Abstract
• Background and Aims The importance of superficial root mats inside the forest floor for the nutrition of Amazonian rain forests has been extensively investigated. The present study was aimed at assessing the function of a root mat adherent to decomposing organic material observed in Eucalyptus plantations.
• Methods The development of the root mat was studied through micromorphological observations of thin litter sections, and the influence of soil microtopography and soil water repellency on root mat biomass was assessed in situ on an area of 5 m2. In addition, input–output budgets of nutrients within the forest floor were established from measurements of litterfall, dissolved nutrients in gravitational solutions, and forest floor nutrient contents.
• Key Findings The amounts of nutrients released during litter decay in this ecosystem during the period of study were, on average, 46, 3, 4, 19 and 17 kg ha–1 year–1 for N, P, K, Ca and Mg, respectively. The simultaneous measurements of the chemical composition of throughfall solutions and leachates beneath the forest floor showed a very quick uptake of nutrients by the root mat during the decomposition processes. Indeed, the solutions did not become noticeably enriched in nutrients during their passage through the holorganic layer, despite large amounts of elements being released during litter decay. The root mat biomass decreased significantly during the dry season, and a preferential development in microdepressions at the soil surface was observed. A strong water repellency observed in these depressions might enhance the ability of the roots to take up water and nutrients during the dry periods.
• Conclusions The root mat was active throughout the year to catch the flux of nutrients from the biodegradation of the forest floor, preventing the transfer of dissolved nutrients toward deeper soil horizons. This mechanism is involved in the successful adaptation of this Eucalyptus hybrid in areas covered by ‘climacic’ savannas in Congo.
Key words: Root mat, Eucalyptus, nutrition, Africa, soil solution, water repellency, biogeochemistry, tropical soil, tropical forest, plantation
INTRODUCTION
In forest ecosystems, and particularly under tropical climates, humus has a crucial function in biological cycling. Indeed, the great majority of tropical soils are highly weathered with only residual minerals which are unable to provide soil solutions with the amounts of nutrients required for plant nutrition. In these conditions, only atmospheric inputs and an intense and efficient biological cycling make it possible to balance the pool of bio‐available elements. Dead organic matter is, therefore, even more than anywhere else, a basic component of soil fertility, being the most favourable support for biological activity. The whole trophic chain is developed from this organic matter, a source of energy for micro‐organisms and a source of nutrients for plants. Ecologists have long observed that tropical forests ‘live from their humus’ (Jordan, 1985; Mabberley, 1992). Despite a global net primary production of zero, growing trees require large amounts of nutrients, provided mainly by the biodegradation of organic matter.
The work reported here concerns the ability of alien plants in a given ecosystem to develop an appropriate strategy that allows them to grow on tropical soils poor in nutrient reserves. In Eucalyptus stands recently planted on the ferrallitic sandy soils of the savanna of the Congolese coast, a dense root mat was observed above the mineral soil, adherent to the fragments of forest floor in the process of decomposition. Various experiments were carried out to assess the function of this root mat for Eucalyptus nutrition. The objective was to test the following hypothesis: that the dense mat of fine roots above the mineral soil in these stands enables the trees to take up efficiently the nutrients brought by throughfall or released during litter decay, these being the major nutrient sources in this ecosystem.
MATERIALS AND METHODS
Ecology
Situation.
The Eucalyptus stand is located on a plateau approx. 40 km to the north of Pointe‐Noire, at an altitude of approx. 80 m and 10 km from the sea. The climate in this region is characterized by air humidity averaging 85 % with low seasonal variation (2 %), a mean annual rainfall of 1200 mm with a marked dry season between May and September (Fig. 1). The average annual temperature is 25 °C with seasonal variations of approx. 5 °C.
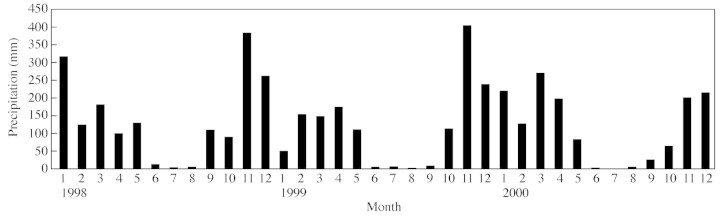
Fig. 1. Time course of rainfall at the site of Kondi over the three years of monitoring.
The soils.
The soils are Ferralic Arenosols (FAO classification) developed on thick depositions of sediments of continental origin dating from Plio‐Pleistocene. They are very deep (several metres), with a high textural homogeneity (sand content >85 %), a very simplified mineralogy (quartz, kaolonite, oxyhydroxides) (Nzila, 2001), an acidic pH (between 4·5 and 5), and a strong chemical paucity (CEC lower than 0·6 cmolc kg–1, very low amounts of ‘exchangeable bases’, generally lower than 0·2 cmolc kg–1) (Table 1).
Table 1.
