Abstract
• Background and Aims Plants with crassulacean acid metabolism (CAM) can be divided into two groups according to the major carbohydrates used for malic acid synthesis, either polysaccharide (starch) or monosaccharide (hexose). This is related to the mechanism and affects energy metabolism in the two groups. In Kalanchoë pinnata and K. daigremontiana, which utilize starch, ATP-dependent phosphofructokinase (tonoplast inorganic pyrophosphatase) activity is greater than inorganic pyrophosphate-dependent phosphofructokinase (tonoplast adenosine triphosphatase) activity, but the reverse is the case in pineapple (Ananas comosus) utilizing hexose. To test the hypothesis that the energy metabolism of the two groups differs, day-night changes in the contents of ATP, ADP, AMP, inorganic phosphate (Pi), phosphoenolpyruvate (PEP) and inorganic pyrophosphate (PPi) in K. pinnata and K. daigremontiana leaves and in pineapple chlorenchyma were analysed.
• Methods The contents of energy-rich compounds were measured spectrophotometrically in extracts of tissue sampled in the light and dark, using potted plants, kept for 15 d before the experiments in a growth chamber.
• Key Results In the three species, ATP content and adenylate energy charge (AEC) increased in the dark and decreased in the light, in contrast to ADP and AMP. Changes in ATP and AEC were greater in Kalanchoë leaves than in pineapple chlorenchyma. PPi content in the three species increased in the dark, but on illumination it decreased rapidly and substantially, remaining little changed through the rest of the light period. Pi content of Kalanchoë leaves did not change between dark and light, whereas Pi in pineapple chlorenchyma increased in the dark and decreased in the light, and the changes were far greater than in Kalanchoë leaves. Light-dark changes in PEP content in the three species were similar.
• Conclusions These results corroborate our hypothesis that day–night changes in the contents of energy-rich compounds differ between CAM species and are related to the carbohydrate used for malic acid synthesis.
Key words: Ananas comosus, ATP, chlorenchyma, crassulacean acid metabolism, inorganic pyrophosphate, Kalanchoë daigremontiana, Kalanchoë pinnata, phosphoenolpyruvate
INTRODUCTION
Crassulacean acid metabolism (CAM) plants can be divided into two groups according to the major carbohydrates used for malic acid synthesis, either polysaccharides (starch) in chloroplasts, or monosaccharides (hexose) in extra-chloroplastic compartments (Kenyon et al., 1985; Black et al., 1996; Christopher and Holtum, 1996). In Kalanchoë pinnata and K. daigremontiana, only starch is used as the precursor for glycolytic phosphoenolpyruvate (PEP) formation. The net energy requirement for 1 mol malic acid accumulation in the dark is 0·5 mol ATP, if the breakdown of starch is phosphorolytic rather than amylolytic, and if 1 mol ATP is consumed in transporting 1 mol malate across the tonoplast (Lüttge et al., 1981; Winter and Smith, 1996). In pineapple (Ananas comosus), mainly hexoses are consumed for malic acid synthesis. Input of hexose into glycolysis via hexokinase (EC 2.7.1.1), instead of hexose-phosphate (hexose-P) produced by phosphorolysis of starch, would increase the ATP requirement to 1 mol ATP per mol malic acid accumulation in the dark (Carnal and Black, 1989). These costs are derived assuming that ATP-dependent phosphofructokinase (ATP-PFK, EC 2.7.1.11), but not inorganic pyrophosphate-dependent phosphofructokinase (PPi-PFK, EC 2.7.1.90), catalyses the phosphorylation of fructose 6-phosphate (F6P). However, in pineapple, the activity of PPi-PFK is 10–20 times greater than that of ATP-PFK (Carnal and Black, 1989; Trípodi and Podestá, 1997). The total ATP requirement for malic acid accumulation in darkness may be reduced if PPi-PFK functions in the glycolytic direction and substitutes for ATP-PFK.
Whereas the efflux of malate from the vacuole is thought to be passive (Lüttge and Smith, 1984), the import of malate into the vacuoles is energy-dependent (Lüttge et al., 1981; Smith et al., 1982, 1996). The energy for transport of malate into the vacuoles is derived from either ATP via an adenosine triphosphatase (ATPase) in the tonoplasts or from inorganic pyrophosphate (PPi) via an inorganic pyrophosphatase (PPase) in the tonoplasts, or a combination of the two (White and Smith 1989; Smith et al., 1996). In leaves of K. pinnata and K. daigremontiana utilizing starch, tonoplast PPase activity is larger than the tonoplast ATPase activity, whereas in the tonoplast of pineapple utilizing hexose PPase activity is smaller than the ATPase activity (Chen and Nose, 2000). Thus, the relative contributions of ATP and PPi to the energy requirement for malate transport across the tonoplast may differ between CAM species utilizing hexose and starch.
In contrast to pineapple, in K. pinnata and K. daigremontiana leaves there are oscillations of citrate and isocitrate in addition to those in malate (Chen et al., 2001, 2002). This may affect cellular energetics: net ATP production is about 7 (or 6) mol when 1 mol of hexose-P (or hexose) is used for the vacuolar citric and isocitric acid accumulation in the dark (Lüttge, 1988; Chen et al., 2002).
