Abstract
• Background and Aims Silica deposition is one of the important characteristics of plants in the family Poaceae. There have been many investigations into the distribution, deposition and physiological functions of silica in this family. Two hypotheses on silica deposition have been proposed based on these studies. First, that silica deposition occurs passively as a result of water uptake by plants, and second, that silica deposition is controlled positively by plants. To test these two apparently contradictory hypotheses, silica deposition in relation to the ageing of leaf tissues in Sasa veitchii was investigated.
• Methods Tissues were examined using a light microscope and a scanning electron microscope equipped with an energy dispersive X‐ray microanalyser.
• Key Results The deposition process differed depending on cell type. In mesophyll tissue, fusoid cells deposited large amounts of silica depending on leaf age after maturation, while chlorenchyma cells deposited little. In epidermal tissue, comprised of eight cell types, only silica cells deposited large amounts of silica during the leaf’s developmental process and none after maturation. Bulliform cells, micro‐hairs and prickle hairs deposited silica densely and continuously after leaf maturation. Cork cells, guard cells, long cells and subsidiary cells deposited silica at low levels.
• Conclusions The significance of these observations is discussed in relation to the two hypotheses proposed for silica deposition in Poaceae. The results of the present study clearly indicate that both hypotheses are compatible with each other dependent on cell types.
Key words: Sasa veitchii, Poaceae, silica deposition, leaf, ageing, epidermis, mesophyll, fusoid cell, clearing method, light microscopy, scanning electron microscopy, X‐ray microanalysis
INTRODUCTION
Silica deposition is one of the important characteristics of plants in the family Poaceae. Many studies have examined the distribution, deposition and physiological functions of silica in this family. Two hypotheses on silica accumulation have been proposed based on reviews by several authors (Jones and Handreck, 1967; Kaufman et al., 1981; Raven, 1983; Hodson and Sangster, 1990; Epstein, 1999; Ma and Takahashi, 2002). The first is that silica deposition occurs passively as a result of water uptake by plants (Frey‐Wyssling, 1930; Yoshida et al., 1962; Jones and Handreck, 1967); i.e. silica is unavoidably absorbed from the soil in the form of silicic acid dissolved in water and deposited in plant tissues through transpiration. The second is that silica deposition is positively controlled by plants as part of physiological activities, such as mechanical stability of tissues (Balasta et al., 1989), protection against micro‐organisms (Belanger et al., 1995; Marschner, 1995) and protection against herbivores (Jones and Handreck, 1967; McNaughton et al., 1985; Cid et al., 1989).
Grasses and bamboos are known to have large deposits of silica in the tissues of leaf blades and inflorescence bracts. It is reported that the silica content in mature leaves of Phyllostachys pubescens increases rapidly during the first early growing season, levels off during the first autumn, and then increases again during the following early spring (Kaneko, 1995), whereas it never increases in P. bambusoides (Ueda and Ueda, 1961). However, it is unclear whether the increase in the second growing season is the result of leaf ageing or to reactivation, because the life span of leaves in both species is only about 1 year. In contrast, leaves of Sasa veitchii have a life span of approx. 2 years, and Motomura et al. (2002) have reported that they continuously accumulate silica throughout their life, not only during the developmental process but also after maturation. This study clarifies the season‐dependent changes in silica content, and shows that the accumulation pattern (rapid in spring and summer and slow in winter) is repeated during the 2 years of the life span. These facts support the first hypothesis that silica deposition is a result of water uptake by plants.
Silica deposition at the tissue level was investigated in young, mature leaves of Pleioblastus chino (about 1–2 months after leaf expansion), and was found to be densest in the epidermis and least in the mesophyll and vascular bundle tissues (Motomura et al., 2000). If silica deposition is a result of water uptake by plants, silica deposits would be expected to be denser in the mesophyll, where a substantial proportion of the water transpires directly, than in the epidermis. However, mesophyll cells do not accumulate much silica, perhaps because they are prevented from doing so as they are actively involved in photosynthesis (Motomura et al., 2000). Consequently, these results support the second hypothesis that silica deposition in tissue systems is positively controlled by the plant.
Most grasses and bamboos accumulate silica in the epidermis, but its distribution is not uniform among the various cell types comprising the epidermis. In Sieglingia decumbens leaves, it is reported that only silica cells, one type of epidermal cell, deposit silica during the developmental process of the leaves but not after their maturation, whilst other types of epidermal cells deposit silica only after leaf maturation (Sangster, 1970). This clearly demonstrates that the silica deposition process is different depending on cell type, but the differences have never been described among the eight or more cell types comprising the epidermis (Metcalfe, 1960; Ellis, 1979).
As reviewed above, the distribution pattern of silica in the leaves of Poaceae seems to be inconsistent with the conclusions from the seasonal changes of silica content shown by Motomura et al. (2002). This inconsistency has arisen because the silica deposition process in each cell type has not been investigated in relation to leaf ageing after maturation. In previous studies, short‐lived leaves (e.g. <2 months in S. decumbens) of grasses or young leaves (e.g. about 1–2 months after leaf expansion in P. chino) of bamboos were sampled. Thus, it is necessary to study silica deposition in each cell type according to the ageing of long‐lived leaves to explain the mechanisms of silica deposition in Poaceae. Within Poaceae, bamboo leaves have a fairly long life‐span, and the silica content, but not distribution, in the leaf blades of S. veitchii has been determined throughout their life. Hence, the present study aimed to clarify the differences in silica deposition within the different cell types in mature leaf blades of S. veitchii using a light microscope and a scanning electron microscope equipped with an energy‐dispersive X‐ray analyser (SEM‐EDXA).
MATERIALS AND METHODS
Leaves were collected from Sasa veitchii (Carrière) Rehder growing in the Botanical Garden of Tohoku University, Sendai, Japan.