Main soil characteristics in the Eucalyptus stand (reference to air‐dry material except for granulometry and total analysis expressed for material dried at 105 °C)
| Granulometry | Total elements * | ||||||||||
| Soil layer (cm) | Clay (%) | Silt (%) | Sand (%) | Organic material (%) | Total N (%) | C/N | CaO (‰) | MgO (‰) | K2O (‰) | P (‰) | Al2O3 (%) |
| A11 0–5 | 7·7 | 2·1 | 87·7 | 1·14 | 0·47 | 14·0 | 0·10 | 0·19 | 0·18 | 0·24 | 2·39 |
| A12 5–50 | 6·5 | 2·1 | 89·5 | 0·66 | 0·31 | 12·3 | 0·12 | 0·17 | 0·17 | 0·14 | 2·51 |
| B1 50–80 | 9·8 | 2·2 | 86·7 | 0·36 | 0·18 | 11·7 | 0·10 | 0·21 | 0·23 | 0·15 | 3·67 |
| B21 80–200 | 10·2 | 2·4 | 86·2 | 0·19 | 0·10 | 11·0 | 0·10 | 0·23 | 0·30 | 0·19 | 3·49 |
| B22 200–600 | 11·3 | 2·7 | 85·1 | 0·14 | 0·11 | 8·1 | 0·11 | 0·24 | 0·35 | 0·16 | 4·20 |
| Exchangeable elements† | |||||||||||
| Soil layer (cm) | pH(eau) | K (cmolc kg–1) | Ca (cmolc kg–1) | Mg (cmolc kg–1) | Mn (cmolc kg–1) | Na (cmolc kg–1) | Al (cmolc kg–1) | BC | CEC | BC/CEC (%) | Available P‡ (‰) |
| A11 0–5 | 4·8 | 0·03 | 0·11 | 0·08 | 0·01 | 0·04 | 0·24 | 0·26 | 0·53 | 49 | 0·05 |
| A12 5–50 | 4·7 | 0·02 | 0·08 | 0·03 | 0·01 | 0·01 | 0·17 | 0·14 | 0·29 | 48 | 0·02 |
| B1 50–80 | 4·9 | 0·01 | 0·08 | 0·03 | 0·01 | 0·00 | 0·14 | 0·12 | 0·31 | 39 | 0·03 |
| B21 80–200 | 5·2 | 0·01 | 0·09 | 0·02 | 0·01 | 0·01 | 0·11 | 0·13 | 0·28 | 46 | 0·02 |
| B22 200–600 | 5·0 | 0·02 | 0·08 | 0·03 | 0·01 | 0·01 | 0·12 | 0·14 | 0·32 | 44 | 0·03 |
* Acid digestion and ICP determination of elements.
† Cobalti‐hexamine extraction, ICP determination of cations.
‡ Duchaufour and Bonneau (1959) methodology.
The stand.
The experimental design was incorporated in a 6‐year‐old clonal stand of Eucalyptus. The stand was planted in January 1992 in a 15‐ha area of savanna at a density of 530 trees per hectare. A starter fertilization (150 g plant–1 of NPK 13 : 13 : 21) was applied, and chemical weeding (with glyphosate) carried out over the first two years after planting led to the lack of understorey. When the stand was 6 years old, its mean height was 26 m, the mean circumference at breast height was 58 cm and its volume over‐bark was 158 m3 ha–1. The clone studied was a Eucalyptus hybrid originating from natural crosses between two or three individuals of Eucalyptus alba Reinw. ex Blume (mother trees) and a group of Eucalyptus hybrids poorly identified from a Brazilian arboretum (father trees). This clone has been widely planted in commercial plantations in Congo. The optimal age of harvesting is 7 years, but logging occurs frequently between 6–9 years, according to changes in the pulpwood market.
Experimental design and observations
An experimental design was set up in autumn 1997 in this stand to study the biogeochemical cycles of nutrients. The pools in the soil and the plants were quantified and the fluxes in solution (rainfall, throughfall, stemflow, transfer in gravitational solutions beneath the forest floor), and in solid form (litterfall), were measured from January 1998 to December 2000.
Experimental area
A comparison of soil texture and mineralogy between the experimental Eucalyptus stand and an adjacent area of native savanna (situated 500 m apart) showed that soils were derived from the same parent material (Nzila, 2001). Even though sampling the top soil at various scales indicated that spatial variability of soil chemical properties was low in the whole area, stand inventories showed a slight gradient of tree growth within the 15 ha, according to the topography (Laclau, 2001). Therefore, three areas of 5 ha (hereafter called ‘plots’) were distinguished in the stand, perpendicular to the slope: plot 1 was situated in an area with a gentle slope (about 2 %), whereas plots 2 and 3 were in a flat area at the bottom of the slope. The studies of litterfall, dissolved nutrients in gravitational solutions, forest floor decomposition, and microscopic observations of root mat inside the forest floor presented in this article were performed in plot 1, to enable comparisons in the same growing conditions. The seasonal variability of root mat biomass inside the forest floor was measured in the whole 15‐ha area, distinguishing the three plots. A flat area was selected in plot 3 to study the effects of soil microtopography on spatial distribution of root mat biomass.
Litterfall and nutrient returns to soil
Litterfall was collected every month from 15 litter traps (0·75 m × 0·75 m) randomly distributed in plot 1. The different components of the litter were separated by hand‐picking: leaves, branches, bark, and ‘miscellaneous’ (occasional components such as fruits, or impossible to identify). After drying at 65 °C until constant weight, samples were ground to 1 mm and mixed.
Nutrient fluxes in throughfall, stemflow, surface run‐off and solutions beneath the forest floor
A complete description of the experimental design set up in plot 1 of this stand is given by Laclau et al. (2003a, c). In brief, three replicates of three PVC gutters (16 cm × 2 m) were installed at various distances from the trees to sample throughfall solutions (Th). Stemflow solutions (Sf) were collected by polyethylene collars positioned at the bottom of the trunk of ten trees, representative of the distribution of the basal area in the stand. The surface run‐off (R) was estimated from five frames of 1 m2 driven into the soil down to a depth of about 5 cm. The downhill side of the frame was perforated at the level of the soil and connected to a tube to collect the run‐off solutions in a container situated in a pit downhill, and protected from light and from sharp variations in temperature (Casenave and Valentin, 1989). These frames were located to sample run‐off at various distances from the planting row.
Gravitational solutions were collected at the litter layer level by four sets of nine thin, tensionless lysimeters (40 × 2·5 cm) to limit the disturbance of the forest floor. The average distance between the replicates of lysimeters was 15 m. They were introduced from four pits which were carefully backfilled after installation with the horizons in their natural arrangement. Solutions were collected in polyethylene containers situated downhill in closed pits. The nutrient fluxes in the leachates beneath the forest floor were calculated by multiplying the flux of water (Th + Sf – R) by the concentration of elements in the solutions collected.
