Abstract
• Background and aims It is well known that resource allocation to male and female functions can be highly variable in hermaphroditic plants. The purpose of this study was to investigate variations in sexual investment at different levels (flower, plant and population) in Polygonatum odoratum, a plant with sequentially opening flowers.
• Methods Pollen and ovule production in base, middle and top flowers of P. odoratum flowering shoots from two natural populations were quantified. Plant measurements of phenotypic and functional gender were calculated in both populations. Total leaf number was used to investigate the relationship between gender assessments and plant size.
• Key results Pollen and ovule production varied depending on flower position, although the precise pattern differed between both studied populations; only investment in female floral function decreased markedly from base to top flowers in both populations. The frequency distribution of phenotypic gender and their relationship with plant size differed between populations. Phenotypic and functional gender were correlated in both populations.
• Conclusions Sexual investment in P. odoratum has shown a marked variability within plants, among plants, and between populations, which confirms the importance of analysing sex expression in plants of this type. Differences in relative investment in male and female components (phenotypic gender) are reflected in the functional gender and it would be expected that the evolution of sexual specialization in Polygonatum odoratum would be promoted.
Key words: Sex allocation, functional gender, phenotypic gender, plant size, Polygonatum odoratum
INTRODUCTION
The capacity of plants to produce both male and female gametes over their lifespan, with variation both in time and in space, undoubtedly constitutes one of their most fascinating characteristics, and has attracted the attention of plant evolutionary biologists ever since Darwin. However, it is only in the last two decades that real efforts have been made to understand sexual investment in hermaphrodite plants, and more particularly to understand how resources available for reproduction are distributed between the male and female functions. The recognition that hermaphroditism is not equivalent to ‘equisexuality’, together with the conceptual distinction between phenotypic and functional gender (Horovitz, 1978; Lloyd, 1980a), has permitted the development of theoretical models of the evolution of reproductive systems, and the integration of sexual investment into general theories of the reproductive ecology of hermaphrodite species (Charlesworth and Charlesworth, 1981; Charnov, 1982; Zhang and Jiang, 2002; Delph, 2003).
Numerous authors have shown that investment in the male and female reproductive functions may vary considerably within hermaphroditic species (e.g. see Charlesworth and Charlesworth, 1981; Lloyd, 1984). Research in this area has adopted four basic approaches.
Some studies have focused on a central prediction of theoretical models of sexual investment, namely that there should be a ‘trade-off’ (i.e. a negative correlation) between investment in male and female function. This prediction is based on the fact that the development of male and female reproductive organs requires limited resources, so that there is ‘inevitably’ a negative relationship between stamen/pollen production and carpel/ovule/fruit production (e.g. see Mazer and Delesalle, 1998; Mazer et al., 1999; Koelewijn and Hunscheid, 2000; and references therein).
Some studies have aimed to evaluate variations in phenotypic and functional gender within and among populations (Lloyd, 1984; Pickering and Ash, 1993; Klinkhamer et al., 1997, Kudo and Maeda, 1998; Dorken and Barrett, 2003), and within individuals among seasons and years (Ashman and Baker, 1992). The extent to which differences among flowers in relative investment in male and female components (i.e. phenotypic gender) are reflected in functional gender is of interest from the evolutionary viewpoint. Little or no correspondence between the two would be expected to inhibit the selection of sexual characteristics and therefore hinder the evolution of sexual specialization in hermaphroditic plants (see Guitián et al., 2003).
Some studies have focused on the relationships existing between sexual investment and plant size [the ‘size allocation model’ (SAM); see references in Klinkhamer et al., 1997; Wright and Barrett, 1999; Sarkissian et al., 2001; García, 2003]. The SAM predicts that sexual investment may depend on the plant's resource status: relative allocations to male and female functions (and indeed the optimal total allocation to reproduction) may differ depending on plant size and/or resource availability (Charnov, 1982; Wright and Barrett, 1999).
An important line of research has been to analyse differences in sexual investment within inflorescences of hermaphrodite plants. Such studies have focused on the importance of resource limitations and/or architectural effects (see Diggle, 1995; Medrano et al., 2000), while others have focused on differences in male and female reproductive success (Devlin and Stephenson, 1987; Brunet and Eckert, 1998). This latter focus, explored in plants with inflorescences showing sequential flower opening, indicates that the different contributions of male and female functions to fitness may exert selective pressure on reproductive investment in terms of flowers (see Brunet and Charlesworth, 1995).
These four different approaches have all highlighted the marked variability of sexual investment in hermaphrodite plants, and represent different perspectives for analysing the multiple selection pressures to which plants are subjected. From the evolutionary viewpoint, the different approaches are consistent with the view that selection should favour efficient investment in resources through the male and female functions (Lloyd, 1984).