In S. veitchii, shoots appear above ground and fully elongate during the first year. In the second year, daughter shoots emerge from nodes located in the middle section of the mother shoots. Such apparent sympodial branching is repeated annually. Each shoot has several leaves arising from nodes located near its tip. Leaf ages can be determined by studying the branching pattern in relation to the sequence of shoot ageing. Three different ages were distinguished in this study, i.e. first‐, second‐ and third‐year leaves.
For light microscope observations, fully expanded leaves were sampled in August 1998 from the second, third and fourth nodes from the top of each of five shoots of three different ages. Each leaf was cut into three equal sections, basal, middle and top, and then three to five pieces, of approx. 5 mm2, were cut from the region between the leaf margin and midrib of the middle sections. These samples were soaked in a 1 : 1 solution of glacial acetic acid and 30 % hydrogen peroxide for 48 h at 60 °C, dehydrated in an ethanol series, stained with safranin, and then observed under a light microscope. In all samples, the numbers of silicified cells and non‐silicified cells in each cell type were counted within an area of 0·3 mm2 in adaxial and abaxial epidermis, as described by Motomura et al. (2000).
For the SEM–EDXA study, samples were collected in August 2000, as described above for optical microscopy, from each of four shoots of three different ages. They were rapidly frozen in liquid nitrogen, freeze‐fractured, then freeze‐dried for 6 h in a freeze‐drying device for chemical analysis (FD‐1, Tokyorikakikai Co. Ltd, Tokyo, Japan). They were then coated with platinum–palladium (80 : 20) in an ion‐sputter‐coater (JFC‐1100, JEOL Co. Ltd, Tokyo, Japan) before being examined under the SEM (JSM‐840, JEOL Co. Ltd) equipped with an EDXA system (JED‐2110, JEOL Co. Ltd).
RESULTS
General anatomy of Sasa veitchii leaf
Sasa veitchii leaves are composed of a single layer of adaxial epidermal cells (ad), two layers of chlorenchyma cells (ch), a single layer of fusoid cells (fu), and a single layer of abaxial epidermal cells (ab) (Fig. 1A and C). Vascular bundles (v) are interspersed among the mesophyll cells (chlorenchyma and fusoid cells), each one being individually surrounded by a bundle sheath consisting of thin‐walled parenchyma cells (bs‐pa) (Fig. 1A). Scleren chyma cells (sc) extend as ‘girders’ from the vascular bundles to both epidermises (Fig. 1A).

Fig. 1. Scanning electron micrographs of transverse and longitudinal sections of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 50 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM–EDX. Relative silicon density is imaged by colour from very low (dark blue) to very high (white) through sky‐blue, yellow and red. (A and B) Transverse fractures; (C and D) longitudinal fractures. ad, Adaxial side; ab, abaxial side; bs‐pa, bundle sheath parenchyma cell; ch, chlorenchyma cell; fu, fusoid cell; gu, guard cell; pa, papillae; sc, sclerenchyma cell; si, silica cell; v, vascular bundle tissue.
Epidermal cells were categorized into eight types; namely, bulliform cells, cork cells, guard cells, long cells, micro‐hairs, prickle hairs, silica cells and subsidiary cells. Cellular composition ratios were different between the adaxial and abaxial epidermises (Fig. 2). Long cells (lo) were the most abundant in both epidermises (approx. 30 %). In the adaxial epidermis, bulliform cells (bu), cork cells (co) and silica cells (si) were also major components (19–29 %), while guard cells (gu) and subsidiary cells (su) were less abundant (0·4 %), and micro‐hairs (mi) and prickle hairs (pr) were absent (Fig. 2). In contrast, in the abaxial epidermis, guard cells and subsidiary cells were the major components (24 %) along with long cells, while cork cells and silica cells were significantly less abundant (Fig. 2). Bulliform cells were absent (Fig. 2), while micro‐hairs and prickle hairs were present only as minor components (3·7 and 1·4 %, respectively).
Fig. 2. Cell composition in the adaxial and abaxial epidermises (measured area = 0·3 mm2). Forty‐five sections from 15 leaves were measured. bu, Bulliform cell; co, cork cell; gu, guard cell; lo, long cell; mi, micro‐hair; pr, prickle hair; si, silica cell; su, subsidiary cell. Bars indicate standard deviations.
Density of silica deposition
Leaf sections. Parts B and D in Fig. 1 show the results of the SEM‐EDXA studies on the same fields as shown in parts A and C, respectively, in Fig. 1. In the sections of first‐year leaves, silica deposition was densest in the abaxial epidermal cell papillae and silica cells, less dense in bundle sheath parenchyma cells and guard cells, and least dense in chlorenchyma cells, fusoid cells and vascular bundle cells (Fig. 1). Silica deposition was also sparse around intercellular spaces under stomata (Fig. 1C and D). In the walls of bundle sheath parenchyma cells (Fig. 3A and B), silica deposition was fairly dense, but was less dense in radial walls (Fig. 3).
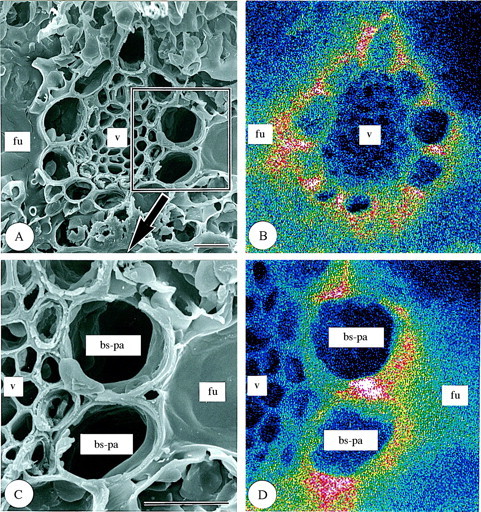
Fig. 3. Scanning electron micrographs of transverse sections of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 10 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM–EDX. (A and B) Vascular bundle tissue; (C and D) bundle sheath parenchyma cells. bs‐pa, Bundle sheath parenchyma cell; fu, fusoid cell; v, vascular bundle tissue.