Forest floor decomposition
Samples from the forest floor were collected after the active mineralization phase, at the end of the rainy season, in 1998, 1999 and 2000. Twenty samples randomly located between two rows were collected in 1998, using a 0·25 m2 frame. The following year, the forest floor was sampled in an adjacent inter‐row, keeping the same position relative to trees (this being an important factor of the holorganic layer structure). The samples were sorted into the following fractions: intact leaves; partly decomposed leaves; bark; dead branches; fruits; and ‘decomposed leaves + roots’ (DLR). This last fraction was sorted by hand‐picking to exclude the maximum amount of Eucalyptus roots adherent to decomposing leaves. However, as the root mat was very dense, it was not possible to exclude all the fine roots. The biomass of fine roots remaining in this fraction was subtracted from the difference in calcium concentration between the ‘decomposing leaves only’ fraction and the DLR fraction. The chemical analyses showed that calcium was the element whose concentrations differed most between the fine roots and the decomposing leaves (being poorer in calcium). The equation used was:
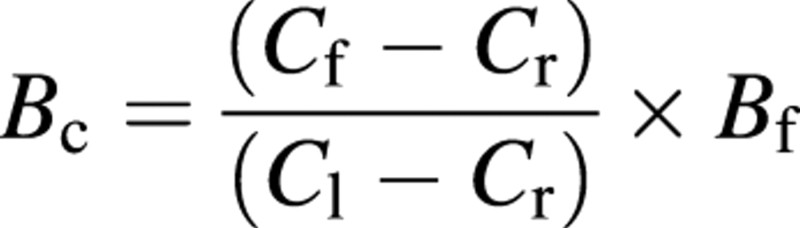
where Bc is the biomass of the corrected DLR fraction (roots excluded), Cf, Cr and Cl are the concentrations of calcium in the DLR fraction, root‐only samples and leaf‐only samples, respectively, and Bf is the biomass of the DLR fraction. Samples were dried until they reached constant weight (65 °C), then ground and mixed. Chemical analyses were performed individually for all the samples collected each year (20 sampling areas × 6 fractions).
The annual rate of litter decay was quantified by comparing the amount of forest floor material over an interval of 1 year, taking into account the amount of litterfall during the same period. The amount (A) of decomposed forest floor is given by the equation:
A = FF1 + L1–2 – FF2(1)
where FF1, FF2 and L1–2 are, respectively, the amounts of forest floor at dates t1 and t2, and the amount of litterfall between t1 and t2. This equation was used to calculate the amount of decomposed biomass as well as to assess the net amount of nutrients released during the period. Nutrients released during fine root decomposition in the forest floor were not taken into account in eqn (1), since A was assessed by the difference between the amounts of nutrients in the forest floor, excluding roots.
Several types of coefficients have been used in the literature to estimate the rate of litter decay in forest stands (Bernhard‐Reversat, 1982). For young stands in which the forest floor is accumulating, the rate of litter decay can be estimated by the coefficient:
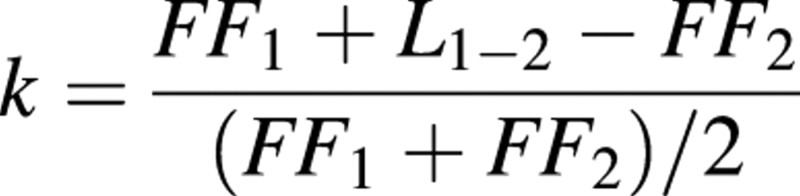
The root mat inside the forest floor
Observation at various scales.
The root mat was examined in the field and in the laboratory in terms of soil micromorphology. The objective was to describe at various scales the morphological characteristics of fine root development inside the structure of decomposing plant material. Thin sections of humus were obtained as follows. Five undisturbed fresh humus samples (i.e. forest floor and upper organo‐mineral layer) were collected in the field in Kubiena boxes (6 × 5 × 9 cm aluminium boxes with a double bottom). The samples were immersed in acetone for 1 week, during which the acetone was changed three times. Samples were then transferred into a desiccator and completely impregnated under vacuum with an epoxy resin (Norsodyne type) according to the method developed by Fitpatrick and Gudmundsson (1978). Resin polymerization took approx. 1 month. Impregnated soil blocks were then finely polished on one side and stuck on a 5 × 10 cm glass slide. The block was then cut with a diamond saw to obtain a 2–3 mm thick layer, which was manually reduced to a thickness of 25 µm using abrasive powder (the thickness was monitored under a polarizing microscope, using quartz which gives a grey colour). The grey colour of quartz is a mineralogical standard showing that the thickness is correct. The thin sections of humus were observed under a polarizing binocular microscope at different magnifications.
Seasonal variability of biomass.
In March 1999, 18 trees with a basal area close to the mean basal area of the stand were selected, six trees in each of the three plots. Border trees and those located close to a missing tree were not selected so as to obtain a sample as homogenous as possible. Every 2 months, one tree out of the six individuals identified in each plot was selected randomly. Four positions were sampled systematically with a 0·25 m2 frame: the bottom of the selected tree, one‐ and two‐thirds of the distance between the tree and the middle of the inter‐row, and the middle of the inter‐row. The roots located above the mineral soil, inside the decomposing forest floor, were separated by hand‐picking and dried at 65 °C until they reached constant weight.
Relationships between microtopography, soil water repellency and the spatial distribution of the root mat.