In the present study, sexual investment was investigated at different levels in two populations of Polygonatum odoratum, and an attempt was made to answer the following specific questions. (1) Is there variation between investment in male function (pollen) and female function (ovules) within flowering shoots and/or between populations? (2) Does phenotypic and/or functional gender differ among individuals and populations? (3) At the plant level is there some relationship between phenotypic and functional gender? (4) Does male/female reproductive investment vary depending on plant size? Polygonatum odoratum is well-suited to studies of this type because (a) shoot leaf number and shoot flower number are correlated, (b) sexual investment varies among flowers, and (c) flowering shoots show sequential flower opening (see Guitián et al., 2001).
MATERIALS AND METHODS
Study species and sites
Polygonatum odoratum (Miller) Druce (Liliaceae) is a clonal rhizomatous geophyte with shoots of up to 50 cm in height, and with very short-stalked leaves arising from the stem. This species is widely distributed in Europe and also occurs in Morocco. It occurs on shady sites on a variety of substrates in both deciduous and evergreen forest. The flowering period commences in April–May. The mean number of cauline leaves on non-flowering shoots was 6·5 ± 1·6 (range 2–10; n = 25), versus 9·4 ± 1·6 (range 6–14; n = 25) on shoots that flowered. These means differ significantly (t = 60·7; P < 0·0001). The species is self-incompatible and pollination is largely by bumblebees (Bombus terrestris, B. pratorum and B. pascuorum). The mean number of flowers produced per flowering shoot is 5·6 ± 4·4 (see Guitián et al., 2001). Although flowers open progressively from the botton to the top of the flowering shoot, there is considerable overlap in the anthesis period of most of the flowers. Within a flowering shoot, axillary flowers are developed at the base of the cauline leaves, where they generally arise grouped in cymes of one, two or sometimes three. Flowering shoots produce either all male flowers, or all hermaphrodite flowers, or varying proportions of both. The yellowish-green to white perianth of male and hermaphrodite flowers is tubular (1·5–3 cm long). Hermaphrodite flowers produce six stamens, a three-locular ovary (with two to eight ovules per locule), and a well-developed slender style (approx. 1 cm long) which is ended by a small three-lobed stigma. Male flowers also have six stamens, but the style is hardly observable at simple sight (absolutely atrophied or absent), and when observed with the stereomicroscope no ovules are developed in their ovaries. The fruit is a black berry of about 1 cm in diameter, containing seven to nine seeds. Throughout this study, the unit of analysis was a flowering shoot. Different flowering shoots can belong to the same individual plant due to the clonal reproduction of the species. To sample different individuals, in all cases selected flowering shoots were always >1 m distant from each other.
The study was performed in two field populations separated by >200 km, Caurel and Santiago (hereafter CAU and SAN). The CAU population is situated at the western end of the Cantabrian Range (Lugo, north-west Spain; 42°36′N, 7°19′W), close to `O alto do Couto', at 1300 m a.s.l, in a mixed deciduous woodland of Quercus robur L., Betula alba L. and Corylus avellana (C. Koch.) Winkl. The SAN population is situated near Santiago de Compostela (A Coruña, north-west Spain; 42°52′N, 8°28′W), at 400 m a.s.l, in a Quercus robur deciduous woodland.
Variation in sexual investment within flowering shoots
Floral sex ratio
To investigate variation in male : hermaphrodite flower ratio within flowering shoots, and whether these patterns of variation differ between the two populations of study, 44 plants were selected in CAU and 58 in SAN. The sexual type of all flowers produced in each inflorescence on all shoots of these plants was monitored and recorded throughout the 2001 flowering period.
Pollen and ovule production
In each population 20 plants were randomly selected and all flowering buds produced were collected 1 or 2 d before anthesis (n = 137 and n = 157, respectively, in CAU and SAN populations). Each bud position in the inflorescence was recorded. Buds were maintained in individual vials containing FAA (formol + acetic acid + 70 % ethanol, at 5 : 5 : 90 v/v) until examination in the laboratory. Ovaries from all collected flower buds were dissected to determine ovule number per flower with the aid of a stereomicroscope. Pollen was counted using a particle counter (Coulter Counter® Z2; Beckman Inc.) equipped with a 100 µm aperture tube and a particle size channellizer accessory. For estimation of pollen production, two anthers from each flower bud (one from each stamen whorl) were examined. Anther content was released into a vial containing 0·5 mL of electrolytic solution (Isoton II®), by mechanical destruction of the theca walls and subsequent vortexing. The wall fragments were then removed and the remaining solution poured into a beaker containing 50 mL of Isoton II. The vial was then refilled with 0·5 mL of Isoton II and shaken to collect any remaining pollen, then emptied into the beaker. The final volume was thus 51 mL (0·5 + 50 + 0·5). Pollen size in P. odoratum had been reported previously to vary between 16 and 46 µm (Valdés et al., 1987). However, pollen size in the present samples ranged between 19 and 29 µm, so the counter was set to count in this range (improving accuracy). For each flower (i.e. each 51 mL pollen suspension sample) five repeat counts were performed, each in 0·5 mL of the pollen suspension sample. Counter accuracy was checked by manual counting under a light microscope: for these counts the pollen from two anthers per flower was suspended in 1 mL of a solution of detergent and safranin, from which ten 5 µL replicates were then obtained for counting (Herrera, 1987).
