Abstract
• Background and aims Intraspecific variation in floral components and reproductive success is often located at the intra-individual level. The arrangement of flowers within inflorescences may explain a great deal of this variation. The variation in number of ovules, fruit set, number of seeds per fruit, seed set, seed weight and seed germination is investigated at different positions within the inflorescence of Silene acutifolia.
• Methods Data were obtained in natural populations, and germination experiments were conducted in a germination chamber.
• Key results The number of ovules, fruit set, number of seeds, seed set and seed weight, showed a significant decline from early (primary) position to later (tertiary) position. The patterns of intra-inflorescence variation were consistent in different populations and years of study. Seed germination showed an opposite pattern, seeds from primary position showed the lowest germination percentages and seeds from tertiary position the highest, although the effect of position on germination was only marginally significant. There was significant among-population variation in number of ovules per flower. Fruit set also varied significantly among populations, with lower fruit set in the smaller and more isolated population. No significant among-population differences were detected in number of seeds per fruit and seed set. Seeds from the smallest and more isolated population (Arnado) were the lightest. Seed germination showed strong differences between populations, seeds from Arnado started to germinate later, and showed the lowest final germination percentages.
• Conclusions Architectural effects or resource competition are the most commonly proposed hypothesis to explain these patterns. Data suggest that there is less pollen available to pollinate tertiary flowers, and that there is not enough outcross pollen in Arnado. The germination percentages suggest that there is variation in the source of pollen within inflorescences, with high probability of receiving outcross pollen in flowers from primary position, and higher probability of geitonogamous crosses in tertiary flowers.
Key words: Caryophyllaceae, fruit set, intra-inflorescence variation, ovule number, seeds per fruit, Silene acutifolia
INTRODUCTION
In many hermaphroditic species, the size and number of reproductive structures and the components of female reproductive success show marked among-flower within-inflorescence variation (Stephenson, 1980; Lee, 1988; Obeso, 1993; Diggle, 1995; Brunet, 1996; for review, see in Ashman and Hitchens, 2000). The pattern most commonly found is a reduction in the number or size of reproductive structures from proximal/early to distal/late flowers within the inflorescence, or a similar reduction in fruit set and/or seed set (Solomon, 1988; Herrera, 1991; Guitián and Navarro, 1996). Lloyd (1980) suggested that these differences reflected serial adjustments in maternal investment at consecutive stages (flower production, ovary development and fruit maturation). Closely related with Lloyd's ideas are hypotheses that attribute differences within inflorescences to phenotypic plasticity in response to different levels of resources (Stephenson, 1981; Lee, 1988; Obeso, 1993). However, some authors have proposed that resource competition is not the only cause of differences at the intra-inflorescence level, but rather that developmental constraints (Wolfe, 1992) or architectural effects (Diggle, 1995; for review, see Diggle, 2002) also have to be considered. According to these hypotheses, differences in floral characteristics from early to late flowers, or from proximal to distal position within the inflorescence, are maintained even in the absence of differential resource allocation, and recent studies have attempted to dissect the causes of intra-inflorescence variation by testing these two hypotheses (i.e. extrinsic resources vs. intrinsic restraints; note of course that both effects may act simultaneously) (Diggle, 1995; Ashman and Hitchens, 2000; Medrano et al., 2000; Wolfe and Denton, 2001). Variations in reproductive traits and female reproductive success may also be attributable to differences in the quantity and quality of pollen (Vaughton and Ramsey, 1995, 1997). These differences may be more pronounced in small populations, in which the number of potential mates is reduced. Differences in the mating environment of flowers may also explain differences in the quality of pollen within plants: e.g. in sequentially blooming plants with protandry, the pollen available for flowers in the female phase may vary between early and late flowers within an inflorescence (Brunet and Charlesworth, 1995). Limited resources have been considered responsible for variation in seed mass (Vaughton and Ramsey, 1997; Jacquemyn et al., 2001). Intra-inflorescence variations in male traits have also been reported (see revision in Diggle, 2002), although variations in the male components are mostly described as changes in sex expression (Diggle, 1991; Emms, 1993; Wolfe, 1998), rather than variation in number of stamens and/or pollen in hermaphrodite flowers within inflorescences.
The main aim of this study was to investigate variation in number of ovules per flower, fruit set, seed set, seed weight and seed germination within inflorescences of Silene acutifolia. Specifically, the following questions were addressed: (1) Is there any reduction in the female components analysed between early- and to later-opening flowers in the inflorescence? (2) Are these patterns consistent among years and populations? (3) Are there among-population differences in the female components analysed? (4) Are these differences correlated with population size?
MATERIALS AND METHODS
Plant and study area
Silene acutifolia Link ex Rohrb. is a polycarpic insect-pollinated herb, endemic to northwest Spain and north and central Portugal. The species is sometimes stoloniferous, with a rosette of basal leaves and a variable number of fertile stems bearing dichasium-type inflorescences. Typical inflorescences have a primary flower with one flower on each side of the central axis (secondary flowers) and one flower on each side of the lateral axis (tertiary flowers); some inflorescences also have quaternary flowers and lateral flowers on the main axis. Flowers in the primary position are basal and open first, while flowers in the tertiary or quaternary position are distal and open later. The flowers are purplish-pink, with five petals, and the ovary usually has three styles. The self-compatible protandrous flowers need pollinators to attain maximal seed set (Buide and Guitián, 2002). The fruits are capsules that contain numerous seeds without any specific type of dispersal mechanism.
