Abstract
• Background and Aims The impedance to root growth imposed by soil can be decreased by both mucilage secretion and the sloughing of border cells from the root cap. The aim of this study is to quantify the contribution of these two factors for maize root growth in compact soil.
• Methods These effects were evaluated by assessing growth after removing both mucilage (treatment I – intact) and the root cap (treatment D – decapped) from the root tip, and then by adding back 2 µL of mucilage to both intact (treatment IM – intact plus mucilage) and decapped (treatment DM – decapped plus mucilage) roots. Roots were grown in either loose (0·9 Mg m−3) or compact (1·5 Mg m−3) loamy sand soils. Also examined were the effects of decapping on root penetration resistance at three soil bulk densities (1·3, 1·4 and 1·5 Mg m−3).
• Key Results In treatment I, mucilage was visible 12 h after transplanting to the compact soil. The decapping and mucilage treatments affected neither the root elongation nor the root widening rates when the plants were grown in loose soil for 12 h. Root growth pressures of seminal axes in D, DM, I and IM treatments were 0·328, 0·288, 0·272 and 0·222 MPa, respectively, when the roots were grown in compact soil (1·5 Mg m−3 density; 1·59 MPa penetrometer resistance).
• Conclusions The contributions of mucilage and presence of the intact root cap without mucilage to the lubricating effect of root cap (percentage decrease in root penetration resistance caused by decapping) were 43 % and 58 %, respectively. The lubricating effect of the root cap was about 30 % and unaffected by the degree of soil compaction (for penetrometer resistances of 0·52, 1·20 and 1·59 MPa).
Key words: Border cell, decapping, lubricating effect, maize, mucilage, root cap, root growth pressure, sloughed cells, soil mechanical impedance, soil compaction, Zea mays
INTRODUCTION
According to Esau (1976), the plant root cap protects the root meristem and assists the growing root in penetrating the soil. Thus, the root cap functions to counteract resistance which soil imposes upon the growing root tip. In a previous study, evidence was provided that the root cap facilitates root penetration, enabling faster root elongation in compact soils (Iijima et al., 2003b). Root growth pressures of intact and decapped maize seminal axes were 0·31 and 0·52 MPa, respectively, when the roots were grown in compacted soil. The mechanism for this may involve both cap border cell release and mucilage lubrication of the root–soil interface.
The outermost root cap cells slough off into the soil during root elongation (Bengough et al., 2001; Iijima et al., 2003a). These detached cap cells are called root border cells (Hawes et al., 1998, 2000). Root border cell production was enhanced by increased soil mechanical impedance, from 1930 to 3220 cells per day per single root axis of maize seedlings (Iijima et al., 2000). The whole of the root cap surface may be covered by these border cells in compacted sand. These cells reduce the friction between root cap surface and surrounding soil particles.
Root cap mucilage has also long been believed to reduce friction at the root–soil interface because it forms a slippery gel when fully hydrated. However, the effect of mucilage on root lubrication in soil has never been quantified before. Mucilage may become tightly appressed to the root surface as it dehydrates. Generally, however, the lubricating properties of cap mucilage are unknown (Guinel and McCully, 1986). It has also been found that mucilage production increases in mechanically impeded roots (e.g. Barber and Gunn, 1974; Iijima and Kono, 1992; Boeuf-Tremblay et al., 1995). The quantity of carbon released from the single root tip of maize to compacted sand increases per millimetre of root elongation as compared with that of the loose sand condition (Iijima et al., 2000).
Enhancement of mucilage production may provide additional lubrication of the interface between soil particles and the root cap, and also between the border cells themselves and the root cap surface. The primary purpose of the present study was to quantify the percentage reduction in root penetration resistance due to the root cap mucilage and the presence of the root cap itself, respectively. The secondary purpose was to evaluate the effects of decapping on root penetration resistance in soil compacted to different bulk densities.
