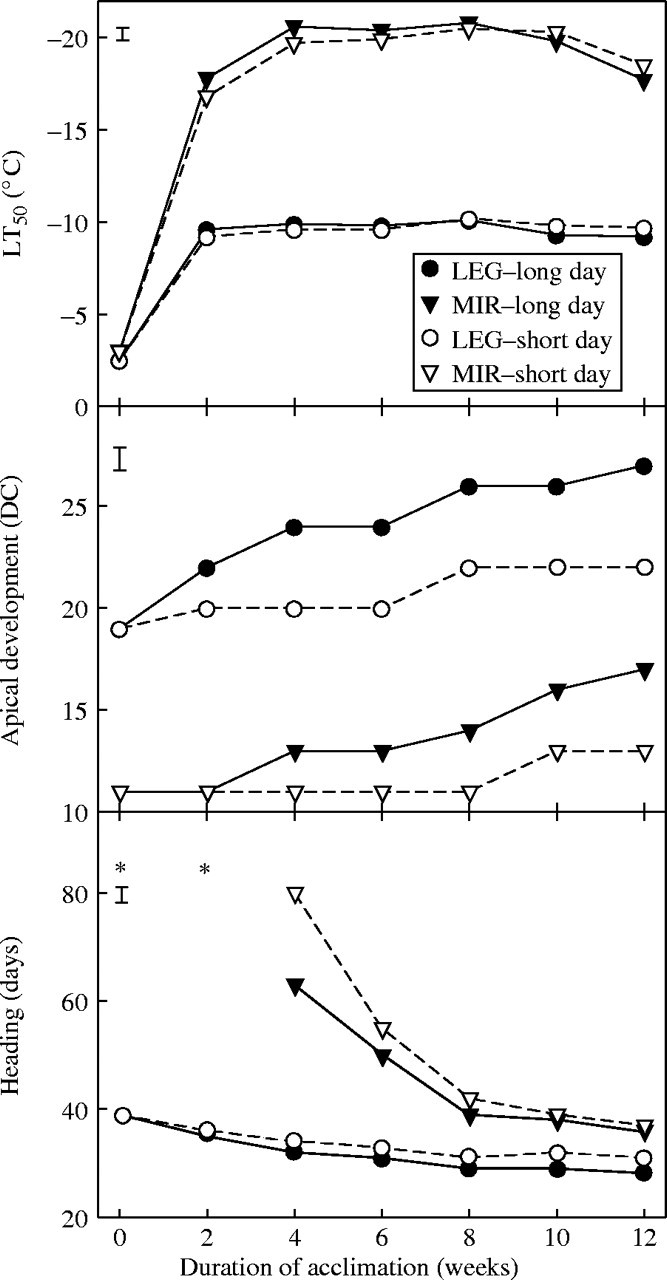
Frost tolerance (LT50), apical development (DC = decimal code) and days to heading of ‘Mironovskaya’ (MIR) winter wheat and ‘Leguan’ (LEG) spring wheat plants during 12 weeks of acclimation at 2 °C and two photoperiods, 16 h (long day) or 8 h (short day). Vertical bars indicate LSD0·05 and asterisks indicate that the plants had not reached heading by 100 d.
‘Leguan’ plants reached the double-ridge stage (DC ≥ 20) after acclimation for only 2 weeks under LD treatment or 8 weeks under SD treatment. ‘Mironovskaya’ shoot apices did not reach the double-ridge stage throughout the whole 12 weeks of acclimation but advanced more under LD than under SD treatment.
‘Leguan’ reached its highest level of frost tolerance after 2 weeks of acclimation and then maintained an LT50 of approx. −9 °C for the rest of the 12-week acclimation period. ‘Mironovskaya’ reached its highest frost tolerance of approx. −20°C after 4 weeks of acclimation and retained this level for 10 weeks. After 12 weeks of acclimation, a significant loss of frost tolerance was detected in ‘Mironovskaya’ plants. In ‘Mironovskaya’, frost tolerance decreased after vernalization saturation but before the morphological manifestation of double ridge formation. ‘Leguan’ reached the double-ridge stage during cold treatment without any change in its frost tolerance. Long days accelerated shoot apex development in each variety, while the level of frost tolerance was not influenced by day length.