The study was carried out in three populations in north-west Spain (Cañones del Sil, Xurés, Arnado). Cañones del Sil and Xurés are considered to be metapopulations with local populations scattered in those microsites with appropriate conditions (fissures and rockfalls of granitic rocks, with a certain degree of humidity). Arnado is the most northerly site known for this species. In the Cañones del Sil population 50 % of plants showed fewer than 12 inflorescences, while in the Arnado population 50 % showed fewer than six inflorescences. The most common type of inflorescence in Cañones del Sil (n = 505) was that with primary, secondary and tertiary flowers (39 %); the most common types in Arnado (n = 222) were that with primary and secondary flowers (44 %) and those with primary, secondary and tertiary flowers (41 %).
Within-inflorescence variation in female allocation
In 1996, 30 plants were marked before the flowering period in each of three populations (Cañones del Sil, Xurés and Arnado) and all fruits and non-fruiting flowers produced at each position in the inflorescence counted. Some of these plants were damaged by herbivores, and data were finally obtained from 23 plants in Cañones del Sil, 26 in Xurés and 26 in Arnado. In 1997, 30 plants in Cañones del Sil were marked, and final data were obtained from 20 plants. In 1998, data were obtained from 38 plants in Cañones del Sil and 29 plants in Arnado. Fruit set was calculated as the percentage of mature fruits in relation to total flowers produced (matured fruits, dead fruits and non-fruiting flowers). Total number of flowers produced is readily counted, because non-fruiting flowers remain on the plant. In 1996, data on number of seeds per fruit were obtained from fruits collected from the primary, secondary and tertiary positions in Cañones del Sil and Arnado. In 1997 and 1998, data on number of seeds per fruit were obtained from fruits collected from the three positions on each marked plant, with a total of 248 fruits from Cañones del Sil in 1997, 420 from Cañones del Sil and 158 from Arnado in 1998. Variation in flower ovule number within the inflorescence was investigated in 420 flowers from Cañones del Sil and 158 from Arnado, in 1998.
Germination and seed weight
Seeds were collected in June and July 1996 from more than 100 plants and more than 300 mature capsules from primary, secondary, tertiary and quaternary position of inflorescences of S. acutifolia from the Xurés, Cañones del Sil and Arnado populations. Seeds from the quaternary position were eliminated from the analysis because no seeds from this position were found in the Arnado population. To assess seed weight at the different positions, 492 of the total of 3552 seeds were dried at 70 °C for 48 h and individually weighed on a microbalance. The remaining 3060 seeds were divided into two groups: half of them were stored in the dark conditions at 5 °C for 25 d, and half were stored in the dark at laboratory temperature (about 20 °C). The temperature of 5 °C was used because it has been considered a very effective temperature for breaking the dormancy of seeds that require cold stratification (Baskin and Baskin, 1989, and references therein). The germination experiments were started in September 1996: seeds (five replications of 30 seeds each for each test condition) were placed on filter paper moistened with distilled water in Petri dishes. The Petri dishes were placed in a climate-control chamber (IBERCEX-G-220) with relative humidity near 100 % and photoperiod of 14 h light at 25 °C and 10 h dark at 10 °C. Subsequent germination was monitored daily from 8 September to 4 December, by which date germination had ceased. The criterion for germination was radicle emergence.
Statistical analyses
Variation in number of ovules per flower with population and position in inflorescence was analysed by repeated measures ANOVA, with position as within-subject factor (three levels: primary, secondary and tertiary position), using the GLM procedure of SPSS; the response variable was transformed (x′ = x2) to meet the assumptions of ANOVA. In 1997 and 1998, the effects of population and position on fruit set, seed number per fruit, and seed set were also analysed using repeated-measures analyses; fruit set, and seed set were transformed (p′ = arcsin √p) to meet the assumptions of ANOVA. In 1996, the data on number of seeds per fruit were calculated from fruits taken haphazardly from primary, secondary and tertiary position from different plants of the Arnado and Cañones del Sil populations; matured seeds were counted, and the data were analysed with a general ANOVA. Population and position effects on seed weight were analysed with a two-way ANOVA, with population and position as fixed factors and Type-III sum of squares, using the SPSS statistical package. The response variable was logarithmically transformed. The seed germination data were analysed by factorial ANOVA with the GLIM 3.77 program, using generalized linear modelling for proportion data (i.e. assuming a binomial error distribution), with a logit link function and with the binomial denominator being total number of seeds per Petri dish (Crawley, 1993). William's adjustment (Williams, 1982; see Crawley, 1993) was applied, prior to statistical inference, to compensate for over-dispersion.
RESULTS
Intra-inflorescence variation in female function
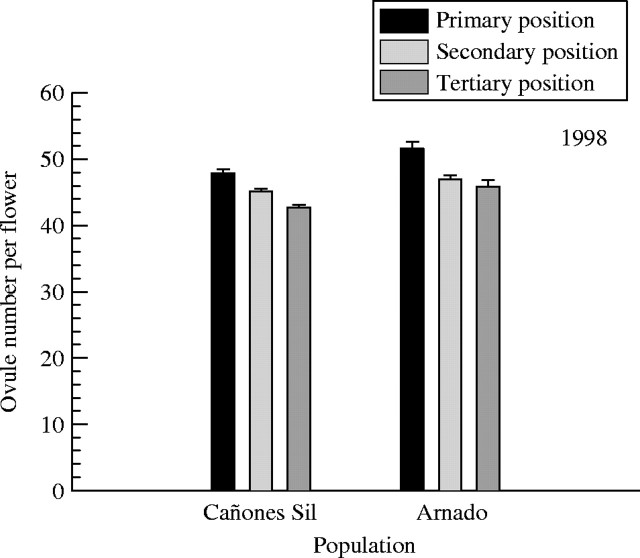
Repeated measures analysis of variance indicated that position in inflorescence and population both had significant effects on number of ovules per flower (Table 1), with a reduction from primary to tertiary positions (Fig. 1), and lower values in Cañones del Sil. No significant interaction between position and population was observed, indicating that the variation among positions is independent of population.