MATERIALS AND METHODS
Seed germination and soil preparation
Young maize (Zea mays L. ‘Robusto 30–71’) plantlets were used for the experiment because their roots have a discrete root cap which can be easily removed. Maize grains were surface-sterilized by immersion in a saturated solution of calcium hypochlorite for 5 min and then washed several times with distilled water. They were placed on blotting paper moistened with distilled water in a Petri dish, and germinated at 28 °C for 48 h in the dark. Loamy sand soil (particle size distribution: sand 87·0 %, silt 9·6 %, clay 3·4 %) was sieved through a 2-mm mesh, and then wetted to a water content of 17–18 % g g−1 (ϕ = −15 to −18 kPa), and left to equilibrate in a plastic bag. Seedlings, each with a straight seminal root 20–35 mm long, were used in all the experiments which were carried out over periods between 4 to 48 h in darkness at 28 °C.
Decapping and mucilage treatments
Evaluation was made of the effects of removing both mucilage (treatment I – intact) and the root cap (treatment D – decapped; technique from Barlow and Hines, 1982) from the root tip, and of adding back 2 µL of mucilage to both intact (treatment IM – intact plus mucilage) and decapped (treatment DM – decapped plus mucilage) roots, grown in either loose (0·9 Mg m−3) or compact (1·5 Mg m−3) loamy sand soils. Mucilage was removed by gently wiping the root cap on filter paper, so as not to damage the cap structure. After decapping, the cap junction was checked under a stereomicroscope, and roots whose cap had come off cleanly were used for further experimentation.
Experiment 1: mucilage removal and its effect on root elongation and diameter
First, the time over which mucilage removal was effective was examined. Wetted soil was packed in a plastic cylinder at two different compaction levels, here termed ‘loose’ and ‘compact’, with respective soil bulk densities of 0·90 Mg m−3 and 1·50 Mg m−3. The cylinder was 100 mm high and 50 mm in diameter for the loose soil treatment, and 50 mm for both height and diameter for the compact soil treatment. Sets of two seedlings of either the IM or I treatments were transplanted into each type of soil and plastic cylinder, and allowed to grow for a further 24 h. Every 4 h after transplanting, the seminal root axis was excavated, and its root tip immersed in distilled water for 30–240 min so as to observe the expansion of root cap mucilage, following the method described by Sealey et al. (1995). At least six replicate roots in each treatment were observed.
The change in root elongation over time was measured for the four treatments, D, DM, I, IM, where the seedlings were grown in soil packed to 1·5 Mg m−3 (compact) to test the appropriate experimental period. One seedling from each of the four treatments was harvested every 4 h, starting at the eighth hour after the transplanting. In total, seven to nine replicates were observed.
Higher soil mechanical impedance usually reduces root elongation growth and thickens the root diameter (Iijima and Kono, 1991; Iijima et al., 1991). Root elongation and root diameter changes of the roots grown for 12 h in both the loose (0·9 Mg m−3) and the compact (1·5 Mg m−3) soil were evaluated to test whether decapping and mucilage treatments affected root growth. Diameters were measured at 2, 3 and 4 mm behind the root apex. In total, nine to 12 replicate plants from the four decapping and mucilage treatments were grown in the two soils of different bulk densities.
Experiment 2: contribution of mucilage and root cap to reduce soil mechanical impedance
Root growth pressure was used to evaluate the contribution of (a) root cap mucilage and (b) presence of an intact root cap without mucilage to the reduction in soil mechanical impedance. Details of root growth pressure measurement are described in Iijima et al. (2003b), though with some modifications. The initial root diameter was measured at 2, 3 and 4 mm behind the apex of the roots under the stereomicroscope. After this the plants were transferred to a seedling holder, as illustrated in fig. 1 of Iijima et al. (2003b). The seminal root axis was held vertically and anchored rigidly behind the zone of elongation. The proximal 1 mm of root tip was inserted into a narrow tapering hole (2 mm deep) in the surface of a soil core. The bulk density of the soil core, packed into a plastic cylinder of 50 mm height and diameter, was same as in the compact treatment of expt I. One seedling was transplanted into each root holder and allowed to grow for a further 12 h. In total, seven to nine replicate plants were grown in the decapping and mucilage treatments. The soil core was placed on an electronic balance so that the pushing force to the soil core was recorded. Balance readings (accurate to within 0·001 g) were taken automatically every 10 min during a 12-h interval by a personal computer interfaced to the balance output. After this, the root was excavated out of the soil core, and the final root diameter was measured, as was the initial diameter. The root growth pressure (QR) was calculated as QR = FR/AR, where FR is the root growth force and AR is the average of the cross-sectional areas of the root at 2, 3 and 4 mm behind the apex.