Gender and size assessments
The basic measure of the phenotypic gender of a plant is the ratio of ovule number to pollen grain number. However, this is necessarily a destructive measure, and assessment of the relationship between phenotypic gender and functional gender requires a non-destructive measure. To non-destructively assess gender, 65 plants were tagged with metal labels in SAN and 50 plants in CAU, and these plants monitored until fruit set (about 2 months after flower opening). For each flower, the position in the inflorescence, and presence or absence of stamen whorls and carpel whorls were also recorded.
Standardized phenotypic gender (Gi) of each plant was calculated as per Lloyd (1980a):
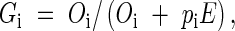 |
where Oi is the number of carpel (female) whorls, pi is the number of stamen (male) whorls, and E is mean Oi/pi in the population. Gi may thus vary between 0 for plants producing only pollen and 1 for plants producing only ovules.
Standardized functional gender (G) of each plant was likewise calculated as per Lloyd (1980a):
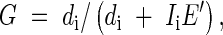 |
where di is the number of fruits produced, Ii is the number of stamen (male) whorls, and E′ is mean di/Ii in the population.
The above formulae assume that stamen whorl number is proportional to pollen number, and that carpel whorl number is proportional to ovule number. To confirm the validity of this assumption, regression analysis was used, considering the 292 flowers for which destructive pollen and ovule counts had been obtained. The result of these analyses (not shown) indicated a strong relationship between a plant's number of female whorls and its number of ovules, and likewise between a plant's number of male whorls and its number of pollen grains. Thus whorl numbers can reasonably be used as indicators of gender.
To investigate relationships between the different gender assessments (phenotypic and functional) and plant size, total leaf number was used as the estimate of plant size. The leaves of P. odoratum develop acropetally and the shoot must reach a certain size and bear a certain number of leaves before becoming a flowering shoot. Considering all flowering shoots, a strong significant positive correlation between leaf number and flower number was previously verified (r = 0·797; P < 0·0001).
Statistical analyses
Within-shoot among-flower variation in pollen and ovule production was explored by fitting generalized linear mixed models (SAS macro GLIMMIX; Littell et al., 1996) to the data. Given that the selected flowering shoots bore varying numbers of flowers, the position of each flower on the shoot was classified in relative terms as ‘base’ (lowest third of inflorescence), ‘middle’ (middle third), or ‘top’ (top third). The factors ‘population’, ‘relative position’, and their interaction were considered as fixed effects in the model, while ‘plant’ nested within ‘population’ was considered as a random effect. All data were analysed using the SAS system (SAS Institute, 1999).
Total variance in pollen and ovule production was partitioned into components due to variation among populations and plants using the MIXED procedure (‘restricted maximum likelihood’ estimation method) in the SAS statistical package. The factors ‘Population’ and ‘Plant’ (nested within ‘Population’) were included in the analyses as categorical factors.
RESULTS
Variation in sexual investment within flowering shoots
Floral sex ratio
Figure 1 shows mean male : hermaphrodite flower ratios in each position of P. odoratum flowering shoots with different numbers of cymes, in both populations studied. In the SAN population, male flowers were only produced in distal positions of the flowering shoot, regardless of number of cymes per shoot. In the CAU population, the distal-most positions also have a greater ratio of male to hermaphrodite flowers than the more basal ones. However, this pattern is most clearly observed in shoots with five to seven cymes, while in shoots with two to four cymes male flowers are also frequently produced in basal positions.
Fig. 1.
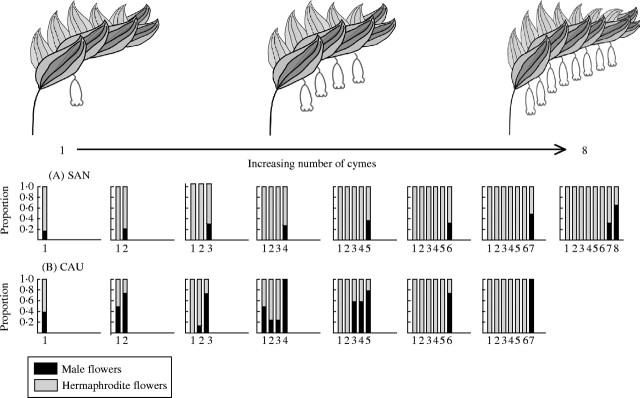
Relative proportions of male and hermaphrodite flowers produced in the different cymes of P. odoratum flowering shoots in (A) SAN and (B) CAU populations.
Pollen and ovule production
Polygonatum odoratum shows wide variation in all traits measured. The mean number of ovules per flower was 12·5 ± 1·9 in SAN and 12·7 ± 2·3 in CAU. The mean number of pollen grains per flower was 42 054 ± 10 683 in SAN and 55 772 ± 13 637 in CAU. Analysis of variance indicated that about 70 % of variance in numbers of ovules is among flowers on the same plant, and the remaining 30 % among plants (not between populations); by contrast, variance in numbers of pollen grains is rather evenly attributable to variation within plants, among plants and between populations (Table 1).