Table 1.
Repeated-measures analyses of variance to investigate factors affecting ovule number in Silene acutifolia, in 1998
| Year |
Source of variation |
d.f. |
MS |
F |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within inflorescence positions | ||||||||||
| Position | 2 | 1527 127·53 | 17·84 | 0·000 | ||||||
| Population × position | 2 | 102 956·43 | 1·20 | 0·307 | ||||||
| Error | 62 | 85 592·67 | ||||||||
| Between populations | ||||||||||
| Population | 1 | 1917 406·56 | 5·12 | 0·031 | ||||||
| Error | 31 | 374 169·67 | ||||||||
Fig. 1.
Ovule number per flower in the primary, secondary and tertiary positions in inflorescences of Silene acutifolia in the Cañones del Sil and Arnado populations. Bars show mean values and standard errors.
As shown in Fig. 2 there was a reduction in fruit set from primary to tertiary flowers in all populations and in all years. The position differences are significant, with the same pattern of variation each year in each population (i.e. no significant interactions) (Table 2). Fruit set also varied significantly among populations, both in 1996 and 1998 (Table 2), with the lowest fruit set in Arnado (Fig. 2).
Fig. 2.
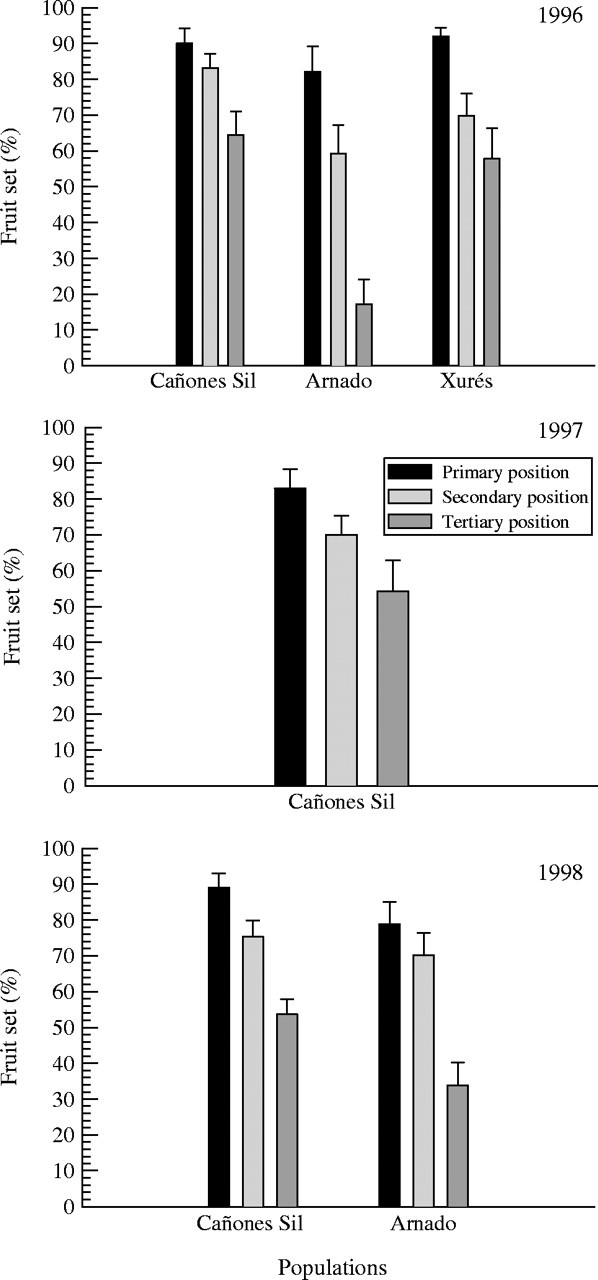
Percentages of fruits produced in the primary, secondary and tertiary positions in inflorescences of Silene acutifolia in 1996 (Cañones del Sil, Arnado and Xurés), 1997 (Cañones del Sil) and 1998 (Cañones del Sil and Arnado). Bars show mean values and standard errors.
Table 2.
Repeated-measures analyses of variance to investigate factors affecting fruit set in Silene acutifolia
| Year |
Source of variation |
d.f. |
MS |
F |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within inflorescence positions | ||||||||||
| 1996 | Position | 2 | 16 770·25 | 36·93 | 0·000 | |||||
| Population × position | 4 | 966·03 | 2·13 | 0·083 | ||||||
| Error | 96 | 454·07 | ||||||||
| 1997 | Position | 2 | 3239·96 | 7·81 | 0·001 | |||||
| Error | 38 | 414·63 | ||||||||
| 1998 | Position | 2 | 16 123·78 | 85·80 | 0·000 | |||||
| Population × position | 2 | 51·60 | 0·28 | 0·760 | ||||||
| Error | 102 | 187·93 | ||||||||
| Between populations | ||||||||||
| 1996 | Population | 2 | 4798·8 | 5·3 | 0·008 | |||||
| Error | 48 | 905·9 | ||||||||
| 1998 | Population | 1 | 8318·9 | 8·796 | 0·005 | |||||
| Error | 51 | 945·8 | ||||||||
As can be seen from Fig. 3, in 1996 there was a marked reduction in number of seeds per fruit between the primary and tertiary positions (Table 3); Tukey tests indicated significant differences between all three positions. The population × position interaction was not significant (Table 3), showing that the reduction from primary to tertiary position was consistent among populations. In 1996, mean number of seeds per fruit in both primary and tertiary positions was slightly higher in Cañones del Sil than in Arnado, but these differences were not significant (Table 3). In 1997 and 1998, number of seeds per fruit varied significantly among positions of the inflorescence (Table 4), but not between populations in 1998 (Table 4). Seed set showed the same pattern (Fig. 4), with a significant reduction from primary to tertiary position in all years and populations (Table 5).