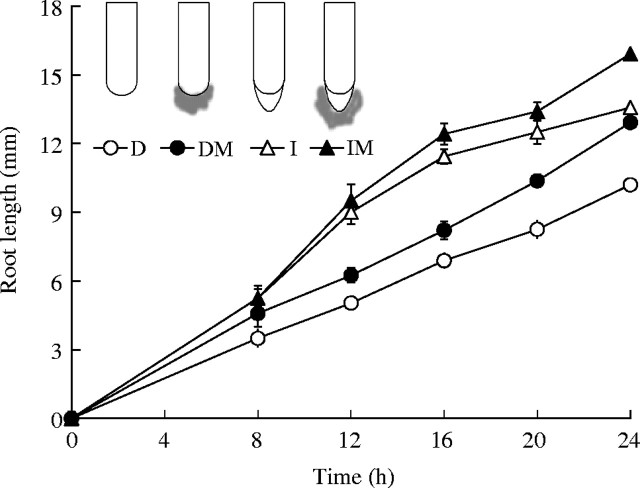
Fig. 1.
Root length increment in compact soil during 24 h growth. Values are from seven to nine replicates. D, Decapped; I, intact; M, 2 µL mucilage added.
In a previous study (Iijima et al., 2003b), the soil resistance experienced by intact roots was found to be smaller than that in the decapped roots. This reduction of soil resistance was termed as a ‘lubricating effect’ due to the root cap, i.e. the percentage decrease in root penetration resistance caused by decapping. The contribution of mucilage alone (eqn 1) and the presence of intact root cap without mucilage (eqn 2) to the lubricating effect were, respectively, evaluated in terms of the soil resistances experienced by roots from each of the experimental treatments, I, IM, D and DM. Thus:
 |
 |
Experiment 3: root cap removal and penetration of soils of different density
The lubricating effect was compared in three different levels of soil compaction; 1·3, 1·4 and 1·5 Mg m−3, here termed light, medium and heavy compaction, respectively. The same size of plastic cylinder (50 mm height and diameter) as in exp II was used. Only the root growth pressure of D and IM treatments were measured in each of the bulk density treatments. Note that the heavy compact is the same as the compact treatment in expts 1 and 2. Five to seven replicates plants were used in the experiment.
Penetrometer resistance
Penetrometer resistance of the soil core was measured using a 0·98 mm diameter probe with a 30° cone angle, pushed into the soil at a rate of 1 mm min−1 to a depth of either 10 or 20 mm for the compact and loose soil treatments, respectively (n = 3). Penetrometer resistance (QP) was calculated as QP = FP/AP, where FP is the penetration force (average value at every 0·16 mm soil depth) and AP is the cross-sectional area of the probe.
Statistical analysis
Differences among the treatments were subjected to either three-way analysis of variance (three-way ANOVA) and to a two-way ANOVA for expts 1, and 2 and 3, respectively. The experiments were not repeated.
RESULTS
Experiment 1: mucilage removal and its effect on root elongation and diameter
After mucilage removal in the intact (I) treatment, the expansion of new root cap mucilage became visible 4 h after planting in the loose soil (data is not shown). In the case of the compact treatment, however, new mucilage appeared on some, but not all, root tips after growing for 12 h in such soil. In this case also, the mucilage expanded after 4 h immersion in water. The delay in mucilage appearance could be attributed to the increased abrasion of the mucilage-producing outermost layers of root cap cells. These cells may therefore be lost and so unavailable for mucilage exudation on the root cap surface. In fact, the number of sloughed cap cells is significantly increased by higher mechanical impedance (Iijima et al., 2000). These results indicate that the mucilage removal treatment is effective for up to 12 h after the planting in the case of the compact soil condition.
Decapped roots in compact soil had relatively constant elongation rates (Fig. 1). Intact roots, however, showed a more sigmoidal growth trend, especially in the case of the IM treatment. Mucilage-treated roots elongated consistently faster than untreated roots (except for IM versus I treatments, at times before 8 h). The decapped roots grew consistently slower than the intact roots.