Silica deposition in third‐year leaves (Fig. 4) was quite different from that in first‐year leaves (Fig. 1). Dense deposition occurred in bundle sheath parenchyma cells and fusoid cells (Fig. 4), the densest deposition being in the cell walls between fusoid cells and bundle sheath parenchyma cells (Fig. 4A and B). Fairly dense silica deposition was found also in cells of both the adaxial and abaxial epidermises, with the densest deposition being present in silica cells and guard cells of the abaxial epidermis (Fig. 4). Larger amounts of silica were detected in the guard cells of third‐year leaves compared with those of first‐year leaves (cf. parts C and D in Fig. 4 with parts C and D in Fig. 1). However, silica deposition was sparse in vascular bundle cells and chlorenchyma cells, including those around intercellular spaces under stomata (Fig. 4).
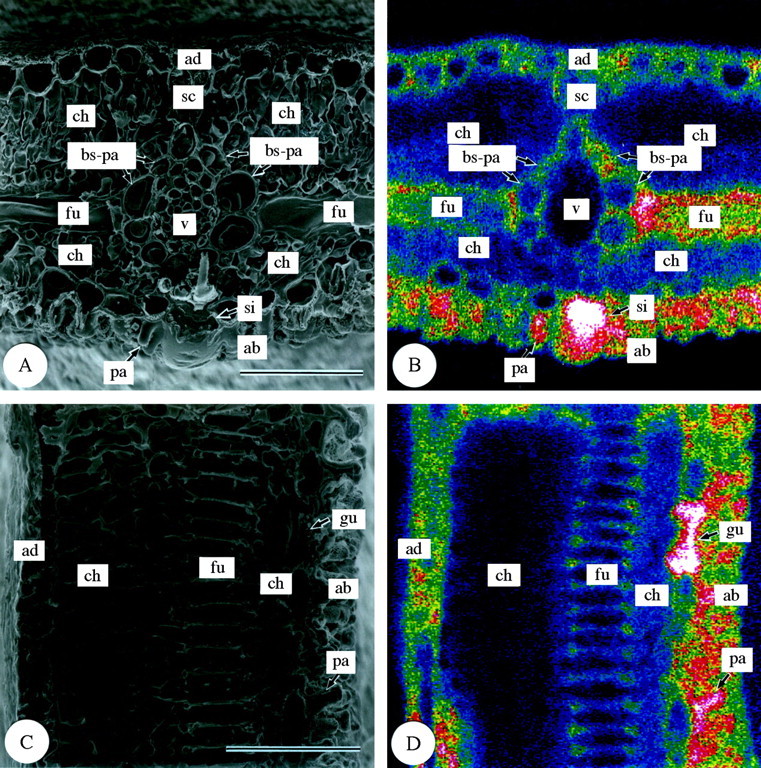
Fig. 4. Scanning electron micrographs of transverse and longitudinal sections of freeze‐dried third‐year leaves of Sasa veitchii. Bar = 50 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Transverse fractures; (C and D) longitudinal fractures. ad, Adaxial side; ab, abaxial side; bs‐pa, bundle sheath parenchyma cell; ch, chlorenchyma cell; fu, fusoid cell; gu, guard cell; pa, papillae; sc, sclerenchyma cell; si, silica cell; v, vascular bundle tissue.
Leaf surfaces. In the surface view of first‐year leaves (Fig. 5), differences in silica deposition were found among the different epidermal cell types. In the adaxial epidermis (Fig. 5A and B), the highest silica deposition was observed in silica cells (si). Silica was also deposited densely in some bulliform cells (bu; Fig. 5A and B), and, within the cell wall, silica deposition was densest in the outer periclinal walls (op; Fig. 6). Silica deposition in other adaxial epidermal cell types was sparse (Fig. 5A and B). In the abaxial epidermis (Fig. 5C and D), silica deposition was similar to that in the adaxial epidermis (Fig. 5A and B). Most silica was deposited in the silica cells (si) (Fig. 5C and D). Large amounts of silica were also deposited in micro‐hairs (mi) and prickle hairs (pr) (Fig. 5C and D). Prickle hairs deposited silica densely either over their entire surfaces (Fig. 5C and D) or at their pointed tips (Fig. 7A and B). Fairly dense silica deposition was found on the cap cells of micro‐hairs (mi‐ca), while deposition in the basal cells (mi‐ba) was less dense (Fig. 7C and D). In the abaxial epidermis, long cells (lo) usually have several papillae (pa). The papillae overhanging the stomata had dense silica deposition on the protrusions (Fig. 5C and D). Little silica deposition was observed in subsidiary cells and only slightly more in guard cells because both categories of cells are shown in longitudinal section in Fig. 8. In guard cells, silica deposition was densest in the middle portion (see centre in Fig. 8C and D). There was little silica deposition in cork cells (co) and long cells (Fig. 5C and D).
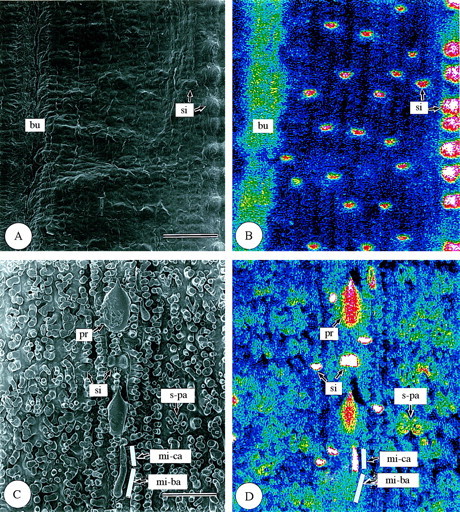
Fig. 5. Scanning electron micrographs of adaxial and abaxial surfaces of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 50 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Adaxial surfaces; (C and D) abaxial surfaces. bu, Bulliform cell; mi‐ba, basal cell of micro‐hair; mi‐ca, cap cell of micro‐hair; pr, prickle hair; si, silica cell; s‐pa, silicified papillae.