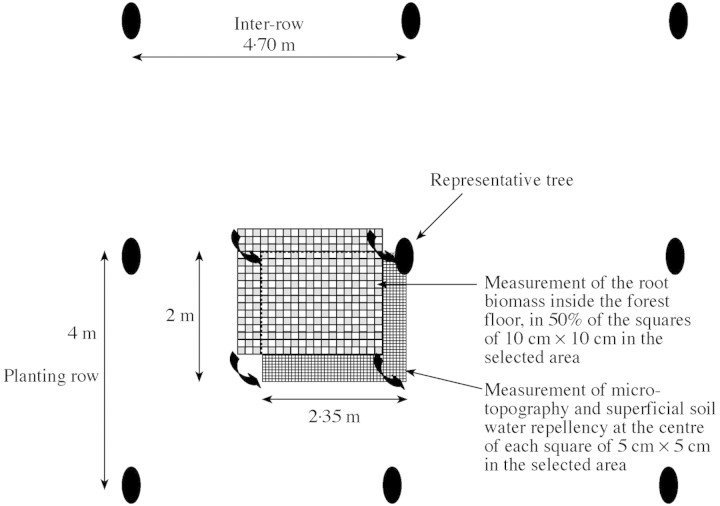
A study was carried out in August 1999 to investigate the parameters likely to explain the spatial distribution of the root mat. The experiment was conducted during the middle of the dry season to experience the highest levels of soil water repellency, which manifests itself when the water content of the soil drops below a critical level. Measurements were made on a soil area of 5 m2 corresponding to a rectangle between the bottom of a tree of average basal area, the middle of the inter‐row and half of the distance up to the next tree in the planting row (Fig. 2).
Fig. 2. Schematic representation of the location of the measurements of the root mat biomass inside the forest floor, as well as the soil microtopography and its superficial water repellency.
Roots above the mineral soil were systematically sampled using a 10 cm × 10 cm frame in 50 % of the squares covering the whole area of 5 m2. They were separated from the forest floor by hand‐picking. The root samples were dried at 65 °C until they reached constant weight, and then weighed.
After having carefully removed the forest floor above the mineral soil, the 5 m2 area selected was divided into 5 × 5 cm squares to measure the spatial variability of microtopography and superficial water repellency. The microtopography was determined by measuring the height between the centre of all the 25 cm2 squares and a reference plane marked by a network of horizontal threads.
Soil water repellency was also measured systematically in all the 25 cm2 squares identified in the 5 m2 area, according to a classical method. The alcohol percentage test was applied by preparing a series of aqueous ethanol solutions ranging in concentration between 0 % and 36 %. In this test, drops of the various solutions were placed at the soil surface, and the degree of actual water repellency was then defined from the alcohol percentage of the weakest solution that still penetrated the soil within 3 s or less. At this concentration, the aqueous ethanol drop has sufficiently low surface tension to overcome the surface water repellency restriction to infiltration. A score of soil hydrophobicity between 1 (non‐water repellent) and 7 (extremely water repellent) was given. The alcohol percentages corresponding to the scores 1, 2, 3, 4, 5, 6 and 7 were 0 %, 3 %, 5 %, 8·5 %, 13 %, 24 % and 36 %, respectively. These concentrations were chosen because they were in agreement with those used in studies elsewhere (Crockford et al., 1991; Doerr et al., 1998) and covered the range of degrees of hydrophobicity encountered in this study.
Nutrient uptake by the root mat inside the forest floor.
The nutrient uptake by the root mat was assessed from an input–output budget, according to the following equation:
U = I + A – DFF – R(2)
where U is the uptake by the root mat inside the holorganic layer, I is the nutrient input above the forest floor with throughfall and stemflow solutions, A is the amount of nutrients released during the decomposition of the forest floor, DFF is the flux of elements in drainage solutions beneath the forest floor and R is the amount of nutrients in surface run‐off.
The chemical analyses performed did not take into account the amounts of elements drained in an organic form in eqn (2). A correction from the concentration of dissolved organic carbon (DOC) that was measured did not seem valid because the chemistry of the organic compounds leached was unknown. The calculations of root uptake inside the forest floor were, therefore, potentially overestimated by an amount corresponding to the organic phase leached. The flux of dissolved organic nitrogen from the forest floor into the mineral soil may represent a large proportion of total dissolved nitrogen in forest ecosystems (Kalbitz et al., 2000; Qualls et al., 2000), and organic phosphorus species have also been observed in leachates from Eucalyptus camaldulensis leaves (Baldwin, 1999). Fungal hyphae may also take up non‐negligible amounts of nutrients in the forest floor (Zeller, 2000). However, the influence of fungal hyphae uptake and microbial immobilization on the assessment of root uptake should be low. Equation (2) estimates root uptake from an input–output budget, and this study was performed at the end of a stand rotation when fungal and microbial biomass should be stabilized.
Chemical analyses
Solution analyses.
Samples were collected once a week: the volume was measured and an aliquot was taken to the laboratory and maintained at 4 °C. Samples collected were pooled proportionally to the volume collected every week to obtain monthly samples for chemical analyses. Three samples of throughfall, one composite sample of stemflow, one sample of run‐off solution, and four replicates of samples collected by lysimeters beneath the forest floor were analysed separately every month. The solutions were filtered (0·45 mm) and measurements of pH (HI 9321) and SO42– by colorimetry (ANA 8 Prolabo) were performed in Congo as quickly as possible. The samples were then acidified with H2SO4 and sent to the CIRAD laboratory in France where nitrate and ammonium were measured by colorimetry (Integral Plus; Alliance instruments), chloride was also measured by colorimetry (Technicon I) and total Si, P, K, Ca, Mg, Na, Al, Mn, Fe by ICP emission spectroscopy (Jobin‐Yvon 50). DOC was measured every month on a Shimadzu TOC 5050, using a composite sample of throughfall, stemflow and soil solution beneath the forest floor. Results presented here focus on the elements N, P, K, Ca and Mg.
Plant material.
Nitrogen was determined by thermal conductivity after combustion (FP‐428) and P, K, Ca, Mg, by a sequential spectrometer ICP (JY 24) after digestion by fluoridric acid and double calcinations. The ash content of root and forest floor samples was determined by combustion for 4 h at 450 °C. All biomass data presented here was corrected to eliminate the effect of remaining soil particles.
Statistical analysis
The GLM procedure of SAS software was used to analyse the variance of root mat biomass throughout the year. The statistical model used was
Bi,j,k = µ + Di + Pj + Lk + θi,j + θi,k + θj,k + ϵi,j,k
where Bi,j,k is the biomass of fine roots inside the forest floor at date i, in plot j and at the location k in the inter‐row; µ is the general mean; D, P and L account for the effects of date, plot and location in the inter‐row, respectively; θi,j the interaction between date i and plot j; θi,k the interaction between date i and location k; θj,k the interaction between plot j and location k; and ϵi,j,k the residual effect. Comparisons of class variables were based on the Newman–Keuls test.