Penetrometer resistance in the loose treatment was only 0·03 MPa, which represents negligible mechanical impedance to root growth. The effects of decapping and mucilage treatment on root morphology were evaluated in the loose treatment after 12 h growth (Table 1). In the loose treatments, neither root elongation nor root diameter was significantly different among the four treatments. This indicates that decapping and mucilage treatments do not affect root growth. In the compact treatment, however, both decapping and mucilage treatments significantly reduced root elongation and increased root diameter.
Table 1.
Root length and diameter of seminal roots as affected by soil bulk density, decapping and mucilage addition
| Decapping treatment (DT) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Compact |
Loose |
|||||||
| Mucilage treatment (MT) |
Decapping |
Intact |
Decapping |
Intact |
||||
| Length (mm) | Mucilage | 6·2 ± 0·6 | 9·5 ± 0·5 | 17·7 ± 1·0 | 17·9 ± 1·2 | |||
| Without mucilage | 5·0 ± 0·4 | 9·0 ± 0·4 | 17·8 ± 1·1 | 18·2 ± 0·9 | ||||
| Diameter (mm) | Mucilage | 1·46 ± 0·01 | 1·02 ± 0·00 | 0·84 ± 0·02 | 0·83 ± 0·02 | |||
| Without mucilage | 1·47 ± 0·01 | 1·21 ± 0·01 | 0·85 ± 0·01 | 0·87 ± 0·01 | ||||
| Length |
Diameter |
|||||||
| Three-way ANOVA | Soil bulk density (S) | *** | *** | |||||
| DT | ** | *** | ||||||
| MT | ns | *** | ||||||
| S × DT | ns | *** | ||||||
| S × MT | ns | *** | ||||||
| DT × MT | ns | ** | ||||||
| S × DT × MT | *** | *** | ||||||
Roots were grown in both loose (0·9 Mg m−3) and compact (1·5 Mg m−3) soil for 12 h.
Soil mechanical impedance (MPa) was 3·18 ± 0·13 for compact and 0·03 ± 0·00 for loose conditions.
Values are the means ± s.e. of nine to 12 replicates.
*** and **, Statistically significant at P < 0·001 and P < 0·01, respectively; ns, not statistically significant at P ≥ 0·05.
Experiment 2: contribution of mucilage and root cap to reduce soil mechanical impedance
Root penetration resistance was evaluated when the root tips were estimated to have grown to about 3 and 5 mm below the soil surface. This was sufficient to eliminate initial soil surface deformation effects and to avoid problems due to the root becoming anchored to soil by its root hairs. Assuming constant root elongation rates, the time required for this amount of root elongation was 2·5–5 h for IM treatment, and 4·8–9·6 h for D treatment, respectively. Growth pressure measurements are summarized in Table 2. In D treatment, the growth pressure was 1·48 times greater than that in IM treatment. The pressure experienced by the growing roots was 4·8 to 7·2 times smaller than the penetrometer resistance. Therefore, the root cap and root elongation characteristics effectively reduced the soil mechanical impedance. The ‘lubricating effect’, the contribution of the root cap to the reduction of soil resistance, was 32·3 % (Table 3). The contributions of mucilage and the presence of an intact root cap without mucilage to this lubricating effect were 43 % and 58 %, respectively.
Table 2.
Root growth pressure (MPa) of seminal roots as affected by decapping and mucilage treatment
| Mucilage treatment (MT) |
Decapping treatment (DT) |
|||
|---|---|---|---|---|
| Decapping |
Intact |
|||
| Mucilage | 0·288 ± 0·005 | 0·222 ± 0·007 | ||
| Non-mucilage | 0·328 ± 0·029 | 0·272 ± 0·006 | ||
| ANOVA | DT | *** | ||
| MT | ** | |||
| DT × MT | ns | |||
Roots were grown in compact (1·5 Mg m–3) soil for 12 h.
Mean soil mechanical impedance at 2–4 mm soil depth was 1·59 MPa.
Values are the means ± s.e. of seven to nine replicates.
*** and **, Statistically significant at P < 0·001 and P < 0·01, respectively; ns, not statistically significant at P ≥ 0·05.
Table 3.