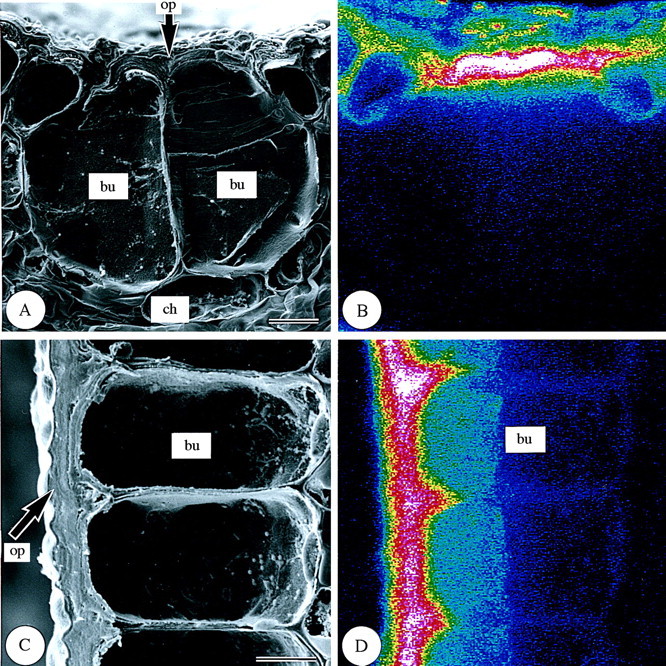
Fig. 6. Scanning electron micrographs of bulliform cells in transverse and longitudinal sections of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 10 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Transverse fractures; (C and D) longitudinal fractures. bu, Bulliform cell; ch, chlorenchyma; op, outer periclinal cell wall.

Fig. 7. Scanning electron micrographs of two cell types in the epidermis of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 10 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Prickle hairs; (C and D) micro‐hairs. mi‐ba, Basal cell; mi‐ca; cap cell.
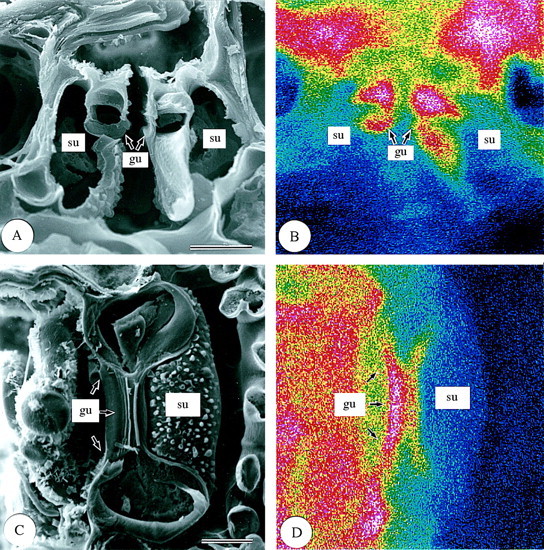
Fig. 8. Scanning electron micrographs of stomata in transverse and longitudinal sections of freeze‐dried first‐year leaves of Sasa veitchii. Bar = 10 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Transverse fractures; (C ang D) longitudinal fractures. gu, Guard cell; su, subsidiary cell.
In the epidermis of third‐year leaves (Fig. 9), the densest silica deposition was observed in silica cells (si) of both the adaxial and abaxial epidermises as in first‐year leaves (Fig. 5). In the adaxial epidermis, very dense deposition was observed in bulliform cells (Fig. 9A and B), the lumina of which were completely filled with granulate silica (Fig. 10). In all other types of adaxial epidermal cells, some silica was deposited (Fig. 9). In the abaxial epidermis, dense silica deposition was observed over the entire surfaces of prickle hairs and in basal and cap cells of micro‐hairs (Fig. 9C and D). Dense silica deposition was also observed on long cell papillae (Fig. 9C and D). Longitudinal sections of guard cells showed dense silica deposition (Fig. 4C and D). Cork cells and subsidiary cells showed some silica deposition (Figs 4C and D and 9C and D).

Fig. 9. Scanning electron micrographs of adaxial and abaxial surfaces of freeze‐dried third‐year leaves of Sasa veitchii. Bar = 50 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Adaxial surfaces; (C and D) abaxial surfaces. bu, Bulliform cell; mi, micro‐hair; pr, prickle hair; si, silica cell; pa, papillae.
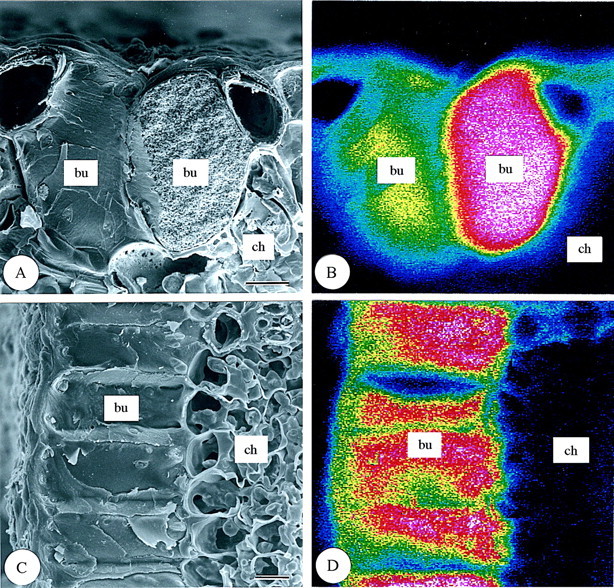
Fig. 10. Scanning electron micrographs of bulliform cells in transverse and longitudinal sections of freeze‐dried third‐year leaves of Sasa veitchii. Bar = 10 µm. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM‐EDX. (A and B) Transverse fractures; (C and D) longitudinal fractures. bu, Bulliform cell; ch, chlorenchyma.