Fig. 3.
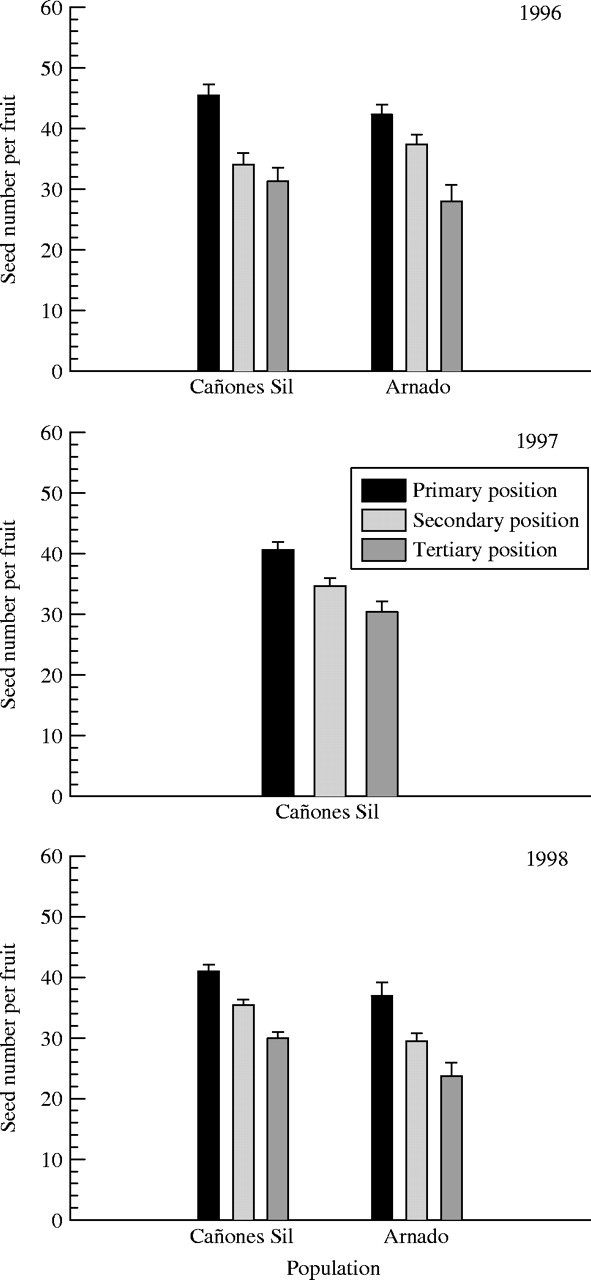
Seed number per fruit in the primary, secondary and tertiary positions in inflorescences of Silene acutifolia in 1996 (Cañones del Sil and Arnado), 1997 (Cañones del Sil), and 1998 (Cañones del Sil and Arnado). Bars show mean values and standard errors.
Table 3.
Two-way analysis of variance to investigate the effects of population and position on number of seeds per fruit in Silene acutifolia in 1996
| Source of variation |
SS |
d.f. |
MS |
F |
P |
|---|---|---|---|---|---|
| Population | 105·3 | 1 | 13·38 | 0·29 | 0·595 |
| Position | 1731·9 | 2 | 854·69 | 18·20 | 0·000 |
| Population × position | 170·3 | 2 | 85·15 | 1·81 | 0·171 |
| Error | 3052·1 | 65 | 46·96 |
Table 4.
Repeated-measures analysis of variance on number of seeds per fruit in Silene acutifolia
| Year |
Source of variation |
d.f. |
MS |
F |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within inflorescence position | ||||||||||
| 1997 | Position | 2 | 467·26 | 19·48 | 0·000 | |||||
| Error | 16 | 23·98 | ||||||||
| 1998 | Position | 2 | 1329·76 | 23·71 | 0·000 | |||||
| Population × position | 2 | 47·68 | 0·85 | 0·432 | ||||||
| Error | 62 | 56·08 | ||||||||
| Between population | ||||||||||
| 1998 | Population | 1 | 187·34 | 1·08 | 0·306 | |||||
| Error | 31 | 173·24 | ||||||||
The position of fruit in the inflorescence is the within-subjects effect, population is the between-subjects effect.
Fig. 4.