Against this background, we hypothesize that day–night changes in the contents of energy-rich compounds will differ between CAM species utilizing hexose and starch. So far, this has been examined in only a limited number of experiments under different conditions (Smith et al., 1982; Pistelli et al., 1987; Lin et al., 1994). For example, Lin et al. (1994) used pineapple plants grown in a glasshouse with natural photoperiod, whereas Pistelli et al. (1987) and Smith et al. (1982) used K. tubiflora, K. daigremontiana and K. blossfeldiana plants grown in growth chambers with controlled conditions. Moreover, PPi and inorganic phosphate (Pi) were not included in the experiment of Lin et al. (1994) and PPi, AMP and PEP were not included in the experiment of Smith et al. (1982). Therefore, it is very difficult to compare data.
In this paper, we tested the hypothesis that day–night changes in the contents of energy-rich compounds differ between CAM species utilizing hexose and starch. This was done by measuring, under controlled environmental conditions, the day–night changes in the contents of ATP, ADP, AMP, PEP, Pi and PPi in the leaves of K. pinnata and K. daigremontiana, which utilize polysaccharides (starch) in chloroplasts, and in the chlorenchyma of pineapple, which utilizes soluble hexose sugars in extra-chloroplastic compartments.
MATERIALS AND METHODS
Plant materials
Pineapple, Kalanchoë pinnata and K. daigremontiana were vegetatively propagated and grown in pots in a heated glasshouse under natural photoperiod. Fifteen days before the experiments, all plants were transferred to a growth chamber (KG-50 HLA, Koito Industrial Co., Ltd, Japan) with a photoperiod of 12 h (0800–2000 h) light and 12 h (2000–0800 h) dark, light intensity at the mid-plant height of 420 to 450 µmol m−2 s−1, and 30 °C in the light and 20 °C in the dark, and a relative humidity of 65 % during the 24 h day–night cycle. Fourth to eighth leaf pairs, counting from the apex of Kalanchoë and fully expanded, mature leaves of pineapple were the materials. Leaf samples of Kalanchoë were taken and immersed in liquid nitrogen immediately until extracted. Pineapple leaves contain a considerable amount of non-chlorophyllous water storage parenchyma (WSP), which is readily and rapidly (about 10 s) separated from the chlorenchyma by hand. Only chlorenchyma was used, after immediate immersion in liquid nitrogen until extracted. To reduce the effects of changing light conditions during leaf harvesting before freezing, the leaves were kept in a similar light intensity while being cut and steeped in liquid nitrogen.
Extraction and measurement of Pi, malate, citrate, isocitrate and PEP
About 2 g frozen samples were ground in liquid nitrogen with a pestle and mortar, 7 mL of ice-cold 4 % (v/v) HClO4 was added to the powder and gently pulverized. The mixture was allowed to thaw slowly on ice. The resulting suspension was kept on ice for 30 min, then centrifuged for 10 min at 20 000 g (P28S rotor, CP75β ultracentrifuge, Hitachi Kiki Co., Ltd, Japan). One millilitre of the supernatant was used for measurement of Pi, spectrophotometrically at 820 nm, using a JASCO V-550 UV/VIS spectrophotometer by the method of Ames (1966).
Four millilitres of supernatant were neutralized at 4 °C with 5 m K2CO3, and the resulting potassium chlorate was removed by 10 min centrifugation at 20 000 g. Thirty-five milligrams of charcoal (activated, washed with HCl) was added to the supernatant, and after 15 min at 4 °C, removed again by 10 min centrifugation at 20 000 g. The supernatant was used for measurement of malate, citrate, isocitrate and PEP. Malate, citrate, isocitrate and PEP were measured according to Chen et al. (2002).
Extraction and measurement of ATP, ADP and AMP
About 2 g frozen samples were ground in liquid nitrogen with a pestle and mortar, 7 mL of ice-cold 10 % (w/v) trichloroacetic acid (TCA) was added to the powder and gently pulverized. The mixture was allowed to thaw slowly on ice. The resulting suspension was kept on ice for 30 min, then centrifuged for 10 min at 20 000 g. Four millilitres of supernatant was neutralized at 4 °C with 5 m K2CO3, 35 mg charcoal was added, and the mixture, kept on ice, was vortexed at least three times during 15 min to adsorb the nucleotides (Obendorf and Marcus, 1974). The charcoal was precipitated by 10 min centrifugation at 20 000 g, then resuspended twice in 10 mL of water. Nucleotides were eluted with 5 mL of ethanol–water–NH4OH (65 : 35 : 0·3) (v/v/v) (Obendorf and Marcus, 1974) during 20 min at 40 °C. The charcoal was removed by 10 min centrifugation at 20 000 g and re-eluted with 4 mL of ethanol–water–NH4OH (65 : 35 : 0·3) (v/v/v). The eluants were combined and evaporated to dryness by vacuum distillation (Model RD 400 centrifugal evaporator; Yamato Scientific Co., Ltd, Japan) at 40 °C, and the residues were dissolved in 1 mL of water and centrifuged to remove the undissolved materials. Aliquots were used directly for measurement of ATP, ADP and AMP.