In addition, one‐way ANOVA was used to determine differences in biomass and nutrient content of each fraction of the forest floor between the dates of sampling.
The cartography of soil microtopography, soil water repellency and root mat dry matter in the 5 m2 area studied was performed with the Splus software according to Akima’s method (Akima, 1978). Overlaying layers of data on the same plots provided a clear visualization of the spatial relationships among the variables.
RESULTS
Decomposition of the forest floor
A 40 % increase in forest floor biomass was observed in the stand between the ages of 6·5 and 8·5 years, representing a rate of about 2·5 Mg ha–1 year–1 (Table 2). This annual rate of accumulation was equal to the mean rate over the previous six years (Laclau, 2001). Litterfall started only at the end of the first year of growth, and the amount of forest floor 5·5 years later had reached 11·6 Mg ha–1. A significant accumulation of N, P, Ca and Mg was observed in the forest floor during the first year of monitoring, mainly in the ‘leaves’ and ‘dead branches’ fractions. By contrast, the amount of nutrients was roughly stable during the following year even though the biomass of forest floor increased largely.
Table 2.
Amounts (kg ha–1) and fluxes (kg ha–1 year–1) of organic matter and nutrients in the various fractions of the forest floor of the Eucalyptus stand at Kondi
| June 1998 to May 1999 | June 1999 to May 2000 | |||||||||
| Leaves | Bark | Branches | Miscellaneous | Total | Leaves | Bark | Branches | Miscellaneous | Total | |
| Biomass | ||||||||||
| FF1: initial amount | 5223a | 1400a | 4942a | 64a | 11628a | 5754b | 1768a | 6703b | 37b | 14262b |
| FF2: final amount | 5754b | 1768a | 6703b | 37b | 14262b | 5847b | 2259b | 8092b | 27b | 16226c |
| L1–2 (kg ha–1 year–1) | 4773 | 1308 | 1297 | 9 | 7386 | 4232 | 1109 | 1706 | 12 | 7058 |
| A (kg ha–1 year–1) | 4242 | 940 | –464 | 36 | 4752 | 4139 | 618 | 317 | 22 | 5094 |
| k (%) | 77 | 59 | – | 71 | 37 | 71 | 31 | 4 | 69 | 33 |
| N | ||||||||||
| FF1: initial amount | 58·9a | 6·8a | 17·5a | 0·5a | 83·7a | 68·6b | 10·1b | 29·9b | 0·3b | 108·8b |
| FF2: final amount | 68·6b | 10·1b | 29·9b | 0·3b | 108·8b | 70·1b | 12·5b | 26·4b | 0·0c | 109·0b |
| L1–2 (kg ha–1 year–1) | 48·1 | 7·0 | 4·6 | 0·0 | 59·7 | 43·5 | 7·0 | 6·5 | 0·2 | 57·2 |
| A (kg ha–1 year–1) | 38·4 | 3·7 | –7·8 | 0·2 | 34·6 | 42·0 | 4·6 | 10·0 | 0·5 | 57·0 |
| k (%) | 60 | 44 | – | 50 | 36 | 61 | 41 | 36 | 333 | 52 |
| P | ||||||||||
| FF1: initial amount | 2·6a | 0·3a | 0·8a | 0·0a | 3·7a | 2·9b | 0·4b | 1·2b | 0·0b | 4·5b |
| FF2: final amount | 2·9b | 0·4b | 1·2b | 0·0b | 4·5b | 3·0b | 0·4b | 1·6c | 0·0c | 5·0c |
| L1–2 (kg ha–1 year–1) | 3·1 | 0·3 | 0·4 | 0·0 | 3·8 | 2·9 | 0·3 | 0·7 | 0·0 | 3·9 |
| A (kg ha–1 year–1) | 2·8 | 0·2 | 0·0 | 0·0 | 3·0 | 2·8 | 0·3 | 0·3 | 0·0 | 3·4 |
| k (%) | 102 | 57 | 0 | – | 73 | 95 | 75 | 21 | – | 72 |
| K | ||||||||||
| FF1: initial amount | 3·4a | 0·7a | 1·8a | 0·1a | 6·0a | 3·3a | 0·5a | 1·9a | 0·0b | 5·7a |
| FF2: final amount | 3·3a | 0·5a | 1·9a | 0·0b | 5·7a | 2·7a | 0·6a | 1·8a | 0·0b | 5·1a |
| L1–2 (kg ha–1 year–1) | 2·8 | 0·3 | 0·3 | 0·0 | 3·4 | 3·0 | 0·4 | 0·2 | 0·0 | 3·6 |
| A (kg ha–1 year–1) | 2·9 | 0·5 | 0·2 | 0·1 | 3·7 | 3·6 | 0·3 | 0·3 | 0·0 | 4·2 |
| k (%) | 87 | 83 | 11 | 200 | 63 | 120 | 55 | 16 | – | 78 |
| Ca | ||||||||||
| FF1: initial amount | 21·5a | 3·7a | 9·3a | 0·2a | 34·6a | 26·3b | 4·6ab | 11·9b | 0·1b | 42·8b |
| FF2: final amount | 26·3b | 4·6ab | 11·9b | 0·1b | 42·8b | 24·1c | 5·3b | 11·0ab | 0·0c | 40·3b |
| L1–2 (kg ha–1 year–1) | 21·1 | 2·4 | 1·8 | 0·0 | 25·4 | 15·1 | 1·9 | 1·7 | 0·0 | 18·6 |
| A (kg ha–1 year–1) | 16·3 | 1·5 | –0·8 | 0·1 | 17·2 | 17·3 | 1·2 | 2·6 | 0·1 | 21·1 |
| k (%) | 68 | 36 | – | 67 | 44 | 69 | 24 | 23 | 200 | 51 |
| Mg | ||||||||||
| FF1: initial amount | 12·5a | 2·1a | 3·2a | 0·2a | 18·4a | 16·4b | 3·0b | 6·4b | 0·1b | 26·0b |
| FF2 : final amount | 16·4b | 3·0b | 6·4b | 0·1b | 26·0b | 14·0c | 3·3b | 6·5b | 0·0b | 23·8b |
| L1–2 (kg ha–1 year–1) | 17·5 | 1·5 | 0·9 | 0·0 | 19·9 | 17·2 | 1·3 | 1·1 | 0·0 | 19·6 |
| A (kg ha–1 year–1) | 13·6 | 0·6 | –2·3 | 0·1 | 12·3 | 19·6 | 1·0 | 1·0 | 0·1 | 21·8 |
| k (%) | 94 | 24 | – | 67 | 55 | 129 | 32 | 16 | 200 | 88 |
The letters a, b and c indicate significant differences (P < 0·05) between sampling dates according to Newman–Keuls test.