Table 1.
Percentages of variance in NO (number of ovules per flower) and NP (number of pollen grains per flower) explained by the different factors for Polygonatum odoratum plants in the two study populations
| NO |
NP |
|||||
|---|---|---|---|---|---|---|
| Factor |
Variance (%) |
P |
Variance (%) |
P |
||
| Population | 0 | — | 41·51 | 0·2498 | ||
| Plant (population) | 30·09 | 0·0009 | 35·82 | <0·0001 | ||
| Residual (= within plant) | 69·91 | <0·0001 | 22·67 | — | ||
The number of ovules per flower declined from basal to top position along the flowering shoot, both in SAN and CAU, whereas the number of pollen grains per flower did not show such a clear pattern: in CAU, the number of pollen grains per flower declined from base to top, whereas in SAN there was no a clear variation along the flowering shoot (Fig. 2). The results of analysis of variance in numbers of ovules and pollen grains with position along the flowering shoot are shown in Table 2. These results show that the number of ovules per flower does not vary significantly between populations, but does vary among the three positions along the shoot; the interaction ‘population × position’ was not significant. Note, however, that male flowers were included in this analysis, and that this result can be affected by their presence mostly in distal positions of the flowering shoots. However, if only hermaphrodite flowers were considered, the pattern of decline in number of ovules per flower with position persisted (F = 4·49; P = 0·015), but differences between populations did not exist (F = 1·95; P = 0·173), and the interaction ‘population × position’ was also not significant (F = 1·28; P = 0·286). The number of pollen grains per flower varied significantly both between populations and among positions, though again the interaction was not significant.
Fig. 2.
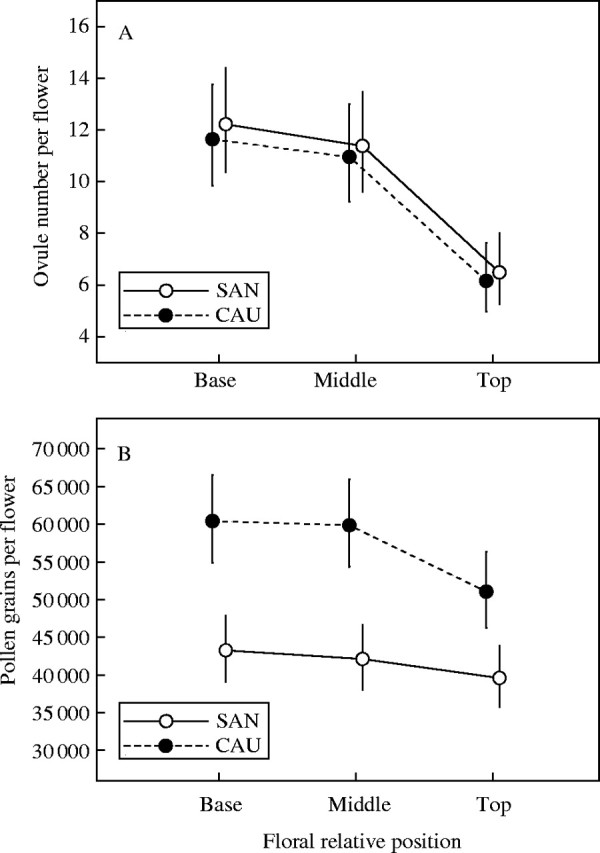
Variation between relative positions (base, middle and top) in (A) number of ovules per flower and (B) number of pollen grains produced per flower for Polygonatum odoratum plants in the study populations. Values plotted are back-transformed model adjusted means obtained from fitting generalized linear models to the data. Vertical lines denote the 95 % parametric confidence intervals around the means.
Table 2.