Frequency of silicified epidermal cells
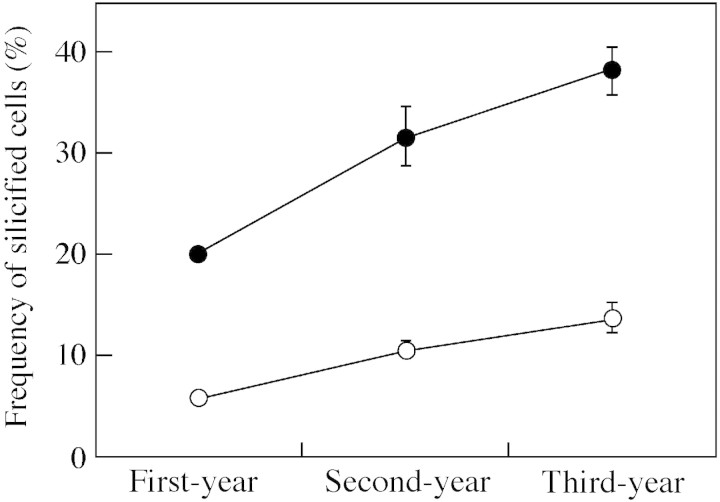
Numbers of silicified cells among all epidermal cell types increased according to leaf age (Table 1 and Fig. 11). In first‐year, fully expanded leaves, silicified cells were observed in all epidermal cell types, the frequency of silicified cells being 20 % in the adaxial epidermis and 5·8 % in the abaxial epidermis (Table 1 and Fig. 11). The frequencies increase markedly in second‐year leaves, with values of 32 % and 11 % in adaxial and abaxial epidermis, respectively. A further increase was observed in third‐year leaves, with values of 38 % and 14 % in adaxial and abaxial epidermis, respectively. The frequency of silicified cells was generally higher among the adaxial epidermal cells than the abaxial ones (Table 1 and Fig. 11).
Table 1.
Frequency of silicified cells in epidermis of leaf blades at three different ages
| First‐year | Second‐year | Third‐year | |||||
| Cell types | % | Mean (±s.d.) | % | Mean (±s.d.) | % | Mean (±s.d.) | |
| (A) Adaxial epidermis | |||||||
| Bulliform cells | C | 18·9 | 116·7 ± 19·2 | 18·6 | 112·9 ± 21·4 | 18·7 | 111·5 ± 13·5 |
| S | 0·0c | 0·0 ± 0·0 | 56·6b | 60·9 ± 29·3 | 87·9a | 98·1 ± 25·0 | |
| Cork cells | C | 29·3 | 180·5 ± 9·2 | 28·4 | 172·2 ± 11·8 | 28·4 | 170·1 ± 8·5 |
| S | 0·0c | 0·0 ± 0·0 | 1·1c | 1·9 ± 1·3 | 2·4b | 4·0 ± 2·9 | |
| Guard cells | C | 0·4 | 2·3 ± 2·0 | 0·4 | 2·7 ± 2·4 | 0·4 | 2·3 ± 1·7 |
| S | 10·0b | 0·1 ± 0·5 | 42·8b | 1·1 ± 1·3 | 65·9a | 1·5 ± 1·3 | |
| Long cells | C | 30·8 | 189·1± 7·9 | 30·7 | 186·2 ± 16·5 | 31·7 | 189·6 ± 8·5 |
| S | 0·0c | 0·0 ± 0·0 | 0·1c | 0·1 ± 0·4 | 1·2b | 2·3 ± 1·3 | |
| Micro‐hairs | C | – | – | – | – | – | – |
| (basal cells) | S | – | – | – | – | – | – |
| Prickle hairs | C | – | – | – | – | – | – |
| S | – | – | – | – | – | – | |
| Silica cells | C | 20·2 | 124·5 ± 11·7 | 21·4 | 129·7 ± 13·4 | 20·5 | 122·8 ± 8·6 |
| S | 98·5a | 122·8 ± 12·5 | 99·0a | 128·4 ± 13·2 | 99·4a | 122·1 ± 8·6 | |
| Subsidiary cells | C | 0·4 | 2·3 ± 2·0 | 0·4 | 2·7 ± 2·4 | 0·4 | 2·3 ± 1·7 |
| S | 0·0c | 0·0 ± 0·0 | 1·1c | 0·1 ± 0·3 | 13·6b | 0·3 ± 0·7 | |
| Total | |||||||
| Cell number | 100 | 615·4 ± 26·5 | 100 | 606·3 ± 28·7 | 100 | 598·6 ± 15·6 | |
| Silicified cell | 20·0C | 122·9 ± 12·5 | 31·7B | 192·5 ± 36·2 | 38·1A | 228·2 ± 26·4 | |
| (B) Abaxial epidermis | |||||||
| Bulliform cells | C | – | – | – | – | – | – |
| S | – | – | – | – | – | – | |
| Cork cells | C | 13·4 | 132·2 ± 15·8 | 14·1 | 141·3 ± 17·1 | 13·2 | 130·9 ± 11·4 |
| S | 0·0b | 0·1 ± 0·3 | 1·8c | 2·6 ± 2·1 | 2·2c | 2·9 ± 1·9 | |
| Guard cells | C | 24·2 | 238·5 ± 19·0 | 23·7 | 237·6 ± 20·1 | 24·4 | 241·3 ± 14·7 |
| S | 0·1b | 0·1 ± 0·5 | 3·1c | 7·5 ± 5·9 | 8·6c | 20·2 ± 17·7 | |
| Long cells | C | 27·1 | 266·9 ± 17·7 | 26·5 | 265·4 ± 15·7 | 26·5 | 262·0 ± 19·2 |
| S | 0·0b | 0·0 ± 0·0 | 0·8c | 2·0 ± 1·9 | 3·0c | 8·0 ± 10·9 | |
| Micro‐hairs | C | 3·4 | 33·6 ± 7·5 | 3·8 | 38·4 ± 6·1 | 3·8 | 37·5 ± 6·4 |
| (basal cells) | S | 0·5b | 0·2 ± 0·6 | 41·1b | 15·9 ± 10·1 | 72·2b | 27·1 ± 7·8 |
| Prickle hairs | C | 1·7 | 17·9 ± 23·7 | 1·4 | 15·0 ± 20·2 | 1·1 | 10·9 ± 11·9 |
| S | 0·0 b | 0·0 ± 0·0 | 56·6b | 7·0 ± 8·7 | 72·5b | 8·7 ± 11·8 | |
| Silica cells | C | 5·9 | 57·9 ± 12·9 | 6·8 | 67·5 ± 14·2 | 6·6 | 65·5 ± 6·8 |
| S | 97·5a | 56·5 ± 12·7 | 99·7a | 67·3 ± 14·3 | 99·7a | 65·3 ± 6·6 | |
| Subsidiary cells | C | 24·2 | 238·5± 19·0 | 23·7 | 237·6 ± 20·1 | 24·4 | 241·3 ± 14·7 |
| S | 0·1b | 0·1 ± 0·0 | 1·6c | 3·7 ± 3·2 | 1·9c | 4·5 ± 2·9 | |
| Total | |||||||
| Cell number | 100 | 985·6 ± 56·4 | 100 | 1002·8 ± 59·6 | 100 | 989·5 ± 48·7 | |
| Silicified cell | 5·8C | 57·0 ± 12·5 | 10·6B | 105·9 ± 15·5 | 13·8A | 136·7 ± 25·8 | |
Measured area = 0·3 mm2, n = 15.