To detect if frost tolerance of ‘Leguan’ declined before the plants reached a more advanced developmental stage, ‘Leguan’ plants were sown at weekly intervals and grown under 17 °C for 2, 3, 4 or 5 weeks. Thus plants at different developmental stages (DC = 19, 24, 28 and 30) were obtained at the initiation of cold acclimation (Fig. 2). After a 3-week acclimation at 2 °C they reached DC values of 26, 27, 29 and 31. Plants with DC < 30 had LT50 values around −9 °C, and a significant increase in LT50 (indicating a decrease in frost tolerance) was observed only at the most advanced developmental stage (DC 31, floret initiation and differentiation) in plants that had been grown for 5 weeks at 17 °C before cold acclimation.
Fig. 2.
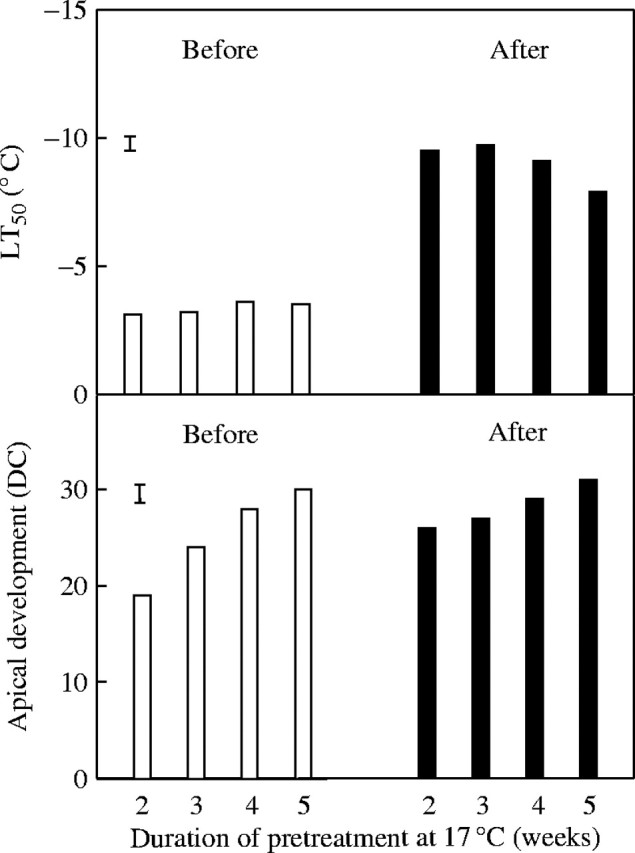
Frost tolerance (LT50) and apical development (DC = decimal code) of ‘Leguan’ spring wheat plants before and after 3 weeks of acclimation at 2 °C and 12-h photoperiod, following 2, 3, 4 or 5 weeks at 17 °C, achieved by progressive sowing. Vertical bars indicate LSD0·05.
Shoot apex development and frost tolerance during cold re-acclimation
The aim of this experiment was to find out to what extent previous vernalization of seedlings affected the subsequent capacity of the plants to re-acclimate to low temperature and to relate this to shoot apex development. ‘Mironovskaya’ and ‘Leguan’ plants were re-acclimated according to experimental procedure II. Heading times before, and frost tolerance and apical development after, cold re-acclimation were recorded (Fig. 3).
Fig. 3.