The decomposition coefficients (k) showed that the release of N and Ca occurred at a rate close to the weight loss during the first year of observation, whereas the rates of P, K and Mg release were quicker. During the following year, all the elements studied were released at a higher rate than mass loss. From age 6·5 to age 8·5 years, the amounts of N, P, K, Ca and Mg released during litter decay were on average 46, 3, 4, 19 and 17 kg ha–1 year–1, respectively. This flux mainly originated from leaf decomposition (>80 %).
Characteristics of the root mat
Observations.
In the field, the Eucalyptus forest floor appeared as a stratified layer with recently fallen litter (OL) in the upper part and increasingly decomposed material (OF) underneath. The OF layer was completely invaded by fine roots, especially during the wet season (Fig. 3).

Fig. 3. Macroscopic view of the root mat into the forest floor.
Under the microscope, the stratification of leaves at increasing stages of degradation is presented in Fig. 4A. Eucalyptus leaves still conserve their structure and have a brown colour in the upper part of the forest floor, while bleached parts appeared in the intermediate layer. White rot activity is characterized by bleaching of intercellular brown pigments and the almost complete degradation of the pecto‐cellulosic walls and conducting vessels (Reisinger et al., 1978). Bleached leaves invaded by mycelia were fragile and easily consumed by the meso‐ and microfauna whose activity was attested to by the presence of faeces, i.e. pellets from acarids, oribatid mites, enchytraeids, collembolas and diptera larvae (Fig. 4). These faeces were recognizable by their shape, size, colour and internal organization (holorganic material or organo‐mineral mixture) (Babel, 1985). The decomposition of the forest floor material was typical of a tropical mull with an OL layer containing limited or no degradation of leaves, and an OF layer (between 30 % and 70 % in volume) with bleached, degraded material with numerous pellets. Some mineral material appeared in places between the leaf layers (Fig. 4A and E).

Fig. 4. (A) Overall view of OL and OF layers. (B) Details of the OL layer. (C) Details of the bottom of the OL layer. (D) Details of the OF layer. Piece of branchwood being transformed. (E) Details of the interface between OF and A layers seen under polarized light. Brl, Brown leaf; Bll, bleached leaf (or part of it); Efr, Eucalyptus fine root; Bcz, branch cortical zone; Bwz, branch wood zone; Ef, enchytreid faeces; Of, oribatid faeces; Tf, termite faeces; q, mineral grain (quartz).
Fine roots were observed near the brown leaves (OL), but mainly in the OF layer near the bleached leaves (Fig. 4B and C). Fine roots were observed inside the plant material, for example inside the leaves (Fig. 4C), and inside branchwood pieces (Fig. 4D). A lot of fine roots were systematically observed at the limit between holorganic (OF) and organo‐mineral (A1) layers (Fig. 4E).
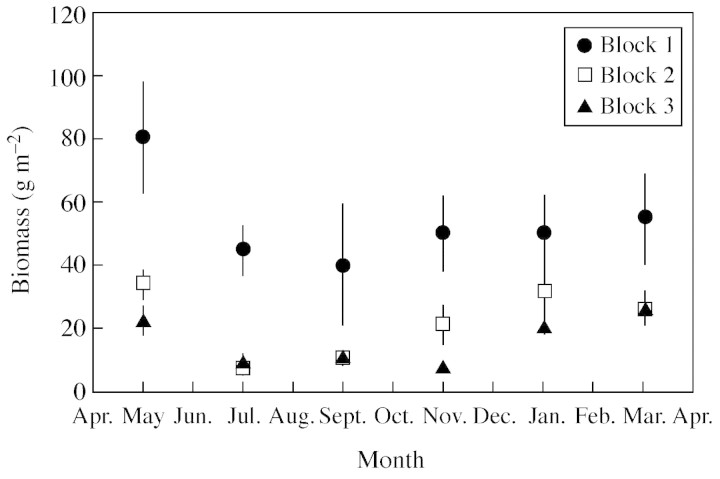
Seasonal variability of the root mat inside the forest floor.
The total mass of the root mat (living and recently dead roots) in this stand averaged 270 kg ha–1 of dry matter over the year (Fig. 5). It was highly dependent on the date of sampling (P < 0·05), and on the plot (P < 0·001), but the effect of the location in the inter‐row was non‐significant, as well as interactions between the factors (at the 5% threshold). The biomass of the root mat was significantly lower (P < 0·05) during the dry season (sampling in June and August) than at the end of the rainy season (sampling in April). Over the course of the year, it ranged from 490 kg ha–1 on average in plot 1 situated on a gentle slope, to 160 kg ha–1 on average in plots 2 and 3 at the bottom of the slope. It was about 75 % higher during the rainy season than during the dry season, taking into account that it was not possible to distinguish correctly living roots and recently dead roots, and that the proportion of the latter in the total biomass measured was probably higher during the dry season.
Fig. 5. Seasonal time course of the biomass of roots inside the forest floor. Mean values ± s.e. (n = 4).
Influence of microtopography and soil water repellency on the root mat localization.