Values with the same superscript are not significantly different at the 0·05 level among cell types by multiple comparisons using Scheffe’s method.
The same capital letters indicates that values are not significantly different at the 0·05 level among cell number in the three ages by multiple comparisons using Scheffe’s method.
Micro‐hairs usually consist of two cells (a cap cell and a basal cell), but most cap cells are easily broken and lost by clearing treatment because of very thin walls even in mature leaf blades.
C, number of all cells; S, number of silicified cells.
Fig. 11. Frequency of silicified cells among all epidermal cell types in leaf blades of Sasa veitchii of three different ages (measured area = 0·3 mm2). Forty‐five sections from 15 leaves were measured. Adaxial epidermis (open circles), abaxial epidermis (closed circles). Bars indicate 95 % confidence intervals.
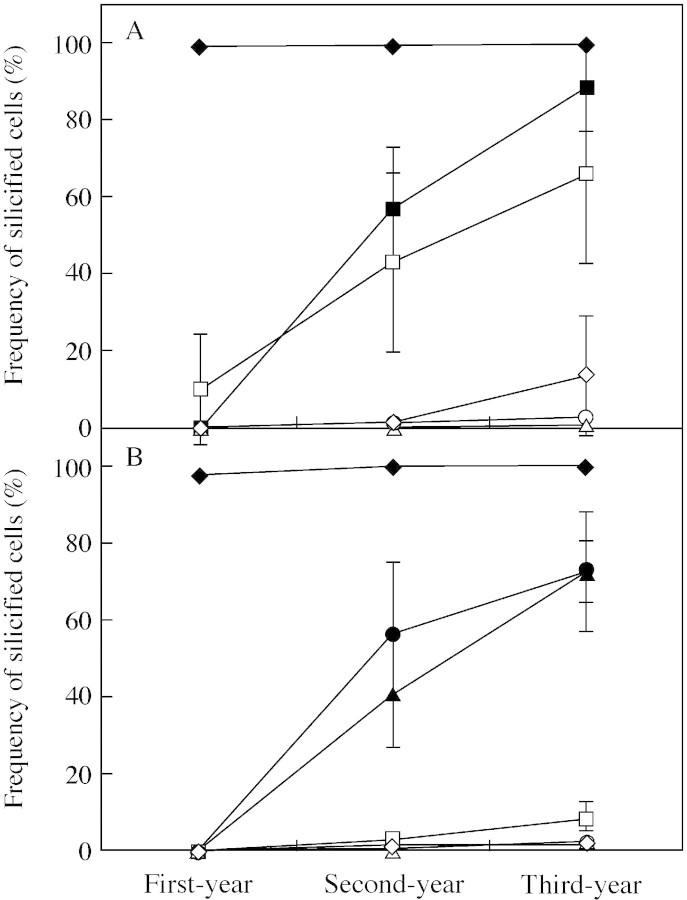
Among the eight types of epidermal cells, frequency of silicified cells was quite different (Table 1). In first‐year leaves, silicified cells were observed in five cell types (cork cells, guard cells, micro‐hairs, silica cells and subsidiary cells), but not in the other three types (bulliform cells, cork cells and prickle hairs). Silica cells were most frequently (100 %) silicified in both the adaxial and abaxial epidermises (Table 1). Other cell types were silicified less frequently (Table 1). Adaxial guard cells (10 %) were more frequently silicified than abaxial guard cells (0·1 %). Adaxial bulliform cells, cork cells and subsidiary cells were not silicified (Table 1). In the abaxial epidermis, <0.5 % of cork cells, guard cells, micro‐hairs and subsidiary cells were silicified, and prickle hairs were not silicified (Table 1). Long cells in both the adaxial and abaxial epidermises were not silicified (Table 1). Thus, these results indicate that in first‐year leaves only a few cell types show signs of silicification.
Comparisons of silicified cell frequencies among leaves of three different ages showed that the silica deposition process after leaf expansion was quite different among the eight cell types. Silica cells were silicified in the first year, and the frequency was not significantly different among the three leaf ages (Table 1 and Fig. 12). This indicates that silica cells cease silica deposition when the leaves becomemature. The other seven cell types were silicified after the completion of leaf expansion. In the adaxial epidermis, the frequency of silicified bulliform cells increased progressively year by year (Table 1 and Fig. 12), the values being 57 % in second‐year leaves and 88 % in third‐year leaves. Guard cells behaved in a similar way (Table 1 and Fig. 12), the corresponding values being 43 % and 66 % in second‐year and third‐year leaves, respectively. These frequencies were variable (0–100 %) in all the ages of leaf, but in the abaxial epidermises they were consistently low (<9 %). In the abaxial epidermis, the frequency of silicified micro‐hairs and prickle hairs increased progressively as leaves aged (Table 1 and Fig. 12), the values being, respectively, 41 % and 57 % in second‐year leaves and 72 % and 73 % in third‐year leaves. In contrast, the frequency of silicified cells in four other cell types (cork cells, guard cells, long cells and subsidiary cells) increased little as leaves aged. Few subsidiary cells on the adaxial side silicified in first‐ and second‐year leaves (0 and 1·1 %, respectively), and <2 % on the abaxial side silicified in leaves of any age, but in third‐year leaves the frequency of those that silicified on the adaxial side inexplicably increased to 14 %. Cork cells and long cells silicified at very low frequencies (<3 %) in both adaxial and abaxial epidermises irrespective of leaf age. Thus, these results indicate that the silica deposition process in the epidermis of expanded leaves has two different patterns. Silica deposition is rapid in bulliform cells, micro‐hairs and prickle hairs and slow in cork cells, guard cells, long cells and subsidiary cells.