ATP was assayed according to Lamprecht and Trautschold (1974) in 1 mL reaction mixture containing 100 mm triethanolamine hydrochloride adjusted to pH 7·6 with NaOH, 4 mm MgCl2, 2 mm glucose, 2 mm NADP, 2·8 units lactate dehydrogenase (EC 1.1.1.27), and 1·8 units hexokinase (EC 2.7.1.1). Both ADP and AMP were measured according to Jaworek et al. (1974) in 1 mL reaction mixture containing100 mm triethanolamine hydrochloride adjusted to pH 7·6 with NaOH, 1 mm PEP, 33·4 mm MgSO4, 0·12 m KCl, 0·36 mm NADH, 24 units lactate dehydrogenase, 18 units pyruvate kinase (EC 2.7.1.40). ADP concentration was determined according to the difference before and after the addition of pyruvate kinase. After this, 16 units myokinase (EC 2.7.4.3) were added to measure AMP.
Extraction and measurement of PPi
PPi was extracted according to Chen and Nose (2001). About 2 g of a frozen sample was ground in liquid nitrogen with a pestle and mortar, 7 mL of ice-cold 5 % (w/v) TCA containing 350 mg polyvinylpolypyrrolidone (PVP) was added to the powder and gently pulverized. The mixture was allowed to thaw slowly on ice. The resulting suspension was kept on ice for 30 min to allow binding of phenolic compounds on the PVP, then centrifuged for 10 min at 20 000 g. Four millilitres of supernatant were added to 35 mg charcoal and the mixture was vortexed at least three times during 15 min. The charcoal was precipitated by 10 min centrifugation at 20 000 g. The supernatant was used for measurement of PPi.
PPi was assayed according to Chen and Nose (2001) in 1 mL assay mixture containing 0·3 mL extract, 0·2 mL 1 m HEPES adjusted to pH 8·2 with KOH, 5 mm ethylene glycol-bis (β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 2 mm MgCl2, 1 mm F6P, 0·15 mm NADH, 0·45 unit aldolase (EC 4.1.2.13), 1·7 unit glycerol-3-phosphate dehydrogenase (EC 1.1.1.8) and 5 units triose-phosphate isomerase (EC 5.3.1.1). The reaction was started by the addition of 0·1 unit PPi-PFK. The final pH of the assay mixture was about 7·4. The blank contained the extract with all reagents except the PPi-PFK, which was replaced by water. Oxidation of NADH was followed by the decrease in absorbance in A340 nm at 30 °C until no further decrease was observed (approx. 20 min).
Recovery experiments
In each experiment duplicate samples of the same batch of the three CAM plants were taken. One sample was pulverized and extracted as described above. To the other, measured amounts of intermediates, comparable to the content of the tissue, were added to the pestle and mortar just before the sample was pulverized. The recoveries of malate, citrate, isocitrate, PEP, PPi and Pi, and of ATP, ADP and AMP were between 96–105 % and 80–93 %, respectively. Metabolite contents were not corrected for recovery except ATP, ADP and AMP.
Calculation of adenylate energy charge (AEC)
Adenylate energy charge (AEC) is calculated according to (Atkinson, 1968):
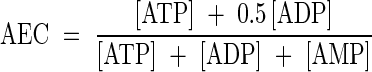 |
Statistical analysis
Experiments were performed with six replicates (one plant per replicate). Results represented the mean ± s.d. for n = 6.
RESULTS
Diurnal changes in malate, citrate and isocitrate
Kalanchoë leaves and pineapple chlorenchyma showed typical features of CAM, with malate increasing in the dark and decreasing in the light (Fig. 1A). In both Kalanchoë species a small increase in citrate occurred in darkness (Fig. 1B) but isocitrate only increased in K. daigremontiana (Fig. 1C). No nocturnal citrate or isocitrate increase was observed in pineapple (Fig. 1B and C).
Fig. 1.
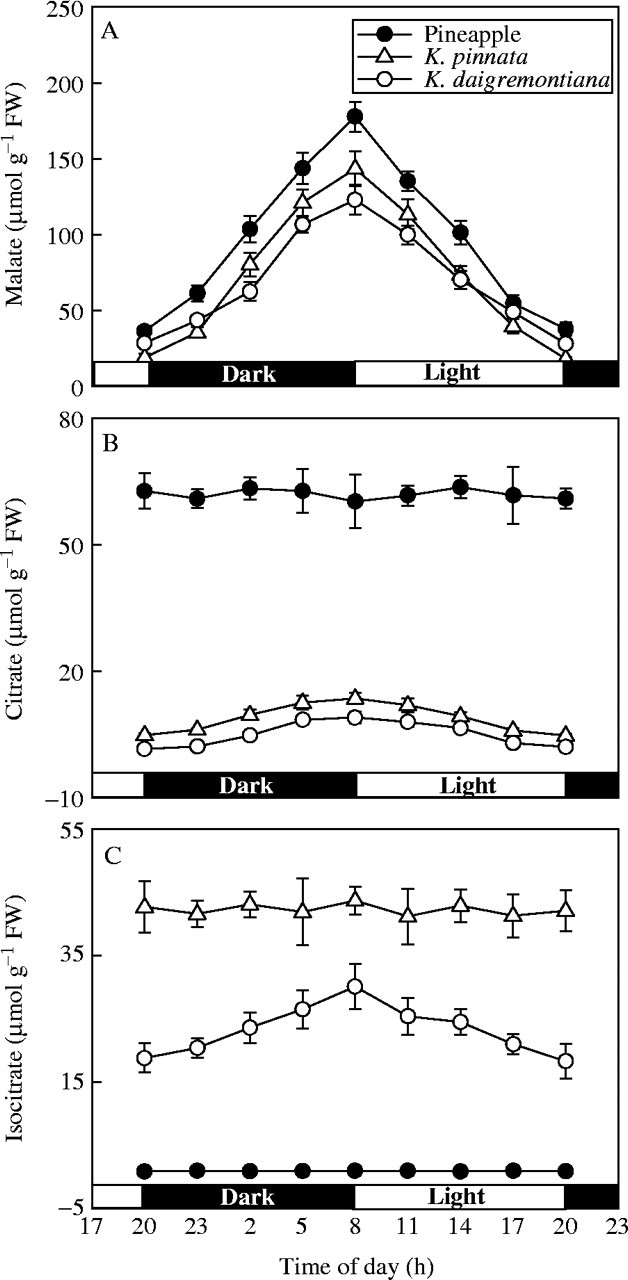
Contents of malate (A), citrate (B) and isocitrate (C) in Kalanchoë pinnata and K. daigremontiana leaves and in pineapple (Ananas comsus) chlorenchyma during the 24-h day–night cycle. Values are means of six experiments ± s.d.