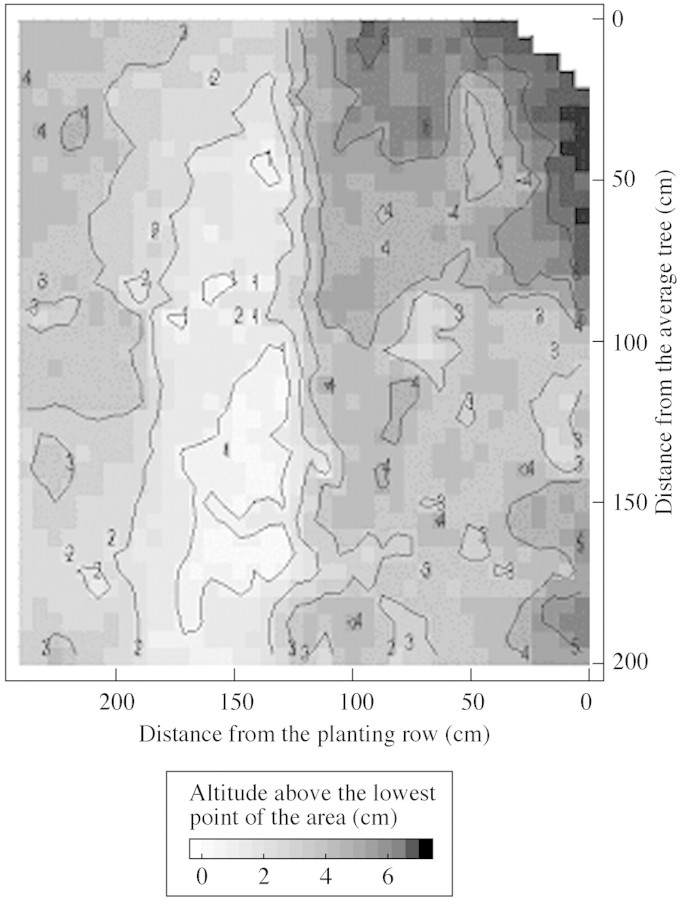
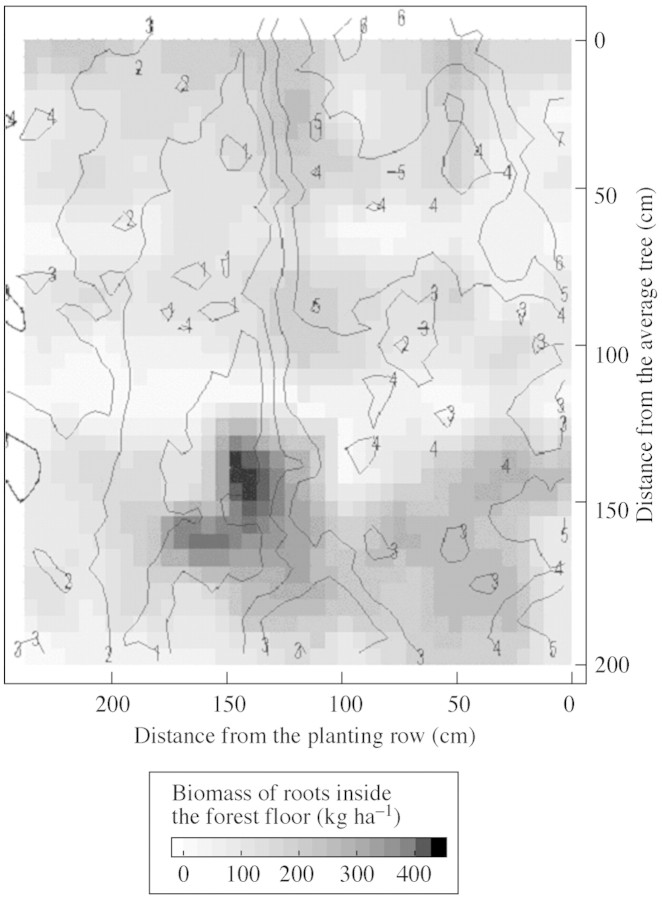
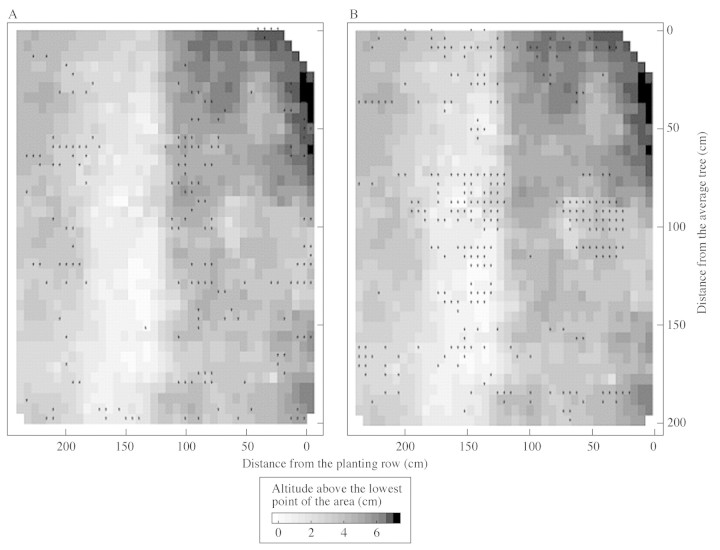
An area of ground depression corresponding to tractor wheel tracks was shown by topography measurements performed for the 5 m2 area studied. Local microdepressions close to the planting row were also observed (Fig. 6). The spatial distribution of the root mat during the dry season exhibited clear patterns: (a) root biomass inside the forest floor was higher in the area of greatest depression (Fig. 7), and (b) soil water repellency was also higher in the soil depression corresponding to tractor wheel tracks (Fig. 8). A similar trend was also observed for local microdepressions closer to the planting row (Fig. 8).
Fig. 6. Two‐dimensional representation of the microtopography of the study area.