Fig. 12. Frequency of silicified cells according to epidermal cell types in leaf blades of Sasa veitchii of three different ages (measured area = 0·3 mm2). Forty‐five sections from 15 leaves were measured. (A) Adaxial epidermis; (B) abaxial epidermis. Bulliform cell, closed square; cork cell, open circle; guard cell, open square; long cell, open triangle; micro‐hair, closed triangle; prickle hair, closed circle; silica cell, closed diamond; subsidiary cell, open diamond). Bars indicate 95 % confidence intervals.
DISCUSSION
Silica deposition in mesophyll cells
In the mesophyll of Sasa veitchii, there was little silica deposition in chlorenchyma cells irrespective of leaf age. Low silica deposition in chlorenchyma cells has been reported in mature leaves of other grasses (Yoshida et al., 1962; Hayward and Parry, 1973; Sakai and Sanford, 1984) and in young leaves of bamboo, such as Pleioblastus chino (Motomura et al., 2000). Since silica deposition was low in third‐year leaves of S. veitchii, it can be concluded that the chlorenchyma cells do not actively accumulate silica throughout their life. In contrast, fusoid cells deposited increasing amounts of silica as they aged. Fusoid cells are characteristically found only in bamboos (Ellis, 1987; Soderstrom and Ellis, 1987), and have been considered as aerenchyma tissues (Takenouchi, 1932). Several authors have shown that fusoid cells degenerate when leaves mature, producing large intercellular spaces between them (Page, 1947; Metcalfe, 1956). As water usually transpires directly from intercellular spaces in the mesophyll, the progressive silica deposition in the fusoid cells after leaf expansion clearly indicates that silica deposition is not a result of physiological activity of the cell but is the result of water uptake by these cells.
Silica deposition in epidermal cells
The epidermis is usually characterized by dense silica deposition in Poaceae. The observation of epidermal cells by SEM–EDXA in the present study revealed various levels of silica deposition from quite sparse to very dense. Among the eight epidermal cell types in S. veitchii, the frequency of cells with dense silica deposits (silicified cells) differed depending on cell type. Only cells with a dense silica deposition were able to be recognized as silicified cells under the light microscope with the clearing method used; those with moderate or inconspicuous deposits were not.
Although there are several studies that report silica deposition in epidermal cells of grasses and bamboos, continued silica deposition after leaf maturation has never been reported. Analysing the distribution of silica in mature leaves of three different ages revealed some new findings. Cork cells, guard cells, long cells and subsidiary cells consistently deposited little silica in mature leaves of S. veitchii. Long cells act as conducting cells in the epidermis and are characterized by having numerous pits in their lateral walls (Sangster, 1970). Guard cells and subsidiary cells form part of the stomatal complex. Although the role of cork cells is still unknown (Fahn, 1990), they usually occur in the epidermis of grasses and bamboos. It is known that dense silica deposition in these four cell types occurs sporadically in the epidermis of grasses and bamboos. As a small amount of water evaporates from the surface of the epidermis, the small silica deposits in these cells do not contradict the hypothesis that silica deposition is a result of water uptake. However, bulliform cells, micro‐hairs and prickle hairs in the epidermis continue to deposit large amounts of silica even after leaf maturation. The functions of bulliform cells are considered to be leaf opening and leaf rolling during, respectively, dry conditions and water storage (Esau, 1965). Micro‐hairs are thought to act as either salt glands or secretory hairs (Thomson et al., 1988). Prickle hairs may play a role in protecting the leaf surface. As dense silica deposition in other epidermal cell types would interfere with the transport of water and nutrients and with gas exchange within leaves, large silica deposits in these cell types in mature leaves indicate that the silica deposition is dependent largely on the physiological significance of cell types.
CONCLUSIONS
There are two hypotheses concerning silica deposition in Poaceae. First, silica deposition occurs passively as a result of water uptake by plants (Frey‐Wyssling, 1930; Yoshida et al., 1962; Jones and Handreck, 1967). Second, silica deposition is controlled positively by plants (Jones and Handreck, 1967; McNaughton et al., 1985; Cid et al., 1989; Belanger et al., 1995; Marschner, 1995). If silica deposition is a result of water uptake, dense silica deposition could be expected in mesophyll cells but not in epidermal cells, as a large volume of uptake‐water evaporates directly from the mesophyll through stomata. In the mesophyll of Sasa veitchii, fusoid cells deposited increasing amounts of silica as cells aged, but chlorenchyma cells did not. Thus, silica deposition in these cells in mature leaves should be a result of water uptake by plants. However, as epidermal cells also deposit large amounts of silica after leaf maturation, silica deposition must be associated with other factors. Among epidermal cell types, only silica cells deposit silica during the developmental process of leaves and not after maturation. It is, therefore, possible that silica deposition in epidermal cells is positively controlled by plants. In the epidermis of bamboo leaves with long life‐spans, bulliform cells, micro‐hairs and prickle hairs deposited large amounts of silica continuously after leaf maturation. The mechanism of silica deposition cannot be explained by just one of the two hypotheses, but can be explained by both depending on cell type. The present study has not resolved how the positive control of silica deposition occurs. It would be necessary to observe the silica deposition process cytologically in order to clarify the physiological significance of silicification in Poaceae.