Diurnal changes in ATP, ADP, AMP and AEC
In Kalanchoë leaves, ATP content always increased in the dark and decreased in the light, but in pineapple chlorenchyma, there were no consistent changes. Day–night changes in ATP were far greater in Kalanchoë than in pineapple (Fig. 2A).
Fig. 2.
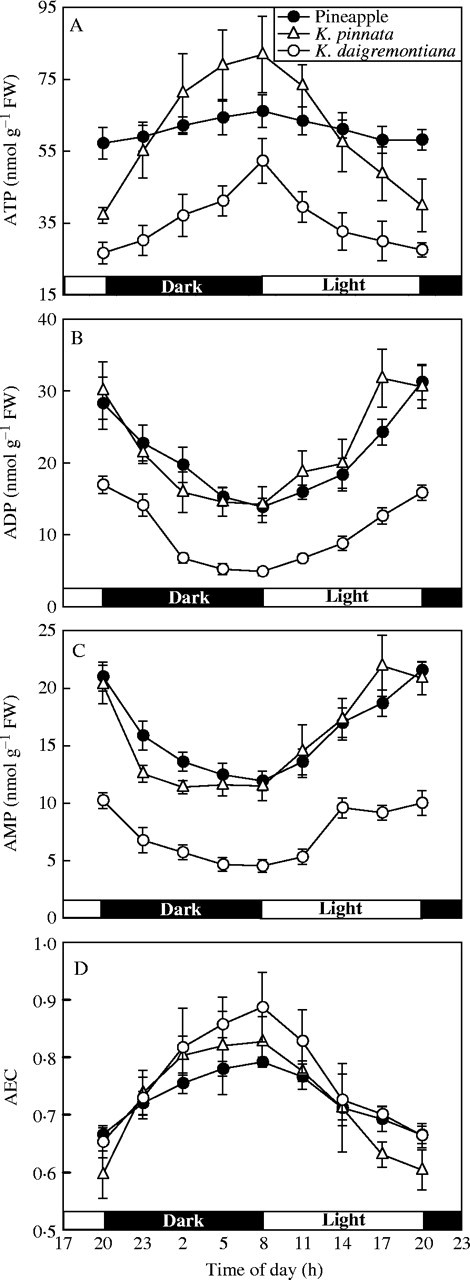
ATP (A), ADP (B) and AMP (C) contents and adenylate energy charge (AEC, D) in K. pinnata and K. daigremontiana leaves and in pineapple chlorenchyma during the 24-h day–night cycle. Values are means of six experiments ± s.d.
ADP and AMP contents in Kalanchoë leaves and pineapple chlorenchyma decreased in the dark and increased in the light (Fig. 2B and C). Since ATP in Kalanchoë and pineapple increased in the dark and decreased in the light (Fig. 2A), AEC also increased in the dark and decreased in the light (Fig. 2D).
Day–night changes in ATP content were far smaller in pineapple chlorenchyma than in Kalanchoë leaves (Fig. 2A), whereas day–night changes in ADP and AMP contents in all the three species were similar (Fig. 2B and C). Therefore, day–night changes in AEC were less in pineapple than in Kalanchoë (Fig. 2D).
Diurnal changes in PPi and Pi
Day–night changes in PPi content in the three species were similar (Fig. 3A): PPi increased in the dark and decreased to a low value during the first part of light period, then remained little changed through the rest of the light period.
Fig. 3.