Root growth pressure and lubricating effect of maize root tips as affected by soil mechanical impedance
| Soil bulk density (g cm−3) | 1·30 | 1·40 | 1·5† | |
|---|---|---|---|---|
| Mean soil mechanical impedance at 2–4 mm soil depth (MPa) | 0·52 | 1·20 | 1·59 | |
| Root growth pressure (MPa) | Decapped (D) | 0·228 ± 0·006 | 0·308 ± 0·021 | 0·328 ± 0·029 |
| Intact (IM) | 0·159 ± 0·005 | 0·213 ± 0·017 | 0·222 ± 0·007 | |
| Lubricating effect (% change in root penetration resistance) |  |
30·0 | 30·8 | 32·3 |
| Two-way ANOVA | Soil bulk density (S) | *** | ||
| Decapping and mucilage (D&IM) | *** | |||
| S × D&IM | ns |
D, Decapped and mucilage not added; IM, intact and mucilage added.
Values are the means ± s.e. of five to nine replicates.
***Statistically significant at P < 0·001; ns, not statistically significant at P ≥ 0·05.
The value is same as in Table 2.
Experiment 3: root cap removal and penetration of soils of different density
The contribution of the root cap to the reduction of soil mechanical impedance was evaluated in the three compaction treatments (Table 3). The resistance which roots experienced during their growth was 2·3–3·3 and 3·9–5·6 times smaller than the penetrometer resistance in the light and medium compaction, respectively. The lubricating effect (percentage decrease in resistance root experience) was independent of soil bulk density, being 30·1, 30·9 and 32·3 % for the light, medium and heavily compaction treatments, respectively, even though the actual difference in soil resistance did increase. Penetrometer resistance was 0·52, 1·20 and 1·59 MPa, respectively, for the 1·3, 1·4, and 1·5 Mg m−3 treatments, in the zone where root growth pressure was measured, 2–4 mm below the soil surface.
DISCUSSION
The results have demonstrated that root cap mucilage and the presence of an intact root cap without mucilage both reduce the soil mechanical impedance to growing roots. The contribution of these two factors to the reduction in soil resistance to maize root growth by the presence of root cap, the lubricating effect, were evaluated as being 43 % for the intact root cap without mucilage and 58 % for the mucilage. These values may alter depending on the experimental condition, such as amount of mucilage on the root cap, the degree of soil mechanical impedance, and soil water status. Because the mucilage added back to the root cap was fully hydrated, it might have not only contributed to the carbohydrate component of the mucilage but also have added water to the soil–root system. Soil mechanical impedance depends on the soil water status, and therefore added mucilage may have reduced soil mechanical impedance in the immediate vicinity of root tip. As stated by Guinel and McCully (1986), mucilage should be a more effective lubricant under conditions of wet soil. Under drier conditions, however, mucilage would be dehydrated by the surrounding rhizosphere soil. Thus, the contribution of mucilage to the reduction in soil resistance to maize root growth may become much smaller under drier condition. Plant root mucilage contains surfactants that may be able to draw water from rhizosphere soil (Read et al., 2003). The wetting or drying function of mucilage to the surrounding rhizosphere soil should therefore be clarified under different soil moisture conditions.
The lubricating effect of the root cap was not affected by soil mechanical impedance (Table 3). In a previous study, the lubricating effect was estimated as 40 % (Iijima et al., 2003b). This could be attributed either to a cultivar difference (‘Mephisto’ vs. ‘Robust 30–71’) or to the different soil textures in the two experiments. The clay content of the soil used in the former study was 8 %, whereas in the present study it was 3·4 %. A higher clay content may result in a higher soil frictional resistance experienced by the roots, and this, in turn, may affect the lubricating effect of the root cap.
The contribution of an intact root cap without mucilage was evaluated by the difference in results from treatments involving the removal of mucilage (treatment I) or the whole root cap plus its mucilage (treatment D). This difference possibly comprises two factors: sloughing of root cap cells and an effect on the shape of the root tips. Decapping results in a blunter shape to the root tip which may, in turn, increase root penetration resistance (Iijima et al., 2003b). However, in the case of metal probes with cone angles of 30–60°, the penetration resistance was relatively unaffected by shape of the tip (i.e. cone angle) (Gill, 1968). Moreover, the root tip itself may not directly push into the soil, but rather it is the lateral portion of the root tip which does so and which deflects soil particles to one side. This effect may be enhanced by root tip nutational movements. Therefore, the major effect of an intact root cap without mucilage should be the sloughing of root cap cells, i.e. the production of the root border cells.