Results of analysis of variance in NO (number of ovules per flower) and NP (number of pollen grains per flower) with factors population and position in the flowering shoot (base, middle, and top), for Polygonatum plants in the two study populations
| Significance test |
||||||
|---|---|---|---|---|---|---|
| Dependent variable |
Effect in the model |
d.f. |
F |
P |
||
| NO | Population | 1,38 | 0·26 | 0·6145 | ||
| Relative position | 2,76 | 30·58 | <0·0001 | |||
| Population × relative position | 2,76 | 0·00 | 0·9959 | |||
| NP | Population | 1,38 | 24·18 | <0·0001 | ||
| Relative position | 2,76 | 13·24 | <0·0001 | |||
| Population × relative position | 2,76 | 1·84 | 0·1658 | |||
Gender and size
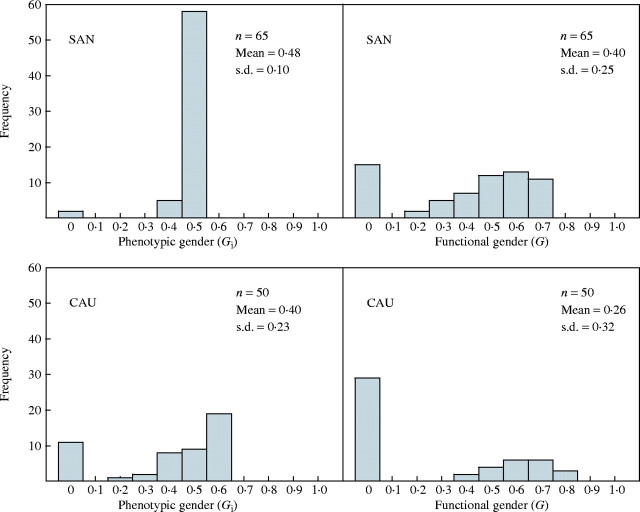
Polygonatum odoratum has flowering shoots with male flowers and hermaphrodite flowers. The female : male whorl ratio (E) was 0·91 in SAN and 0·65 in CAU. Standardized phenotypic gender (Gi) ranged from 0 (i.e. totally male plants) to 0·52 (plants with all flowers hermaphrodite) in SAN, and from 0 to 0·60 in CAU. The percentage of flowers that set fruit ranged between 0 and 100 in both populations (0·46 ± 0·35 in SAN and 0·17 ± 0·25 in CAU). The fruit: stamen ratio (E′) was 0·47 in SAN and 0·22 in CAU. Standardized functional gender (G) ranged between 0 and 0·68 in SAN, and between 0 and 0·82 in CAU. The distribution of both Gi and G differed markedly between the two populations (Fig. 3). Gi was more homogenous among individuals in SAN than in CAU. In SAN the distribution of Gi was basically unimodal, with most plants equisexual, whereas in CAU the distribution was markedly bimodal, with large numbers of both equisexual and male-only plants. Gi and G were significantly correlated both in SAN (r = 0·283; P = 0·022) and CAU (r = 0·514; P < 0·0001).
Fig. 3.
Observed frequency distributions of phenotypic (Gi) and functional gender (G) in both Polygonatum odoratum populations studied.
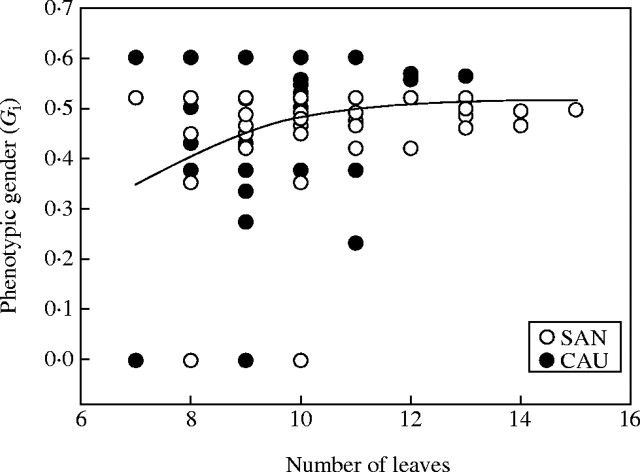
The relationship between phenotypic gender (Gi) and plant size, as assessed on the basis of number of leaves, is show in Fig. 4. The line shown is a cubic spline fitted to the data for both populations. As can be seen, the relationship between gender and plant size differs between the two populations being more predictable in the SAN population (see also Fig. 1).
Fig. 4.
Plot of phenotypic gender (Gi) against plant size, defined as number of leaves. Open circles, plants for SAN population; filled circles, plants from the CAU population. The line is a cubic spline fitted to the data for both populations.
DISCUSSION
Sex allocation theory predicts that sexually plastic individuals will be able to increase their fitness by adjusting their relative investment in male and female sexual function in response to variation in environmental conditions (Charnov, 1979). In Polygonatum odoratum, sexual variability is seen both in the male : female gamete ratio at the flower level, and in male : hermaphrodite flower ratio at the plant level.
Pollen and ovule production
The results of the present study indicate that P. odoratum shows marked sexual variability in both traits considered (ovule number, pollen grain number) and at all levels considered (flower, plant and population). Variations in sexual investment at the individual level have been documented in numerous hermaphroditic species and have been attributed to changes in age and resource status of plants and/or genetic factors (Lloyd and Bawa, 1984; Meagher, 1988; Charlesworth, 1999; Mazer and Dawson, 2001). However, there have been few studies of how this variation is distributed among different possible levels (however, see Guitián et al., 2003, and references therein). The results presented here indicate that the two traits analysed show very different patterns of variation: the number of ovules per flower showed high within-plant variation, whereas the number of pollen grains per flower showed high among-plant within-population variation and high between-population variation. Consequently, the male components of gender in P. odoratum appear to be more stable at the individual level than the female components. This is in line with findings obtained for other species (Rhaphanus sativus, Mazer, 1992; Campanula rapunculoides, Vogler et al., 1999; Helleborus foetidus, Guitián et al., 2003).