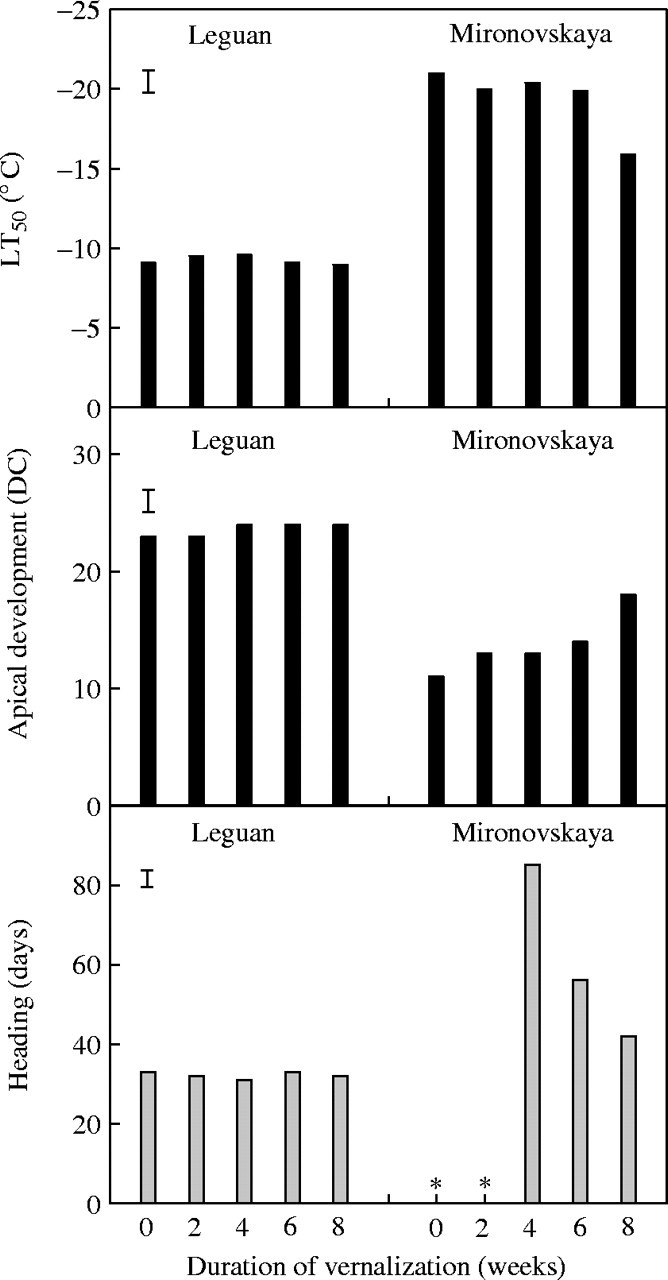
Frost tolerance (LT50), apical development (DC = decimal code) and days to heading of ‘Mironovskaya’ winter wheat and ‘Leguan’ spring wheat plants after 3 weeks of re-acclimation at 2 °C and 12-h photoperiod, following vernalization for 0, 2, 4, 6 or 8 weeks at 2 °C, achieved by progressive sowing. Vertical bars indicate LSD0·05 and asterisks indicate that the plants had not reached heading by 100 d.
Heading time and frost tolerance at the end of the 3-week re-acclimation period was the same for all ‘Leguan’ treatments, regardless of the previous vernalization period, confirming that ‘Leguan’ did not have a vernalization requirement. Shoot apex development of ‘Leguan’ plants had crossed the double-ridge stages in all vernalization treatments without any effect on the level of frost tolerance.
‘Mironovskaya’ plants that had been vernalized for 0 or 2 weeks did not head. They headed after 4 weeks of vernalization, the number of days to heading decreased with additional vernalization and was lowest in plants vernalized for 8 weeks, confirming the vernalization saturation of the variety. The LT50 values reached the lowest level of approx. −20 °C after 3 weeks of re-acclimation, and this level remained unchanged for all pre-vernalization treatments except for the 8 weeks of vernalization. LT50 values were significantly higher for the last treatment where plants showed progressive shoot apex differentiation. The shoot apex of ‘Mironovskaya’ did not reach double ridges (i.e. DC ≥ 20) indicating that fully vernalized ‘Mironovskaya’ plants were not able to develop the full degree of frost tolerance during re-acclimation even though their shoot apices did not reach the double-ridge stage.
To know if vernalized plants developed shoot apices more quickly and lost the ability to develop high frost tolerance during re-acclimation more rapidly than unvernalized plants, both varieties were investigated during 5 weeks of re-acclimation at 2 °C after a 0-, 4- or 8-week vernalization pre-treatment (Fig. 4). ‘Leguan’ plants were already after the double-ridge stage at the beginning of the acclimation process. During re-acclimation their apical development progressed slowly and reached a value close to DC 30 (floret initiation) in the oldest plants vernalized for 8 weeks. LT50 values of ‘Leguan’ reached the lowest level of around −9 °C after 1·5 weeks of re-acclimation, and there was a small but insignificant decrease in frost tolerance in the oldest plants by the end of re-acclimation time. ‘Mironovskaya’ plants did not reach the double-ridge stage following the 0- and 4- week vernalization pre-treatment, but the plants vernalized for 8 weeks did, reaching a more advanced developmental stage during the whole re-acclimation time. These plants with saturated vernalization requirement had LT50 values which were significantly higher than those of plants without it, indicating that saturation of vernalization requirement in ‘Mironovskaya’ plants led to a more rapid development of the shoot apex and to the loss of their ability to establish a high level of frost tolerance during re-acclimation.