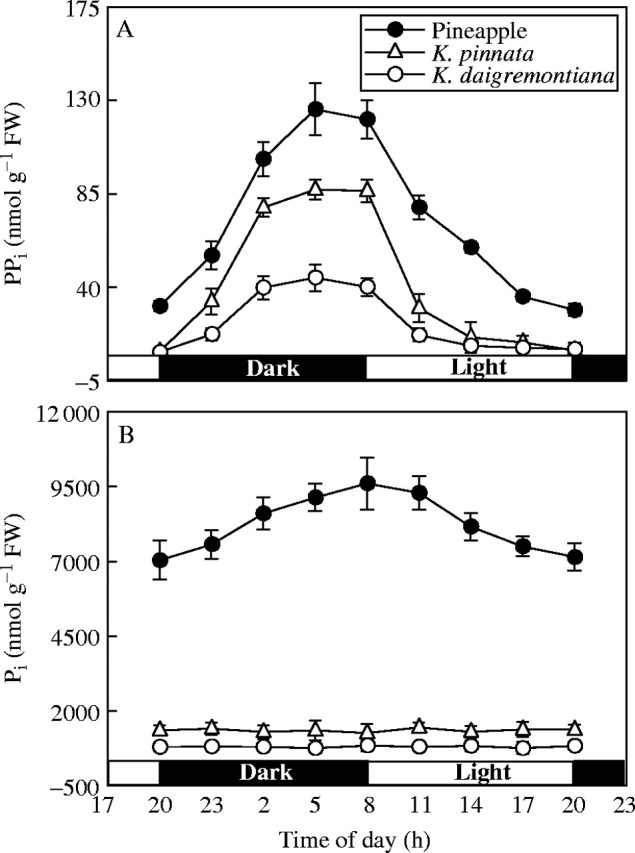
Contents of inorganic pyrophosphate (PPi) (A) and inorganic phosphate (Pi) (B) in K. pinnata and K. daigremontiana leaves and in pineapple chlorenchyma during the 24-h day–night cycle. Values are means of six experiments ± s.d.
Pi content in pineapple chlorenchyma was far greater than in Kalanchoë leaves. In Kalanchoë, Pi did not show any clear changes during the 24-h day–night cycle. However, Pi content in pineapple increased in the dark and decreased in the light (Fig. 3B).
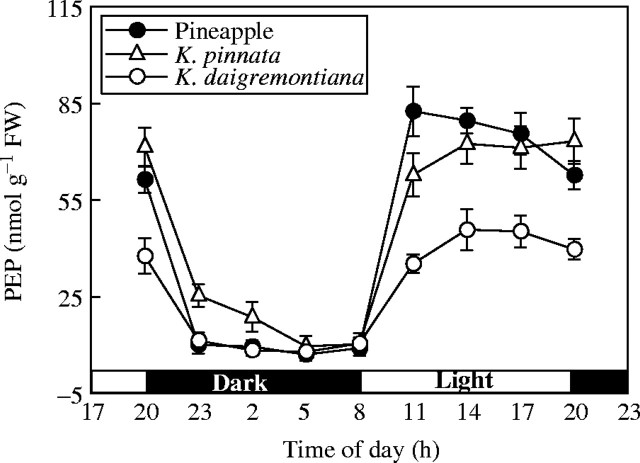
Diurnal changes in PEP
Day–night changes in PEP content in Kalanchoë leaves and pineapple chlorenchyma were basically similar, dropping drastically to a low value in the dark and remaining little changed through the rest of the dark period. PEP then increased rapidly during the first part of the light period to substantially greater content, remaining little changed in Kalanchoë but decreasing slightly in pineapple through the rest of the light period (Fig. 4).
Fig. 4.
Content of phosphoenolpyruvate (PEP) in K. pinnata and K. daigremontiana leaves and in pineapple chlorenchyma during the 24-h day–night cycle. Values are means of six experiments ± s.d.
DISCUSSION
Although ATP content of pineapple chlorenchyma increased in the dark, the extent was far less than in Kalanchoë leaves (Fig. 2A). The cause is not known, but the difference may be related to several factors, including: (a) the major carbohydrates used in the daily cycle and the glycolytic pathway; (b) the relative contribution of tonoplast ATPase and PPase to malate transport across tonoplast; and (c) the accumulation of citric acid and isocitric acid.
In K. pinnata and K. daigremontiana, the CO2 acceptor, PEP, is formed from hexose-P, the product of starch phosphorolysis, and hexose, the product of starch amylolysis, whereas in pineapple PEP is mainly derived from free hexose, which requires ATP-consuming hexokinase (Carnal and Black, 1989; Christopher and Holtum, 1996; Häusler et al., 2000; Chen et al., 2001). Assuming that 1 mol ATP is consumed in transporting 1 mol malate across the tonoplast (Lüttge et al., 1981; Smith et al., 1996), the net ATP requirement for accumulating 1 mol malic acid in the dark is 0·5 mol when starch breakdown is phosphorolytic or 1 mol when it is amylolytic in Kalanchoë. However, the net ATP requirement is 1 mol when ATP-PFK, but not PPi-PFK, catalyses the phosphorylation of F-6-P or 0·5 mol when PPi-PFK, but not ATP-PFK, catalyses the phosphorylation of F-6-P in pineapple (Carnal and Black, 1989). Since the activity of PPi-PFK is very large in pineapple leaves and is considered to function in the glycolytic direction, it might substitute for ATP-PFK (Carnal and Black, 1989; Trípodi and Podestá, 1997). If so, net ATP requirement for 1 mol malic acid accumulation in pineapple in the dark would be 0·5 mol (Carnal and Black, 1989). In addition, pineapple may use starch for malic acid accumulation (Christopher and Holtum, 1998), so the net ATP requirement for malic acid accumulation in the dark will be reduced. Thus, the difference in the extent of nocturnal ATP increase observed among the three CAM species cannot be explained in this way.