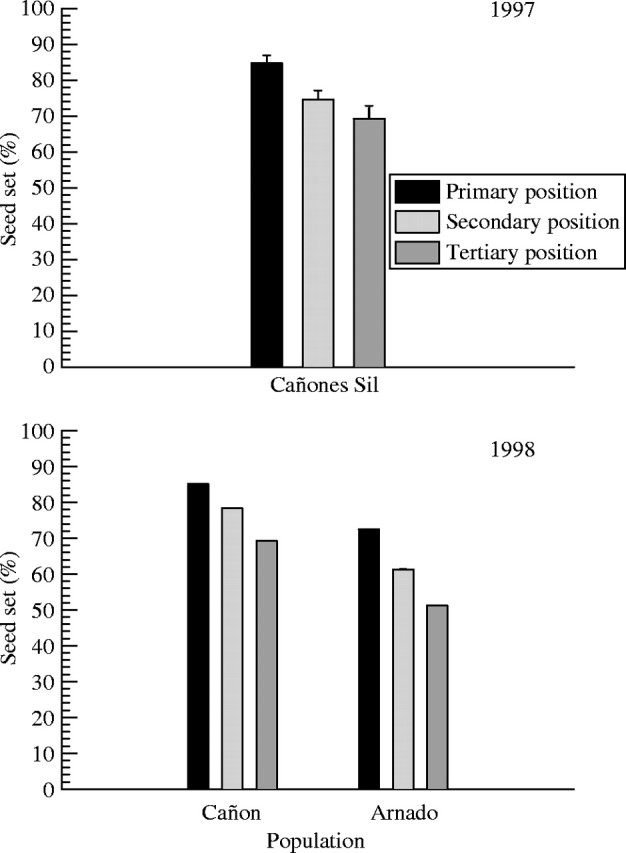
Seed set in the primary, secondary and tertiary positions in inflorescences of Silene acutifolia in 1997 (Cañones del Sil) and 1998 (Cañones del Sil, Arnado). Bars show mean values and standard errors.
Table 5.
Repeated-measures analysis of variance on seed set in Silene acutifolia
| Year |
Source of variation |
d.f. |
MS |
F |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within inflorescence position | ||||||||||
| 1997 | Position | 2 | 549·87 | 9·48 | 0·002 | |||||
| Error | 16 | 58·03 | ||||||||
| 1998 | Position | 2 | 1639·05 | 15·51 | 0·000 | |||||
| Population × position | 2 | 67·70 | 0·64 | 0·531 | ||||||
| Error | 62 | 105·70 | ||||||||
| Between population | ||||||||||
| 1998 | Population | 1 | 1234·48 | 3·57 | 0·068 | |||||
| Error | 31 | 345·87 | ||||||||
The position of fruit in the inflorescence is the within-subjects effect, population is the between-subjects effect.
Seed weight and germination
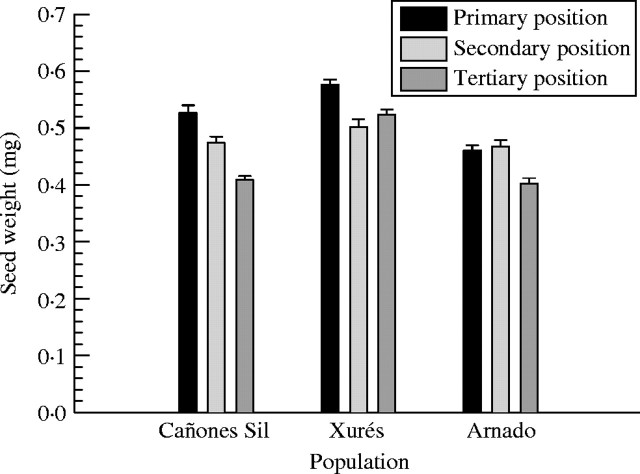
Seed weight varied between 0·770 and 0·228 mg, with a mean of 0·48 mg (n = 450). Seed weight varied significantly among populations (P < 0·0001): the heaviest seeds were those from Xurés, and the lightest those from Arnado; seed weight also varied significantly among positions (P < 0·0001), from early to late. The patterns of variation differed among populations (i.e. significant population × position interaction, P < 0·0001) (Fig. 5). Pairwise comparisons (Tukey tests) indicated significant differences between all populations and positions.
Fig. 5.
Weight of seeds from primary-, secondary- and tertiary-position fruits of Silene acutifolia in the three populations of study. Values shown are means ± 2 s.e.
Data on the proportion of seeds germinated were fitted to a generalized linear model with factors population, position and cold treatment (Table 6), which accounted for 59·13 % of the total sum of squares. The effect of population was highly significant (Table 6), seeds from Arnado started to germinate later, and showed the lowest final germination percentages (Fig. 6). The cold treatment had a marginally significant effect (Table 6), seeds stored at room temperature started to germinate first, and showed higher germination percentages than seeds stored at 5 °C (Fig. 6). The intra-inflorescence position had a marginally significant effect (Table 6), seeds from tertiary position showed the highest germination percentages, and seeds from primary position the lowest (Fig. 6).
Table 6.
The results of a generalized linear model of variance in proportions of seeds germinated
| Source |
LLR |
d.f. |
P |
|---|---|---|---|
| Population | 117·5 | 1 | <0·0001 |
| Treatment | 3·628 | 1 | 0·057 |
| Position | 3·555 | 1 | 0·059 |
| Population × treatment | 2·009 | 1 | 0·156 |
| Population × position | 1·141 | 1 | 0·285 |
| Treatment × position | 0·055 | 1 | 0·815 |
| Population × treatment × position | 0·361 | 1 | 0·548 |
The full model was significant (LLR = 241·7, d.f. = 9, P < 0·0001).
The contribution of each source was tested in an analysis of deviance, by deletion of (a) each main factor from a model including all main factors only and, (b) the three-way interaction from the full model and, (c) the two-way interaction from a full model without the three-way interaction.
Fig. 6.