ACKNOWLEDGEMENTS
We thank the Forestry and Forest Products Research Institute of Japan for providing facilities, and Dr Koji Yonekura and Mr Nabin Acharya, Tohoku University, Sendai, Japan for critically reviewing the manuscript prior to publication.
Received: 2 July 2003;; Returned for revision: 24 September 2003; Accepted: 11 November 2003, Published electronically: 26 January 2004
References
- BalastaMLFC, Perez CM, Juliano BO, Villareal CP, Lott JNA, Roxas DB.1989. Effects of silica level on some properties of Oryza sativa straw and hull. Canadian Journal of Botany 67: 2356–2363. [Google Scholar]
- BelangerRR, Bowen PA, Ehret DL, Menzies JG.1995. Soluble silicon. Its role in crop and disease management of greenhouse crops. Plant Disease 79: 329–336. [Google Scholar]
- CidMS, Detling JK, Brizuela MA, Whicker AD.1989. Patterns in grass silicification: response to grazing history and defoliation. Oecologia 80: 268–271. [DOI] [PubMed] [Google Scholar]
- EllisRP.1979. A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 12: 641–671. [Google Scholar]
- EllisRP.1987. A review of comparative leaf blade anatomy in the systematics of the Poaceae: the past twenty‐five years. In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME, eds. Grass systematics and evolution Washington, DC: Smithsonian Institution Press, 3–10. [Google Scholar]
- EpsteinE.1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50: 641–664. [DOI] [PubMed] [Google Scholar]
- EsauK.1965.Plant anatomy 2nd edn. New York: John Wiley and Sons. [Google Scholar]
- FahnA.1990.Plant anatomy 4nd edn. Oxford: Pergamon Press. [Google Scholar]
- Frey‐WysslingA.1930. Vergleich Zwischen der Ausscheidung von Kieselsäure und Kalziumsalzen in der Pflanze. Berichte der Deutschen Botanischen Gesellschaft 48: 184–191. [Google Scholar]
- HaywardDM, Parry DW.1973. Electron‐probe microanalysis studies of silica distribution in barley (Hordeum sativum L.). Annals of Botany 37: 579–591. [Google Scholar]
- HodsonMJ, Sangster AG.1990. Techniques for the microanalysis of higher plants with particular reference to silicon in cryofixed wheat tissues. Scanning Microscopy 4: 407–418. [Google Scholar]
- JonesLHP, Handreck KA.1967. Silica in soils, plants, and animals. Advances in Agronomy 19: 107–149. [Google Scholar]
- KanekoS.1995. Seasonal change of nutrient concentrations in Phyllostachys bambusoides and Phyllostachys pubescens Bamboo Journal 13: 27–33 [in Japanese]. [Google Scholar]
- KaufmanPB, Dayanandan P, Takeoka Y, Bigelow WC, Jones JD, Iler R.1981. Silica in shoots of higher plants. In: Simpson TL, Volcani BE, eds. Silicon and siliceous structures in biological systems New York: Springer, 409–449. [Google Scholar]
- McNaughtonSJ, Tarrants JT, McNaughton MM, Davis RH.1985. Silica as a defense against herbivory and a growth promoter in African grasses. Ecology 66: 528–535. [Google Scholar]
- MaJF, Takahashi E.2002.Soil, fertilizer, and plant silicon research in Japan. Amsterdam: Elsevier Science. [Google Scholar]
- MarschnerH.1995.Mineral nutrition of higher plants. San Diego: Academic Press. [Google Scholar]
- MetcalfeCR.1956. Some thoughts on the structure of bamboo leaves. Botanical Magazine, Tokyo 69: 391–400. [Google Scholar]
- MetcalfeCR.1960.Anatomy of the monocotyledons. I. Gramineae. Oxford: Clarendon Press. [Google Scholar]
- MotomuraH, Fujii T, Suzuki M.2000. Distribution of silicified cells in the leaf blades of Pleioblastus chino (Franchet et Savatier) Makino (Bambusoideae). Annals of Botany 85: 751–757. [Google Scholar]
- MotomuraH, Mita N, Suzuki M.2002. Silica accumulation in long‐lived leaves of Sasa veitchii (Carrière) Rehder (Poaceae: Bambusoideae). Annals of Botany 90: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PageVM.1947. Leaf anatomy of Streptochaeta and the relation of this genus to the bamboos. Bulletin of the Torrey Botanical Club 74: 232–239. [Google Scholar]
- RavenJA.1983. The transport and function of silicon in plants. Biological Review 58: 179–207. [Google Scholar]
- SakaiWS, Sanford WG.1984. A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscopy and energy dispersive X‐ray analysis. American Journal of Botany 71: 1315–1322. [Google Scholar]
- SangsterAG.1970. Intracellular silica deposition in mature and senescent leaves of Sieglingia decumbens (L.) Bernh. Annals of Botany 34: 557–570. [Google Scholar]
- SoderstromTR, Ellis RP.1987. The position of bamboo genera and allies in a system of grass classification. In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME, eds. Grass systematics and evolution Washington, DC: Smithsonian Institution Press, 225–238. [Google Scholar]
- TakenouchiY.1932.The study on bamboos. Tokyo: Youkendo [in Japanese]. [Google Scholar]
- ThomsonWW, Faraday CD, Oross JW.1988. Salt glands. In: Baker DA, Hall JL, eds. Solute transport in plant cells and tissues Harlow: Longman, 498–537. [Google Scholar]
- UedaK, Ueda S.1961. Effect of silicic acid on bamboo‐growth. Bulletin of the Kyoto University Forests 33: 79–99 [in Japanese]. [Google Scholar]
- YoshidaS, Ohnishi Y, Kitagishi K.1962.Histochemistry of silicon in plant. II. Localization of silicon within rice tissues. Soil Science and Plant Nutrition 8: 36–41. [Google Scholar]