In general, in Polygonatum odoratum both number of ovules and number of pollen grains declined from proximal to distal positions in the flowering shoot. A decline in pollen and/or ovule production per flower has been recorded in numerous previous studies (e.g. Ashman and Hitchens, 2000; Mazer and Dawson, 2001; Ishii and Sakai, 2002; García, 2003; Ishii, 2004); in the present study, however, the pattern observed differed between the two populations. In CAU, both ovule number and pollen grain number showed a clear decline from proximal to distal positions, whereas in SAN pollen grain number showed little change with position in the inflorescence, so that distal flowers are proportionally more male. In other words, the positional variation in ovule number in SAN leads to a variation in sex expression within the flowering shoots. More interestingly, in both populations of P. odoratum studied quantitative variation in gender among morphologically hermaphrodite flowers exists, because when flowers without ovules are excluded from the analysis, the decline in ovule production from proximal to distal positions remains significant.
In hermaphroditic plants with sequentially opening inflorescences, the contribution to fitness via the male and female functions may vary among flowers in different positions, as a result of differences in the flowers' ‘mating environment’ (Brunet and Charlesworth, 1995). Factors such as the spatial separation of sexual phases (dichogamy) or the spatial directionality of pollinator movement may modify the mating environment and thus alter the relative fitnesses of male and female gender. In P. odoratum flowering stems show sequential opening, and pollinators (basically Bombus species) show clear directionality, starting with proximal flowers and continuing to more distal flowers. Thus the first flower of the shoot is likely to receive xenogamous pollen only, while the remaining flowers will receive varying proportions of xenogamous and geitonogamous pollen, which will limit pollination effectiveness, since this is a self-incompatible species (for more on the negative effects of geitonogamy, see Klinkhamer and de Jong, 1993). In this situation, the probability that a flower in distal-most position will receive effective pollination is very small, except when this is the only flower remaining open (by which time the attractiveness of the inflorescence as a whole is greatly reduced). Thus the flowers in a flowering shoot of P. odoratum have different probabilities of ‘siring’ offspring: distal flowers are more likely to donate pollen to the flowers of neighbouring plants.
Gender variation and size
The size sex allocation model predicts an increase in female function with increasing plant size, so that large plants will be more female in terms of both phenotypic and functional gender (de Jong and Klinkhamer, 1989; Wright and Barrett, 1999; Sarkissian et al., 2001). As shown in Fig. 4, the relationship between size and gender differs between the two populations. In the SAN population the number of male flowers does not seem to be affected by the plant size (see Klinkhamer et al., 1997), whereas the number of hermaphrodite flowers increased with increasing size; in CAU, by contrast, there appears to be a threshold size below which plants have only male flowers. This ‘male-only strategy’ may be an adaptative strategy to conserve resources (for additional explanations, see Huang et al., 2002). Various authors have reported variations in phenotypic and/or functional gender at different levels in hermaphrodite plants, with both unimodal and bimodal population patterns generally attributable to differences in the size and resources of the plants within the population (Solomon, 1985; Eriksson, 1987; Kudo and Maeda, 1998; Huang et al., 2002; Dorken and Barrett, 2003). Presumably, the distributions of phenotypic and functional gender indicated by our results reflect different degrees of control of within-shoot maternal investment (number of flowers with ovary, number of ovaries that produce fruit) in response to spatial and temporal variations in resource availability.
Evolutionary consequences
Andromonoecy (i.e. presence of male-only and hermaphrodite flowers within a single plant) has been suggested to be a mechanism for adjusting sexual phenotype in response to changing environmental conditions (Lloyd and Bawa, 1984). In line with this model, it would be expect that (a) it will occur in species in which the cost of producing mature fruit is high and the optimal number of male flowers is higher than the number of potentially fruit-producing flowers (see Diggle, 1993), and (b) sexual expression in andromonoecious plants may vary among individuals, among populations and among years (Diggle, 1994). The results of the present study indicate considerable variation in sex expression at the different levels considered, and previously reported results (Guitián et al., 2001) have shown a decline in fruit set levels in distal flowers of P. odoratum attributable to competition for resources. Consequently, flowers at different positions in the inflorescence will vary as regards the quantity of resources destined for reproduction. In line with Lloyd (1980b), when there are morphological gradients along shoots or within inflorescences giving some flowers a predictable advantage over others, producing a small proportion of hermaphrodite flowers is more advantageous than hermaphroditism combined with low fruit set.
Various authors have considered the possible role of pollinator movement directionality in the evolution of sexual systems in plants with sequentially opening inflorescences, stressing the importance of differences in the probability of pollen transfer at each position in the inflorescence (e.g. Lloyd and Webb, 1986; Brunet, 1996; Kudo et al., 2001; Ishii, 2004). In self-incompatible plants such as P. odoratum, this effect may be even greater, since pollen deposited on the stigma of another flower will not fertilize ovules. In line with Brunet and Charlesworth (1995), the male specialization of the distal flowers of P. odoratum, and the consequent functional andromonoecy, may be attributable to differences in available resources and probabilities of pollen transfer at each position in the inflorescence. Transition between functional and morphological andromonoecy must require a pre-anthesis mechanism for determination of ovary development in functionally female sterile flowers (Miller and Diggle, 2003).