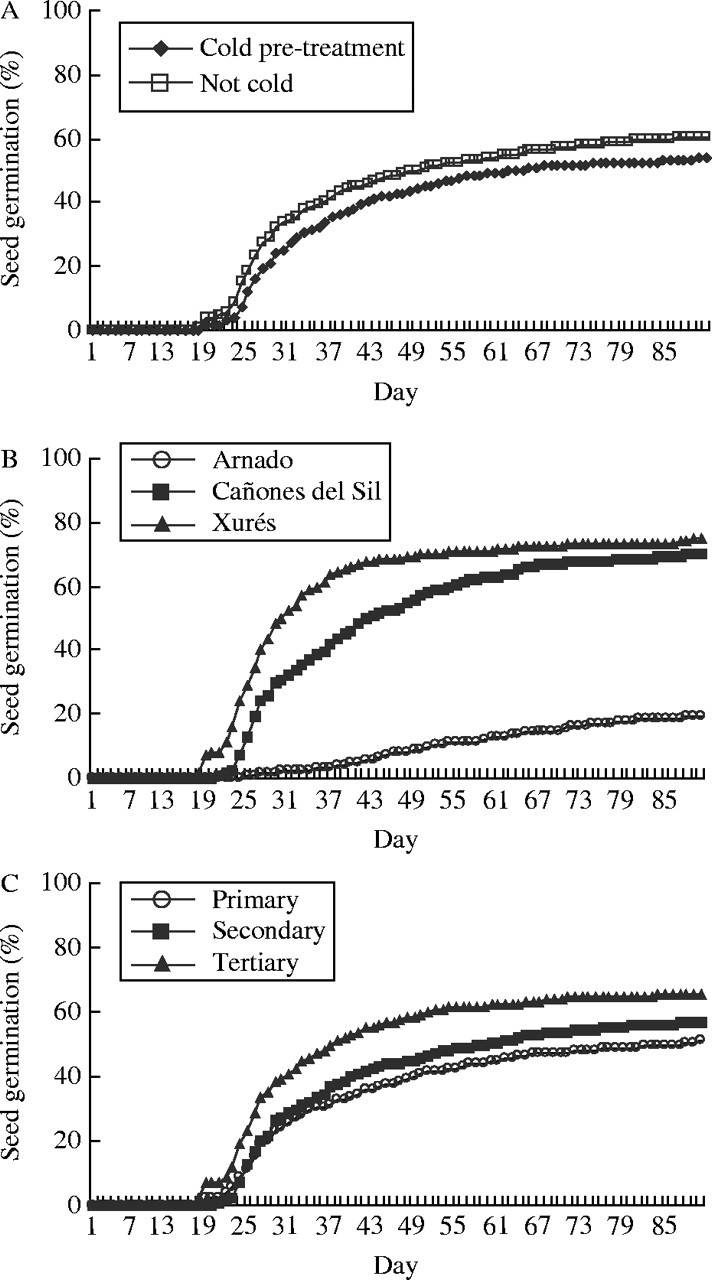
Cumulative seed germination percentages in Silene acutifolia: (A) with or without cold pretreatment, (B) in the different populations, and (C) at different positions in the inflorescence.
DISCUSSION
Intra-inflorescence variation
The flowers of the dichasium-type inflorescence of Silene acutifolia open sequentially from central (primary position) to lateral (secondary, tertiary, etc.). The present results show that there is significant intra-inflorescence variation in the number of ovules per flower, fruit set, number of seeds per fruit, seed set and seed weight in S. acutifolia, with marginally significant differences in proportion of seeds germinated. The patterns of variation in all variables except seed germination percentage, show a decline from primary to tertiary positions. The observed decline was consistent over the years and populations studied. Intra-inflorescence variation is a common pattern in plants (Stephenson, 1980; Lee, 1988; Solomon, 1988; Berry and Calvo, 1991; Herrera, 1991; Obeso, 1993; Diggle, 1995; Brunet, 1996; for reviews, see Ashman and Hitchens, 2000; Medrano et al., 2000). Although most studies have been of raceme-type inflorescences, similar patterns have also been described in species with dichasium-type inflorescences (Guitián and Navarro, 1996; Ashman and Hitchens, 2000). Intra-inflorescence variation has been shown in both floral structure and floral function; e.g. Solomon (1988) reported a decrease in ovary size from basal to distal positions, while Miller and Diggle (2003) have reported variation in sex expression in some Solanum species. Intra-inflorescence variation is also detected in components of reproductive success (e.g. fruit maturation and seed set): in Lavandula stoechas, for example, early-opening flowers have higher fruit set than later flowers (Herrera, 1991).
As noted, the two hypotheses most commonly advanced to explain these patterns are resource competition (Stephenson, 1981; Solomon, 1988; Obeso, 1993; Medrano et al., 2000; Guitián et al., 2001) and architectural effects (Wolfe, 1992; Diggle, 1995; Wolfe and Denton, 2001). Hormonal factors may be involved in seed abortion: in Syzygium cuminii, Arathi et al. (1996) have shown that low seed sets were mediated by ethylene, through embryo abortion. Differences in pollen quantity and quality have also been considered to be a cause of variation in fruit and seed set between early and late flowers. In a parallel study of the Cañones del Sil S. acutifolia population in 1998 and 1999, pollen quantity was not a limiting resource for primary- and secondary-position flowers, and pollen quality had no effect on fruit and seed set (Buide and Guitián, 2002). However, tertiary flowers may be pollen-limited in the female phase, especially in the Arnado population, the most isolated population and that with the lowest number of reproductive individuals. This is probably because S. acutifolia is a sequential blooming plant with protandry at flower level (Buide and Guitián, 2002); thus when tertiary flowers are in female phase, little pollen remains in the population. Pollen limitations in later flowers due to protandry have been demonstrated by Brunet and Charlesworth (1995). In relation to seed mass variations, Winn (1991) suggests that within-individual variation in Prunella vulgaris is a consequence of the unequal distribution of limited resources among flowers within inflorescences. In a recent experiment measuring the effects of experimental elimination of primary and secondary flowers in supplementary pollinated tertiary flowers of S. acutifolia, architectural effects were found to be the cause of intra-inflorescence variation in number of ovules, fruit set and seed set (M. L. Buide, unpubl. res.).