Fig. 4.
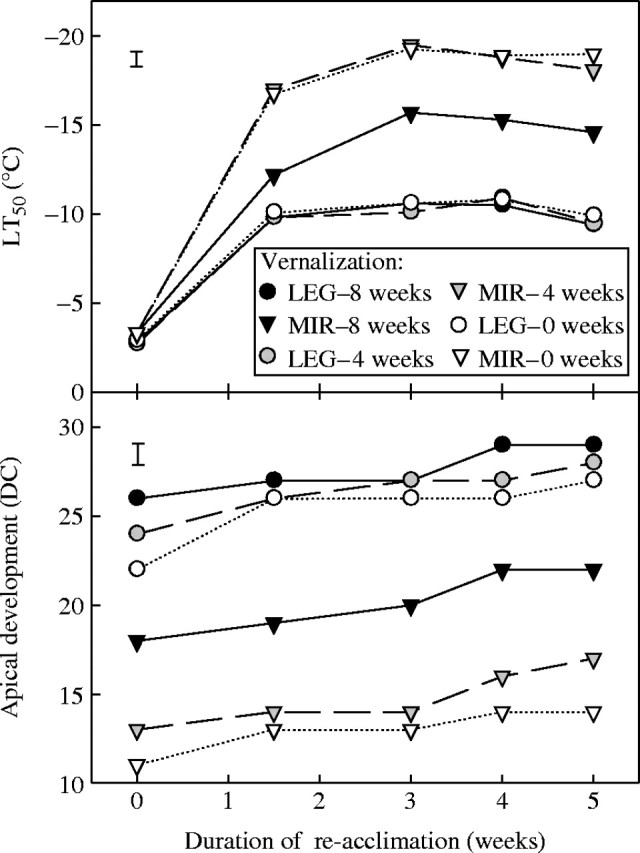
Frost tolerance (LT50) and apical development (DC = decimal code) of ‘Mironovskaya’ (MIR) winter wheat and ‘Leguan’ (LEG) spring wheat plants during 5 weeks of re-acclimation at 2 °C and 12-h photoperiod, following vernalization for 0, 4, or 8 weeks at 2 °C, achieved by progressive sowing. Vertical bars indicate LSD0·05.
DISCUSSION
The dynamics of low-temperature acclimation in wheat grown in controlled environments under uniform conditions have been described in a number of studies (Tsenov, 1973; Roberts, 1979; Veisz and Sutka, 1989; Fowler et al., 1996a). At first there is a gradual increase in frost tolerance, which is more rapid and lasts longer in hardier varieties of wheat. After a certain period of time, frost tolerance reaches its highest value (usually between 6 and 11 weeks) followed by a gradual decrease. Vágújfalvi et al. (1999) described a more rapid decline in frost tolerance for less hardy genotypes of wheat. Recent studies of frost tolerance dynamics in cereals have revealed that wheat varieties cold-acclimated at a rapid rate for the first 3 weeks at a constant low temperature, after which tolerance declined slowly (spring varieties) or was maintained at a high level for a further 6–7 weeks before declining (winter varieties) (Fowler et al., 1996a, b; Mahfoozi et al., 2001a, b). Similarly, in this experiment, the frost tolerance of ‘Mironovskaya’ winter wheat increased for at least 3 weeks and then was maintained at the highest level for the next 9 weeks before a decrease occurred (Fig. 1). The less hardy ‘Leguan’ spring wheat reached its highest frost tolerance after 10 d at 2 °C, and it did not decrease over the whole period of 12 weeks of acclimation, indicating that the spring variety had a different pattern of frost tolerance from that of the winter variety during cold acclimation.