The accumulation of malic acid in the vacuoles of the photosynthetic cells could be energized by the tonoplast ATPase or PPase, or a combination of the two (White and Smith, 1989; Smith et al., 1996). We have shown that the tonoplast PPase activity of both K. pinnata and K. daigremontiana leaves is greater than the tonoplast ATPase activity (Chen and Nose, 2000), whereas the PPase activity in pineapple chlorenchyma is less than tonoplast ATPase activity (Chen and Nose, 2002). As summarized in Table 1, the relative contribution of PPi to the energy requirement for malate transport across the tonoplast may be greater in Kalanchoë leaves than in pineapple chlorenchyma, which may reduce energy-demand for malic acid accumulation in Kalanchoë in the dark.
Table 1.
Energy requirement for malate transport across tonoplast in Kalanchoë pinnata and K. daigremontiana leaves and in pineapple chlorenchyma in the dark
| Pineapple |
K. pinnata |
K. daigremontiana |
||||
|---|---|---|---|---|---|---|
| Ratio of tonoplast PPase activity to ATPase activity | 0·45 : 1 | 1·22 : 1 | 1·42 : 1 | |||
| Ratio of potential transport activity of tonoplast PPase to ATPase | 0·225 : 1 | 0·61 : 1 | 0·71 : 1 | |||
| Net energy requirement for 1 mol malate transport across tonoplast was as follows: | ||||||
| Pineapple: 0·816 ATP + 0·368 PPi → 0·817 ADP + 1·552 Pi; | ||||||
| K. pinnata: 0·621 ATP + 0·758 PPi → 0·621 ADP + 2·137 Pi; | ||||||
| K. daigremontiana: 0·585 ATP + 0·830 PPi → 0·585 ADP + 2·245 Pi. | ||||||
The stoichiometry of H+ : PPi and H+ : ATP is 1 and 2, respectively. The stoichometry of malate2− : H+ is 2 (Lüttge, 1988; Chen et al., 2002).
Data of tonoplast PPase and ATPase activities are taken from Chen and Nose (2000, 2002).
In Kalachoë, a small increase in citrate (Fig. 1B) and in K. daigremontiana a small increase in isocitrate (Fig. 1C), occurred nocturnally. About 7 or 6 mol ATP is produced when 1 mol of hexose-P or hexose, respectively, is used for the vacuolar citric (isocitric) acid accumulation in the dark (Lüttge, 1988; Chen et al., 2002). Thus, citric or isocitric acid accumulation, which provides ATP, may have contributed to the difference in the extent of nocturnal ATP increase observed between Kalanchoë and pineapple.
PPi content in all the three CAM species increased in the dark and decreased in the light (Fig. 3A). Similar results were obtained in K. daigremontiana leaves (Pistelli et al., 1987). PPi is required for malate transport in all the three species (Table 1), suggesting that PPi may be produced by other metabolic pathways in the dark, leading to nocturnal PPi increase. This may facilitate malic acid accumulation in vacuoles by stimulating tonoplast ATPase activity (Lüttge et al., 1995) and by providing sufficient substrates for PPi-PFK and tonoplast PPase.
Pi content was far smaller in Kalanchoë leaves than in pineapple chlorenchyma (Fig. 3B). Previous studies showed that fructose 2,6-bisphosphate (F-2,6-P2) concentrations in pineapple leaves were higher than those in K. tubiflorum phyllodes or in C3 or CAM-Mesembryanthemum (Fahrendorf et al., 1987; Theodorou and Kruger, 2001). F-2,6-P2 is synthesized by fructose 6-phosphate 2-kinase (F-6-P 2-kinase, EC 2.7.1.105) and degraded by fructose 2,6-bisphosphatase (F-2,6-P2ase, EC 3.1.3.46), and its concentration can be altered by changing the activities of these enzymes (Stitt, 1990). Pi stimulates F-6-P 2-kinase and inhibits F-2,6-P2ase, favouring an increase of F-2,6-P2 concentration (Stitt et al., 1987). Therefore, substantial Pi content in pineapple chlorenchyma may be related to the fact that pineapple leaves contain large concentrations of F-2,6-P2.
In Kalanchoë leaves, Pi content did not change significantly through the CAM cycle (Fig. 3B). Similar results have been obtained with K. tubiflora (Smith et al., 1982) and K. daigremontiana (Pistelli et al., 1987). However, Pi content of pineapple chlorenchyma increased in the dark and decreased in the light (Fig. 3B). Net energy requirement for 1 mol malic acid accumulation is 0·5 mol ATP → 0·5 mol ADP + 0·5 mol Pi (starch phosphorolysis) or 1 mol ATP → 1 mol ADP + 1 mol Pi (starch amylolysis) in Kalanchoë if 1 mol ATP is consumed in transport of 1 mol malate and F-6-P is phosphorylated by ATP-PFK, and 0·5 mol ATP + 0·5 mol PPi → 0·5 mol ADP + 1·5 mol Pi in pineapple if 1 mol ATP is consumed in transport of 1 mol malate and F-6-P is phosphorylated by PPi-PFK (Carnal and Black, 1989). When ATP requirement for malic acid accumulation balances ATP production, 1 mol Pi is generated per mol malic acid accumulated in the vacuoles of pineapple leaves. However, no net Pi is generated under the same circumstance in Kalanchoë (Carnal and Black, 1989). Therefore, the difference of day–night changes in Pi content among the three species may be related to the glycotylic pathway. More CAM species need to be investigated if nocturnal increase in Pi is a universal phenomenon of CAM species with high PPi-PFK activity.