The lower, although not significant, germination percentage of seeds from fruits in the primary position could be due to higher levels of self-pollen in this position: when the primary-position flowers are in female phase, the secondary-position flowers are in male phase, increasing the probability of geitonogamous crosses (Buide and Guitián, 2002); when tertiary-position flowers are in female phase, fruits are developing in the primary and secondary positions, so that less pollen is available, though it is more likely to be outcross pollen. Although S. acutifolia has a high self-compatibility index in terms of fruit and seed set (Buide and Guitián, 2002), the effect of self-pollen on seed germination may be different.
Among-population variation
In addition to within-plant variation, S. acutifolia showed among-population variation in number of ovules per flower, fruit set, seed weight and seed germination percentage. Fruit set, seed weight and seed germination percentage were lower in the Arnado population than in the other populations. Jacquemyn et al. (2001) found among-population differences in seed weight of Primula elatior, though in their study the smallest population showed the highest seed weight. They also found differences in seed number (fewer seeds per fruit in the smallest populations). They explained these results as a trade-off between seed size and seed weight. In S. acutifolia the number of seeds per fruit did not vary significantly among populations, and the lower values in the Arnado population cannot be explained as a trade-off. Arnado is the northernmost population known for this species, far from the main area of distribution and very isolated from the other populations. Silene acutifolia is self-compatible, but seed set is much reduced in flowers bagged without hand-pollination (Buide and Guitián, 2002), thus, pollen limitation may occur in the absence of pollen vectors. Variation in female reproductive success among populations of the same species has been attributed to differences in quality and/or quantity of pollen (Campbell, 1987; Oostermeijer et al., 2000; Jacquemyn et al., 2001), and furthermore genetic problems may reduce fecundity in small isolated populations. For example, fecundity reduction in small populations has been demonstrated in Primula veris and Gentiana lutea (Kéry et al., 2000), with pollen limitation or inbreeding depression the most likely causes. When population size is small and the population is isolated, plant–pollinator mutualisms may be altered, and this can reduce fitness and eventually the probability of population survival (Lamont et al., 1993), certainly, theoretical models predict the great importance of pollinator limitations for the conservation of small populations of rare species (Ingvarsson and Lundberg, 1995; Ågren, 1996; Kéry et al., 2000). Small populations may show low levels of gene flow, loss of rare alleles and increased expression of deleterious alleles, which may limit fitness (Barrett and Kohn, 1991). Moreover, small populations may be affected by demographic and environmental stochasticity (Menges, 1991; Hendrix and Kyhl, 2000). An alternative explanation for the observed among-population differences is that differences in the level of resources in Arnado reduce female reproductive success directly or through an effect on plant size. In this way, large plants may attract more pollinators, and this may result in a larger or genetically more diverse seed crop (de Jong and Klinkhamer, 1994).
Acknowledgments
M.L.B. was supported during the field work by a doctoral fellowship from the Galician Government, and during the early stages of preparation of this manuscript by a post-doctoral fellowship from the University of Santiago de Compostela. I thank J. Guitián for discussions on the first manuscript, and T. M. Meagher and another (anonymous) reviewer for their suggestions.
LITERATURE CITED
- Ågren J. 1996. Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria Ecology 77: 1779–1790. [Google Scholar]
- Arathi HS, Ganeshaiah KN, Uma Schaanker R, Hegde SG. 1996. Factors affecting embryo abortion in Syzygium cumnii (L.) skeels (Myrtaceae). International Journal of Plant Sciences 157: 49–52. [Google Scholar]
- Ashman T-L, Hitchens MS. 2000. Dissecting the causes of variation in intra-inflorescence allocation in a sexually polymorphic species, Fragaria virginiana (Rosaceae). American Journal of Botany 87: 197–204. [PubMed] [Google Scholar]
- Barrett SCH, Kohn JR. 1991. Genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Falk DA, Holsinger KE, eds. Genetics and conservation of rare plants. New York: Oxford University Press, 3–30. [Google Scholar]
- Baskin JM, Baskin CC. 1989. Seed germination ecophysiology of Jeffersonia diphylla, a perennial herb of mesic deciduous forests. American Journal of Botany 76: 1073–1080. [Google Scholar]
- Berry PE, Calvo RN. 1991. Pollinator limitation and position dependent fruit set in the high Andean orchid Myrosmodes cochleare (Orchidaceae). Plant Systematics and Evolution 174: 93–101. [Google Scholar]
- Brunet J. 1996. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 77: 2458–2471. [Google Scholar]
- Brunet J, Charlesworth D. 1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Buide ML, Guitián J. 2002. Breeding system in the dichogamous hermaphrodite Silene acutifolia (Caryophyllaceae). Annals of Botany 90: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR. 1987. Interpopulational variation in fruit production: the role of pollination-limitation in the Olympic Mountains. American Journal of Botany 74: 269–272. [Google Scholar]