The length of the photoperiod (LD vs. SD) during acclimation did not influence the course of frost tolerance significantly in either variety (Fig. 1). This corresponds to the general conclusion that, in contrast to woody plants, photoperiod does not play an important role in cold-acclimation of herbs (Kacperska, 1985). A longer photoperiod also means a longer daily period of time for the formation of photosynthetic products and other metabolites, which play an important function in the survival of cell dehydration. This explains why some studies, especially those which monitored the development of frost tolerance in green leaves, have shown influence of day length on the induction of frost tolerance (Griffith and McIntyre, 1993). In this experiment, long days reduced the time to heading and accelerated shoot apex differentiation in both varieties of wheat indicating that apical morphogenesis is more sensitive to photoperiod than the measured frost tolerance of the whole plant during cold acclimation.
The saturation of vernalization in ‘Mironovskaya’ (8 weeks under low temperature) did not lead immediately to a decrease in frost tolerance. An additional 4 weeks at low temperature were necessary for the decrease (Fig. 1) and, as the re-acclimation experiments showed (Figs 3 and 4), about 2 weeks of growth at 17 °C (after full vernalization of plants) caused a decrease in the ability to re-establish a great degree of frost tolerance. In these experiments, ‘Mironovskaya’ did not, in general, reach the morphologically differentiated double-ridge stage (DC = 20). Similarly Mahfoozi et al. (2001a, b) found out that the period of saturation of vernalization preceded the double-ridge stage. These observations indicate the following sequence of events in cold-acclimated winter varieties: saturation of vernalization, decrease of frost tolerance and then appearance of the double-ridge stage. The double ridge does not signal the start of the reproductive development (this corresponds to a much earlier initiation of the spikelets) but rather the point at which is no going back to the production of leaves (Hay and Ellis, 1998).
A strong relationship has been shown between growth habit and frost tolerance in wheat (Roberts, 1990; Braun, 1997). The delay in the transition to the more sensitive reproductive phase due to vernalization requirement may be involved in the higher frost tolerance of the plants with winter growth habit. This model, supported by Fowler et al. (1996a, 1999), is based on the hypothesis that vernalization genes Vrn (as well as photoperiodic genes Ppd) act to control the duration of expression of low-temperature-induced genes (Lti, Cor etc.) related to the induction of frost tolerance. The level of tolerance achieved is, therefore, determined by the time and extent to which these structural genes are promoted. For winter wheat, due to a longer vegetative phase, these genes are expressed for a longer time and at a higher level than in spring wheat. Recently a candidate Vrn product (TaVRTt-1), regulating the expression of Cor genes in wheat, has been published (Danyluk et al., 2003). This model can explain why a winter wheat variety, in contrast to a spring variety, is more capable of enhancing, maintaining and re-establishing frost tolerance before saturation of the vernalization requirement.
From field experiments it is known that winter wheat can be very frost tolerant in the autumn, whereas it responds, after overwintering, to a drop in temperature with an increase in tolerance in a manner similar to that of spring wheat (Gusta and Fowler, 1979). Furthermore the double ridge was here not associated with a loss in frost tolerance in the cold tender spring wheat variety ‘Leguan’. Only a much more advanced developmental stage (DC > 30) was associated with a decrease in tolerance (Fig. 2). These observations indicate that the low-temperature system for the induction of frost tolerance in wheat is also functioning during the reproductive phase, but lower levels of tolerance are generated. The regulation of frost tolerance by the reproductive phase is not yet understood.
Acknowledgments
We thank Prof. D. Brian Fowler (University of Saskatchewan, Canada) for helpful discussion and Prof. Karl Dörffling (University of Hamburg, Germany) for valuable suggestions and reading the text. This research was supported by the grant 522/01/0510, Grant Agency of the Czech Republic.