The mechanism of glycolytic regulation probably involves F 2,6-P2 which binds to an activator site of PPi-PFK and activates the forward and reverse reactions in parallel by increasing the affinity of PPi-PFK for F6P and F-1,6-P2. The direction of net flux will depend on the mass ratio [F6P][PPi]/[F-1,6-P2][Pi] and the equilibrium constant of the reaction (Nielsen, 1995; Stitt, 1989). Because nocturnal Pi increase in pineapple chlorenchyma was less than nocturnal PPi increase, the ratio of PPi : Pi increased (Fig. 3A and B). Glyolytic activity of PPi-PFK during the dark period may be promoted in pineapple chlorenchyma.
Our finding that PEP content in Kalanchoë leaves, and in pineapple chlorenchyma decreased in the dark (Fig. 4) is in agreement with the view that nocturnal decrease in PEP and increase in ATP reflect the regulation of the total balance of energy-rich phosphates (Smith et al., 1982). In addition, we found that total energy-rich compounds (2ATP + ADP + PPi + PEP) increased by about 40, 86 and 41 nmol g−1 f. wt in the dark for pineapple, K. pinnata and K. daigremontiana, respectively, if changes of ATP are doubled because of the two energy-rich ∼P-bonds in the molecule and the differences in the standard Gibbs free energy of ATP, ADP, PEP and PPi are neglected. This suggests that nocturnal increase in ATP and PPi is sufficient to balance the decrease in ADP and PEP, similar to the result of Pistelli et al. (1987). In view of the tight energy budget in the dark (Lüttge and Ball, 1987), it remains unexplained, however, why the total energy-rich compounds increase in the dark. There is a need to examine if the nocturnal increase in total energy-rich compounds is a typical phenomenon of CAM.
In conclusion, ATP content in the three CAM species increased in the dark, but the extent of nocturnal increase in ATP content was far less in the chlorenchyma of pineapple utilizing hexose than in the leaves of K. pinnata and K. dagremontiana utilizing starch. The difference may be related to several factors, including: (a) the major carbohydrates used in their daily cycle and the glycolytic pathway; (b) the relative contribution of tonoplast ATPase and PPase to malate transport across tonoplast; and (c) the accumulation of citric acid and isocitric acid. In Kalanchoë leaves, Pi content did not show day–night changes, whereas Pi content in pineapple chlorenchyma increased in the dark and decreased in the light. Pi content in pineapple chlorenchyma was far greater than in Kalanchoë leaves. The data are consistent with the hypothesis that day–night changes in the contents of energy-rich compounds differ between CAM species utilizing hexose and starch, and the mechanisms by which this occurs.
Supplementary Material
Acknowledgments
This research was supported by a post-doctoral fellowship for foreign researchers in Japan from the Japan Society for the Promotion of Science (ID No. P97415). We thank Dr Lailiang Cheng, Cornell University, USA, for critical reading of the manuscript.
LITERATURE CITED
- Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatase. Methods in Enzymology 8: 115–118. [Google Scholar]
- Atkinson DE. 1968. The energy charge of the adenylate pool as a regular parameter: interaction with feedback modifiers. Biochemistry 7: 4030–4034. [DOI] [PubMed] [Google Scholar]
- Black CC, Chen J-Q, Doong RL, Angelov MN, Sung SJS. 1996. Alternative carbohydrate reserves used in the daily cycle of crassulacean acid metabolism. In: Winter K and Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag, 31–45. [Google Scholar]
- Carnal NW, Black CC. 1989. Soluble sugars as the carbohydrate reserve for CAM in pineapple leaves: implications for the role pyrophosphate:6-phosphofructokinase in glycolysis. Plant Physiology 90: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Nose A. 2000. Characteristics of adenosinetriphosphatase and inorganic pyrophosphatase in tonoplast isolated from three CAM species, Ananas comosus, Kalanchoë pinnata and K. daigremontiana Plant Production Science 3: 24–31. [Google Scholar]
- Chen L-S, Nose A. 2001. An improved method for extraction and measurement of the inorganic pyrophosphate in leaves of crassulacean acid metabolism (CAM) plants. Plant Production Science 4: 15–19. [Google Scholar]
- Chen L-S, Nose A. 2002. Characteristics of tonoplast adenosinetriphosphatase and inorganic pyrophosphatase in the chlorenchyma and the water storage parenchyma of Ananas comosus leaves. Journal of Plant Physiology and Molecular Biology 28: 28–36. [Google Scholar]
- Chen L-S, Lin Q, Nose A. 2002. A comparative study on diurnal changes in metabolite levels in the leaves of three crassulacean acid metabolism (CAM) species, Ananas comosus, Kalanchoë daigremontiana and K. pinnata Journal of Experimental Botany 53: 341–350. [DOI] [PubMed] [Google Scholar]
- Chen L-S, Qi Y-P, Nose A. 2001. Diurnal changes in metabolite levels in the chlorenchyma and water storage parenchyma of Ananas comosus leaves. Acta Phytophysiologica Sinica 27: 253–260. [Google Scholar]