In conclusion, sexual investment in P. odoratum showed a marked variability within plants, among plants, and between populations, which confirms the importance of analysing sex expression in plants of this type. Differences in relative investment in male and female components (phenotypic gender) are reflected in the functional gender and it would be expected that the evolution of sexual specialization in Polygonatum odoratum be promoted.
Acknowledgments
We thank José Luis Medina and Marina García (Estación Biológica de Doñana) for pollen grains and ovule counting. Carlos Herrera, José Luis Garrido, Pablo Guitián and two anonymous referees made useful comments on an early version of the manuscript. During this work the authors were receiving funding from the Ministerio de Ciencia y Tecnología (BOS2000-1122-C03) and the Fundación RAMON ARECES. While conducting this work, M.M. was supported by a post-doctoral grant from the Ministerio de Educación y Cultura (EX2001 35313421) and a post-doctoral contract from the Consejo Superior de Investigaciones Científicas (I3P Program), financed by the Fondo Social Europeo.
LITERATURE CITED
- Ashman T-L, Baker I. 1992. Variation in floral sex allocation with time of season and currency. Ecology 73: 1237–1243. [Google Scholar]
- Ashman T-L, Hitchens MS. 2000. Dissecting the causes of variation in intra-inflorescence allocation in a sexually polymorphic species, Fragaria virginiana (Rosaceae). American Journal of Botany 87: 197–204. [PubMed] [Google Scholar]
- Brunet J. 1996. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 77: 2458–2471. [Google Scholar]
- Brunet J, Charlesworth D. 1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Brunet J, Eckert CG. 1998. Effect of floral morphology and display on outcrossing in blue columbine, Aquilegia caerulea (Ranunculaceae). Functional Ecology 12: 596–606. [Google Scholar]
- Charlesworth D. 1999. Theories of evolution of dioecy. In Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. New York: Springer-Verlag, 33–60. [Google Scholar]
- Charlesworth D, Charlesworth B. 1981. Allocation of resources to male and female functions in hermaphrodites. Biological Journal of the Linnean Society 15: 57–74. [Google Scholar]
- Charnov EL. 1979. Simultaneous hermaphroditism and sexual selection. Proceedings of the National Academy of Sciences of the United States of America 76: 2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL. 1982.The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. 1989. Size dependency of sex-allocation in hermaphroditic, monocarpic plants. Functional Ecology 3: 201–206. [Google Scholar]
- Delph L. 2003. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evolution and Development 5: 34–39 [DOI] [PubMed] [Google Scholar]
- Devlin B, Stephenson AG. 1987. Sexual variation among plants of perfect-flowered species. American Naturalist 130: 199–218. [Google Scholar]
- Diggle PK. 1993. Developmental plasticity, genetic variation, and the evolution of andromonoecy in Solanum hirtum (Solanaceae). American Journal of Botany 80: 967–973. [Google Scholar]
- Diggle PK. 1994. The sex expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenic contingency. American Journal of Botany 81: 1354–1365. [Google Scholar]
- Diggle PK. 1995. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology and Systematics 26: 531–552. [Google Scholar]
- Dorken ME, Barrett SCH. 2003. Gender plasticity in Sagittaria sagittifolia (Alismataceae), a monoecious aquatic species. Plant Systematics and Evolution 237: 99–106. [Google Scholar]
- Eriksson O. 1987. Regulation of seed-set and gender variation in the hermaphroditic plant Potentilla anserina Oikos 49: 165–171. [Google Scholar]
- García MB. 2003. Sex allocation in a long-lived monocarpic plant. Plant Biology 5: 203–209. [Google Scholar]
- Guitián J, Guitián P, Medrano M. 2001. Causes of fruit set variation in Polygonatum odoratum (Liliaceae). Plant Biology 3: 637–641. [Google Scholar]
- Guitián J, Medrano M, Herrera CM, Sánchez-Lafuente AM. 2003. Variation in structural gender in the hermaphrodite Helleborus foetidus (Ranunculaceae): within- and among-population patterns. Plant Systematics and Evolution 241: 139–151. [Google Scholar]
- Herrera J. 1987. Flower and fruit biology in southern Spanish Mediterranean shrublands. Annals of Missouri Botanical Garden 74: 69–78. [Google Scholar]
- Horovitz A. 1978. Is the hermaphrodite flowering plant equisexual? American Journal of Botany 65: 485–486. [Google Scholar]
- Huang S-Q, Sun S-G, Takahashi Y, Guo Y-H. 2002. Gender variation of sequential inflorescences in monoecious plant Sagittaria trifolia (Alismataceae). Annals of Botany 90: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii HS. 2004. Increase of male reproductive components with size in an animal-pollinated hermaphrodite, Narthecium asiaticum (Liliaceae). Functional Ecology 18: 130–137. [Google Scholar]