LITERATURE CITED
- Braun HJ. 1997. Winter hardiness of bread wheats derived from spring×winter crosses. Acta Agronomica Hungarica 45: 317–327. [Google Scholar]
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F. 2003. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiology 132: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delécolle R, Hay RKM, Guérif M, Pluchard P, Varlet-Grancher C. 1989. A method of describing the progress of apical development in wheat, based on the time-course of organogenesis. Field Crops Research 21: 147–160. [Google Scholar]
- Fowler DB, Breton G, Limin AE, Mahfoozi S, Sarhan F. 2001. Photoperiod and temperature interaction regulate low-temperature-induced gene expression in barley. Plant Physiology 127: 1676–1681. [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Chauvin LP, Limin AE, Sarhan F. 1996. The regulatory role of vernalization in the expression of low-temperature-induced genes in wheat and rye. Theoretical and Applied Genetics 93: 554–559. [DOI] [PubMed] [Google Scholar]
- Fowler DB, Limin AE, Ritchie JT. 1999. Low-temperature tolerance in cereals: model and genetic interpretation. Crop Science 39: 626–633. [Google Scholar]
- Fowler DB, Limin AE, Wang SY, Ward RW. 1996. Relationship between low-temperature tolerance and vernalization in wheat and rye. Canadian Journal of Plant Science 76: 37–42. [Google Scholar]
- Galiba G, Quarrie SA, Sutka J, Morgounov A, Snape JW. 1995. RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theoretical and Applied Genetics 90: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Griffith M, McIntyre CH. 1993. The interrelationship of growth and frost tolerance in winter rye. Physiologia Plantarum 87: 335–344. [Google Scholar]
- Gusta LV, Fowler DB. 1979. Cold resistance and injury in winter cereals. In: Mussel H, Staples RC, eds. Stress physiology in crop plants. New York: John Wiley and Sons, 160–178. [Google Scholar]
- Hay RKM, Ellis RP. 1998. The control of flowering in wheat and barley: what recent advances in molecular genetics can reveal. Annals of Botany 82: 541–554. [Google Scholar]
- Hay RKM, Kirby EJM. 1991. Convergence and synchrony – a review of the coordination of development in wheat. Australian Journal of Agricultural Research 42: 661–700. [Google Scholar]
- Janáček J, Prášil I. 1991. Quantification of plant frost injury by nonlinear fitting of an s-shaped function. Cryo-Letters 12: 47–52. [Google Scholar]
- Kacperska A. 1985. Biochemical and physiological aspects of frost hardening in herbaceous plants. In: Kaurin Å, Junttila O, Nilsen J. eds. Plant production in the north. Tromso: Norwegian University Press, 99–115. [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB. 2001. Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Science 41: 1006–1011. [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB. 2001. Developmental regulation of low-temperature tolerance in winter wheat. Annals of Botany 87: 751–757. [Google Scholar]
- Nátrová Z, Jokeš M. 1993. A proposal for a decimal scale of the inflorescence development of wheat. Rostlinná výroba 39: 315–328. [Google Scholar]
- Prášil I, Zámečník J. 1991. The effect of weather on the overwintering of winter crops. In: Petr J, ed. Weather and yield. Amsterdam: Elsevier Science Publishers, 141–151. [Google Scholar]
- Roberts DWA. 1979. Duration of hardening and cold hardiness in winter wheat. Canadian Journal of Botany 57: 1511–1517. [Google Scholar]
- Roberts DWA. 1990. Identification of loci on chromosome 5A of wheat involved in control of cold hardiness, vernalization, leaf length, rosette growth habit, and height of hardened plants. Genome 33: 247–259. [Google Scholar]
- Snape JW, Sarma R, Quarrie SA, Fish L, Galiba G, Sutka J. 2001. Mapping genes for flowering time and frost tolerance in cereals using precise genetic stocks. Euphytica 120: 309–315. [Google Scholar]
- Sutka J. 2001. Genes for frost resistance in wheat. Euphytica 119: 167–172. [Google Scholar]
- Tsenov A. 1973. Duration of hardening of different winter wheat varieties under constant temperature and light conditions. In: Rajki S. ed. Proceedings of a colloquium on the winter hardiness of cereals. Budapest: Agricultural Research Institute of the Hungarian Academy of Sciences, 61–70. [Google Scholar]
- Vágújfalvi A, Kerepesi I, Galiba G, Tischner T, Sutka J. 1999. Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Science 144: 85–92. [Google Scholar]
- Veisz O, Sutka J. 1989. The relationships of hardening period and the expression of frost resistance in chromosome substitution lines of wheat. Euphytica 43: 41–45. [Google Scholar]