- Christopher JT, Holtum JAM. 1996. Patterns of carbon partitioning in leaves of crassulacean acid metabolism species during deacidification. Plant Physiology 112, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher JT, Holtum JAM. 1998. Carbohydrate partitioning in leaves of Bromeliaceae performing C3 photosynthesis or crassulacean acid metabolism. Australian Journal of Plant Physiology 25, 371–376. [Google Scholar]
- Fahrendorf T, Holtum JAM, Mukherjee U, Latzko E. 1987. Fructose 2,6-bisphosphate, carbohydrate partitionng and crassulaceaan acid metabolism. Plant Physiology 84: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Baur B, Scharte J, Teichmann T, Eicks M, Fisher KL, Flügger U-I, Schubert S, Weber A, Fischer K. 2000. Plastidic metabolite transporters and their physiological functions in the inducible crassulacean acid metabolism plant Mesembryanthemum crystallinum Plant Journal 24: 285–296. [DOI] [PubMed] [Google Scholar]
- Jaworek D, Gruber W, Bergmyer HU. 1974. Adenosine 5-diphosphate and adenosine 5-monophosphate. In: Bergmeyer HU, ed. Methods of enzymatic analysis, Vol. 4. Weinhein: Verlag Chemic, 2127–2131. [Google Scholar]
- Kenyon WH, Severson RF, Black CC Jr. 1985. Maintenance carbon cycle in crassulacean acid metabolism plant leaves: source and compartmentation of carbon for nocturnal malate synthesis. Plant Physiology 77: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht W, Trautschold I. 1974. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, ed. Methods of enzymatic analysis, Vol. 4. Verlag Chemic Weinhein, 2101–2110. [Google Scholar]
- Lin ZF, Peng C-L, Lin G-Z, Li S-S. 1994. Diurnal changes of phosphoenolpyruvate carboxykinase activity, oxalacetate content and adenosine pool in pineapple leaves. Acta Phytophysiologica Sinica 20: 353–359. [Google Scholar]
- Lüttge U. 1988. Day-night changes of citric-acid levels in crassulacean acid metabolism: phenomenon and ecophysiological significance. Plant Cell & Environment 11: 445–451. [Google Scholar]
- Lüttge U, Ball E. 1987. Dark respiration of CAM plants. Plant Physiology and Biochemistry 25: 3–10. [Google Scholar]
- Lüttge U, Smith JAC. 1984. Mechanism of passive malic-acid efflux from vacuoles of the CAM-plant Kalanchoë daigremontiana Journal of Membrane Biology 81: 149–158. [Google Scholar]
- Lüttge U, Fischer-Schliebs E, Ratajczak R, Kramber D, Berndt E, Kluge M. 1995. Functioning of the tonoplast in vacuolar C-storage and remobilization in crassulacean acid metabolism. Journal of Experimental Botany 46: 1377–1388. [Google Scholar]
- Lüttge U, Smith JAC, Marigo G, Osmond CB. 1981. Energetics of malate accumulation in the vacuoles of Kalanchoë tubiflora FEBS Letters 126: 81–84. [Google Scholar]
- Nielsen TH. 1995. Fructose-1,6-bisphosphate is an allosteric activator of pyrophosphate:fructose-6-phosphate 1-phosphotransferase. Plant Physiology 108: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf RL, Marcus A. 1974. Rapid increase in adenosine 5-triphosphate during early wheat embryo germination. Plant Physiology 53: 779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistelli L, Marigo G, Ball E, Lüttge U. 1987. Day-night changes in the levels of adenine nucleotides, phosphoenolpyruvate and inorganic pyrophosphate in leaves of plants having crassulacean acid metabolism. Planta 172: 479–486. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Ingram J, Brakla BJ. 1996. Transport across the vacuolar membrane in CAM plants. In: Winter K and Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag, 53–71. [Google Scholar]
- Smith JAC, Marigo G, Lüttge U, Ball E. 1982. Adenine-nucleotide levels during crassulacean acid metabolism and the energetics of malate accumulation in Kalanchoë tubiflora Plant Science Letters 26: 13–21. [Google Scholar]
- Stitt M. 1989. Product inhibition of potato tuber pyrophosphate:fructose-6-phosphate phosphotransferase by phosphate and pyrophosphate. Plant Physiology 89: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. 1990. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annual Review of Plant Physiology and Plant Molecular Biology 41: 153–185. [Google Scholar]
- Stitt M, Gerhardt R, Wike I, Heldt HW. 1987. The contribution of fructose-2,6-bisphosphate to the regulation of sucrose synthesis during photosynthesis. Physiologia Plantarum 69: 377–386. [Google Scholar]
- Theodorou ME, Kruger NJ. 2001. Physiological relevance of fructose 2,6-bisphosphate in the reglation of spinach leaf pyrophosphate:fructose 6-phosphate 1-phosphotransferase. Planta 213: 147–157. [DOI] [PubMed] [Google Scholar]
- Trípodi KEJ, Podestá FE. 1997. Purification and structural and kinetic characterization of the pyrophosphate:fructose-6-phosphate 1-phosphotransferase from the crassulacean acid metabolism plant, pineapple. Plant Physiology 113: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Smith JAC. 1989. Proton and anion transport at the tonoplast in crassulacean-acid-metabolism plants: specificity of the malate-influx system in Kalanchoë daigremontiana Planta 179: 265–274. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC. 1996. Crassulacean acid metabolism: current status and perspectives. In: Winter K and Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag, 389–426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.