- Crawley MJ. 1993.GLIM for ecologists. Oxford: Blackwell Science. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. 1994. Plant size and reproductive success through female and male function. Journal of Ecology 82: 399–402. [Google Scholar]
- Diggle PK. 1991. Labile sex expression in andromonoecious Solanum hirtum: floral development and sex determination. American Journal of Botany 78: 377–393. [Google Scholar]
- Diggle PK. 1995. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology and Systematics 26: 531–552. [Google Scholar]
- Diggle PK. 2002. Architectural effects on floral form and function: a review. In: Stuessy T, Hörandl E, Mayer V, eds. Deep morphology: toward a renaissance of morphology in plant systematics. Königstein: Koeltz. [Google Scholar]
- Emms SK. 1993. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. American Journal of Botany 80: 914–923. [Google Scholar]
- Guitián J, Guitián P, Medrano M. 2001. Causes of fruit set variation in Polygonatum odoratum (Liliaceae). Plant Biology 3: 637–641. [Google Scholar]
- Guitián J, Navarro L. 1996. Allocation of reproductive resources within inflorescences of Petrocoptis grandiflora (Caryophyllaceae). Canadian Journal of Botany 74: 1482–1486. [Google Scholar]
- Hendrix SD, Kyhl JF. 2000. Population size and reproduction in Phlox pilosa Conservation Biology 14: 304–313. [Google Scholar]
- Herrera J. 1991. Allocation of reproductive resources within and among inflorescences of Lavandula stoechas (Lamiaceae). American Journal of Botany 78: 789–794. [Google Scholar]
- Ingvarsson RK, Lundberg S. 1995. Pollinator functional response and plant population dynamics: pollinators as a limiting resource. Evolutionary Ecology 9: 421–428. [Google Scholar]
- Jacquemyn H, Brys R, Hermy M. 2001. Within and between plant variation in seed number, seed mass and germinability of Primula elatior: effect of population size. Plant Biology 3: 561–568. [Google Scholar]
- Kéry M, Matthies D, Spillmann H-H. 2000. Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea Journal of Ecology 88: 17–30. [Google Scholar]
- Lamont BB, Klinkhamer PGL, Witkowski, ETF. 1993. Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect. Oecologia 94: 446–450. [DOI] [PubMed] [Google Scholar]
- Lee TD. 1988. Patterns of fruit and seed production. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive biology: patterns and strategies. New York: Oxford University Press, 179–202. [Google Scholar]
- Lloyd DG. 1980. Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytologist 86: 69–79. [Google Scholar]
- Medrano M, Guitián P, Guitián J. 2000. Patterns of fruit and seed set within inflorescences of Pancratium maritimum (Amaryllidaceae): nonuniform pollination, resource limitation, or architectural effects? American Journal of Botany 87: 493–501. [PubMed] [Google Scholar]
- Menges ES. 1991. The application of minimum viable population theory to plants. In: Falk DA, Holsinger KE, eds. Genetics and conservation of rare plants. New York: Oxford University Press, 45–61. [Google Scholar]
- Miller JS, Diggle PK. 2003. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. American Journal of Botany 90: 707–715. [DOI] [PubMed] [Google Scholar]
- Obeso JR. 1993. Seed mass variation in the perennial herb Asphodelus albus: sources of variation and position effect. Oecologia 93: 571–575. [DOI] [PubMed] [Google Scholar]
- Oostermeijer JGB, Luijten SH, Petanidou T, Kos M, Ellis-Adam AC, Den Nijs HCM. 2000. Pollination in rare plants: is population size important? In: Totland Ø, Armbruster S, Fenster C, Molau U, Nilsson A, Olesen JM, Ollerton J, Philipp M, Ågren J, eds. The Scandinavian Association for Pollination Ecology honours Knut Fægri. Oslo: The Norwegian Academy of Science and Letters, 201–213. [Google Scholar]
- Solomon BP. 1988. Patterns of pre- and postfertilization resource allocation within an inflorescence: evidence for interovary competition. American Journal of Botany 75: 1074–1079. [Google Scholar]
- Stephenson AG. 1980. Fruit set, herbivory, fruit reduction, and the fruiting strategy of Catalpa speciosa (Bignoniaceae). Ecology 61: 57–64. [Google Scholar]
- Stephenson AG. 1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12: 253–279. [Google Scholar]
- Vaughton G, Ramsey M. 1995. Pollinators and seed production. In: Kigel J, Galili G, eds. Seed development and germination. New York: Marcel Dekker, Inc., pp. 475–490. [Google Scholar]
- Vaughton G, Ramsey M. 1997. Seed mass variation in the shrub Banksia spinulosa (Proteaceae): resource constraints and pollen source effects. International Journal of Plant Sciences 158: 424–431. [Google Scholar]
- Williams DA. 1982. Extra-binomial variation in logistic linear models. Applied Statistics 31: 144–148. [Google Scholar]
- Winn AA. 1991. Proximate and ultimate sources of within-individual variation in seed mass in Prunella vulgaris (Lamiaceae). American Journal of Botany 78: 838–844. [Google Scholar]
- Wolfe LM. 1992. Why does the size of reproductive structures decline through time in Hydrophyllum appendiculatum (Hydrophyllaceae)?: developmental constraints vs resource limitation. American Journal of Botany 79: 1286–1290. [Google Scholar]
- Wolfe LM. 1998. Regulation of sex expression in desert and meditterranean populations of an andromonoecious plant (Gagea chlorantha, Liliaceae). Israel Journal of Plant Sciences 46: 17–25. [Google Scholar]
- Wolfe LM, Denton W. 2001. Morphological constraints on fruit size in Linaria canadensis International Journal of Plant Sciences 162: 1313–1316. [Google Scholar]