- Ishii HS, Sakai S. 2002. Temporal variation in floral display size and individual floral sex allocation in racemes of Narthecium asiaticum (Liliaceae). American Journal of Botany 89: 441–446. [DOI] [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong TJ. 1993. Attractiveness to pollinators: a plant's dilemma. Oikos 66: 180–184. [Google Scholar]
- Klinkhamer PGL, de Jong TJ, Metz H. 1997. Sex and size in cosexual plants. Trends in Ecology and Evolution 12: 260–265. [DOI] [PubMed] [Google Scholar]
- Koelewijn HP, Hunscheid MPH. 2000. Intraspecific variation in sex allocation in hermaphroditic Plantago coronopus L. Journal of Evolutionary Biology 13: 302–315. [Google Scholar]
- Kudo G, Maeda T. 1998. Size-dependent variation in phenotypic gender and functional gender of a spring ephemeral, Anemone debilis Fish. Plant Species Biology 13: 69–76. [Google Scholar]
- Kudo G, Maeda T, Narita K. 2001. Variation in floral sex allocation and reproductive success within inflorescences of Corydalis ambigua (Fumariaceae): pollination efficiency or resource limitation? Journal of Ecology 89: 48–56. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. 1996.SAS system for mixed models. Cary, NC: SAS Institute. [Google Scholar]
- Lloyd DG. 1980. Sexual strategies in plants. III. A quantitative method for describing the gender of plants. New Zealand Journal of Botany 18: 103–108. [Google Scholar]
- Lloyd DG. 1980. Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytologist 86: 69–79. [Google Scholar]
- Lloyd DG. 1984. Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhán J, eds. Perspectives on Plant Population Ecology. Sunderland, MA: Sinauer Associates, 277–300. [Google Scholar]
- Lloyd DG, Bawa KS. 1984. Modification of the gender of seed plants in varying conditions. Evolutionary Biology 17: 255–338. [Google Scholar]
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- Mazer SJ. 1992. Environmental modifications of gender allocation in wild radish: consequences for sexual and natural selection. In: Wyatt R, ed. Ecology and evolution of plant reproduction: new approaches. New York: Chapman and Hall, 181–225. [Google Scholar]
- Mazer SJ, Delesalle VA. 1998. Contrasting variation within and covariation between gender-related traits in autogamous versus outcrossing sepecies: alternative evolutionary predictions. Evolutionary Ecology 12: 403–425. [Google Scholar]
- Mazer SJ, Dawson KA. 2001. Size-dependence sex allocation within flowers of the annual herb Clarkia unguiculata (Onagraceae): ontogenic and among-plant variation. American Journal of Botany 88: 819–831. [PubMed] [Google Scholar]
- Mazer SJ, Delesalle VA, Neal PR. 1999. Response of floral traits to selection on primary sexual investment in Spergularia marina: The battle betweeen the sexes. Evolution 53: 717–731. [DOI] [PubMed] [Google Scholar]
- Meagher TR. 1988. Sex determination in plants. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive ecology. New York: Oxford University Press, 125–138. [Google Scholar]
- Medrano M, Guitián P, Guitián J. 2000. Patterns of fruit and seed set within inflorescences of Pancratium maritimum (Amarylidaceae): non-uniform pollination hypothesis, resource limitation, or architectural effects? American Journal of Botany 87: 493–501. [PubMed] [Google Scholar]
- Miller JS, Diggle PK. 2003. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. American Journal of Botany 90: 707–715. [DOI] [PubMed] [Google Scholar]
- Pickering CM, Ash JE. 1993. Gender variation in hermaphrodite plants: evidence from five species of alpine Ranunculus Oikos 68: 539–548. [Google Scholar]
- Sarkissian TS, Barrett SCH, Harder L. 2001. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology 82: 360–373. [Google Scholar]
- SAS Institute. 1999.SAS/STAT users guide, 8th edn. Cary, NC: SAS Institute. [Google Scholar]
- Solomon BP. 1985. Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology 66: 1321–1332. [Google Scholar]
- Valdés B, Díez MJ, Fernández I. 1987.Atlas polínico de Andalucía occidental. Sevilla: Instituto de Desarrollo Regional, Universidad de Sevilla, Excma. Diputación de Cádiz. [Google Scholar]
- Vogler DW, Peretz S, Stephenson AG. 1999. Floral plasticity in an iteroparous plant: the interactive effects of genotype, environment, and ontogeny in Campanula rapunculoides (Campanulaceae). American Journal of Botany 86: 482–494. [PubMed] [Google Scholar]
- Wright SI, Barrett SCH. 1999. Size-dependent gender modification in a hermaphroditic perennial herb. Proceedings of the Royal Society of London Series B 266: 225–232. [Google Scholar]
- Zhang DY, Jiang XH. 2002. Size-dependent resource allocation and sex allocation in herbaceous perennial plants. Evolutionary Biology 15: 74–83. [Google Scholar]