In conclusion, this study has quantified the contribution of intact root cap without mucilage (i.e. root border cells) and root cap mucilage in reducing soil resistance to root elongation growth. The contribution of root border cells was relatively larger than the effect of adding mucilage. The percentage reduction in soil resistance due to the presence of a root cap was constant in soils of different densities.
Acknowledgments
We thank Dr Glyn Bengough, SCRI, UK, and Prof. Shigenori Morita, Tokyo University, Japan, for helpful comments. The Japanese Society of Promotion of Science (B2-12460010) and the Royal Society of London provided funding for the visit to UK by Dr M. Iijima and Mr T. Higuchi.
LITERATURE CITED
- Barber DA, Gunn KB. 1974. The effect of mechanical forces on the exudation of organic substances by the roots of cereal plants grown under sterile conditions. New Phytologist 73: 39–45. [Google Scholar]
- Barlow PW, Hines ER. 1982. Regeneration of the root cap of Zea mays L. and Pisum sativum L.: a study with the scanning electron microscope. Annals of Botany 49: 521–539. [Google Scholar]
- Bengough AG, Iijima M, Barlow PW. 2001. Image analysis of maize root caps—estimating cell numbers from 2-D longitudinal sections. Annals of Botany 87: 693–698. [Google Scholar]
- Boeuf-Tremblay V, Planturcux S, Guckert A. 1995. Influence of mechanical impedance on root exudation of maize seedlings at two development stages. Plant and Soil 172: 279–287. [Google Scholar]
- Esau K. 1976.Anatomy of seed plants, 2nd edn. New York: John Wiley & Sons. [Google Scholar]
- Gill WR. 1968. Influence of compaction hardening of soil on penetration resistance. Transactions of the American Society of Agricultural Engineers 11: 741–745. [Google Scholar]
- Guinel FC, McCully ME. 1986. Some water-related physical properties of maize root-cap mucilage. Plant Cell and Environment 9: 657–666. [Google Scholar]
- Hawes MC, Brigham LA, Wen F, Woo HH, Zhu Y. 1998. Function of root border cells in plant health: pioneers in the rhizosphere. Annual Review of Phytopathology 36: 311–27. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X. 2000. The role of root border cells in plant defence. Trends in Plant Science 5: 128–133. [DOI] [PubMed] [Google Scholar]
- Iijima M, Kono Y. 1991. Interspecific differences of the root system structures of four cereal species as affected by soil compaction. Japanese Journal of Crop Science 60: 130–138. [Google Scholar]
- Iijima M, Kono Y. 1992. Development of Golgi apparatus in the root cap cells of maize (Zea mays L.) as affected by compacted soil. Annals of Botany 70: 207–212. [Google Scholar]
- Iijima M, Barlow PW, Bengough AG. 2003. Root cap structure and cell production rates of maize (Zea mays L.) roots in compacted sand. New Phytologist 160: 127–134. [DOI] [PubMed] [Google Scholar]
- Iijima M, Griffith B, Bengough AG. 2000. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist 145: 477–482. [DOI] [PubMed] [Google Scholar]
- Iijima M, Higuchi T, Barlow PW, Bengough AG. 2003. Root cap removal increases root penetration resistance in maize (Zea mays L.). Journal of Experimental Botany 54: 2105–2109. [DOI] [PubMed] [Google Scholar]
- Iijima M, Kono Y, Yamauchi A, Pardales JR Jr. 1991. Effects of soil compaction on the development of rice and maize root systems. Environmental and Experimental Botany 31: 333–342. [Google Scholar]
- Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang, X. 2003. Plant roots release phospholipids surfactants that modify the physical and chemical properties of soil. New Phytologist 157: 315–326. [DOI] [PubMed] [Google Scholar]
- Sealey LJ, McCully ME, Canny MJ. 1995. The expansion of maize root-cap mucilage during hydration. 1. Kinetics. Physiologia Plantarum 93: 38–46. [Google Scholar]