Fig. 7. Contour lines indicating the microtopography of the area studied overlaying a two‐dimensional representation of the biomass of roots inside the forest floor.
Fig. 8. Marks of soil water repellency overlaying a two‐dimensional representation of the microtopography of the area. * Class of water repellency = 4 (8·5 % ethanol) in A and 6 (24 % ethanol) in B. The class was 5 (13 % ethanol) in most of the other squares of measurements in the study area. Scores lower than 4 were found only exceptionally.
Nutrient uptake by the root mat inside the forest floor.
The fluxes of most of the nutrients dissolved in gravitational solutions (throughfall + stemflow vs. run‐off + leachates) were modified little during their transfer through the forest floor (Table 3). The uptake by the root mat, calculated from eqn (2), represented, on average, from June 1998 to May 2000: 46, <1, 5, 27 and 16 kg ha–1 year–1 for N, P, K, Ca and Mg, respectively. Even if the fluxes of elements in an organic form (not taken into account here) probably led to overestimating the N uptake by the root mat, these results showed an intense uptake of nutrients in the decomposing forest floor.
Table 3.
Amounts of nutrients (kg ha–1 year–1) taken up by the superficial root mat during litter decay, estimated from eqn (2)
| From June 1998 to May 1999 | From June 1999 to May 2000 | |||||||||
| N | P | K | Ca | Mg | N | P | K | Ca | Mg | |
| Litter decay | 35 | 3·0 | 4 | 17 | 12 | 57 | 3·2 | 4 | 21 | 22 |
| Throughfall + stemflow | 3 | 1·2 | 6 | 17 | 4 | 4 | 1·1 | 7 | 15 | 3 |
| Run‐off | 0 | 0·0 | 0 | 0 | 0 | 0 | 0·0 | 0 | 0 | 0 |
| Leaching beneath the forest floor | 3 | 3·2 | 5 | 9 | 5 | 4 | 4·8 | 7 | 7 | 4 |
| Uptake inside the forest floor | 35 | 1·0 | 5 | 25 | 11 | 57 | –0·5 | 4 | 29 | 21 |
DISCUSSION
Litter decay
The biomass of dead branches represented about 50 % of the forest floor dry matter at the end of the stand rotation (Table 2). The annual accumulation of dead branches amounted to 1·5 Mg ha–1 year–1, which was approximately equivalent to the biomass of branches in litterfall during the same period. A slight underestimation of the amount of branches in litterfall, due to the shape of litter traps, might explain the lack of decomposition of the ‘dead branches’ fraction in the forest floor estimated during the first year of monitoring. Even if the litter traps were situated close to the soil so that branches would remain supported on the trap when they fell, some of these branches might have fallen outside. Large size litter‐traps are recommended to collect branches that do not break up on the trees before falling.
A strong increment in the N, Ca and Mg contents of the forest floor was observed during the first year of monitoring, whereas the amounts of these elements remained roughly stable in the second year. Changes in the decomposition stage of the ‘dead branches’ fraction between the two years might be involved in the nutrient accumulation dynamics observed for the whole forest floor. Similar decomposition rates were observed in other Eucalyptus stands in Congo (Bernhard‐Reversat, 1993; Nzila et al., 2002).
Studies in tropical rain forests suggest that root mats inside the forest floor are likely to influence the rates of litter decay. Cuevas and Medina (1988) observed in an Amazonian forest on oxisols that litter contact with the dense root mat significantly increased the rates of disappearance for biomass, Ca and Mg, as compared with litter lifted weekly from the root mat to avoid root attachment. They concluded that there must be a nutrient release mechanism mediated by these roots and/or their associated micro‐organisms. In an African forest growing on sandy, acidic and low‐phosphorus soils, Chuyong et al. (2002) showed differential effects of the root mat for mineralization rates of Mg, according to the abundance of ectomycorrhizal trees. They suggested that the active root mat of ectomycorrhizal trees that invaded the litter bags caused a greater sink of Mg, and that differences in mineralization rates of Mg between forest types was possibly related to an increase of polyphenol inhibition by the root mat of ectomycorrhizal trees. A broad range of fungal taxa form ectomycorrhizae with Eucalyptus species (Grove et al., 1996) and the litter is characterized by high polyphenol content (Chapuis‐Lardy et al., 2002). The influence of the root mat inside the forest floor on nutrient releases during litter decay deserves further investigation in these plantations.
Localization of the root mat
Very thick root mats inside forest litter have been described in undisturbed tropical forests growing on atypical soils such as giant podzols, but less dramatic root mats can be found beneath forests on more typical oxisols and ultisols (Montagnini and Buschbacher, 1989; Kingsbury and Kellman, 1997; Chuyong et al., 2002). Smith et al. (2002) found non‐significant differences in root mat biomass after forest conversion to tree plantations in lowland Amazonia, but the influence of root mats on the nutrition of tropical tree plantations has been little investigated.
In the present study, a large number of faeces attested to the activity of meso‐ and micro fauna during litter decay (Fig. 4). The development of the root mat inside the forest floor might be influenced by the activity of decomposers, e.g. white rot fungi excreted droplets rich in nutrients (Ca) which might represent a ‘trophic’ tropism for fine roots (Toutain, 1992, unpublished data in French Guyana forest). Solutions at the base of the forest floor were relatively concentrated in nutrients compared with soil solutions (Laclau et al., 2003a) and might also represent a ‘trophic’ tropism for fine roots. Studies of root mats in Amazonian forests have shown that root proliferation is stimulated by dead organic matter (St John, 1983; Cuevas and Medina, 1988), but Kingsbury and Kellman (1997) observed no evidence that low soil fertility contributes significantly to stimulating upward root proliferation into the forest floor layer on well‐drained sites.
Throughout the year in the present study, root biomass inside the forest floor on a slight slope (plot 1) remained higher than values measured at the bottom of the slope in the two other plots (Fig. 5). The opposite trend was observed for tree growth, which was lower in the area of low slope (Laclau, 2001). The higher root biomass in the forest floor of plot 1 might be a result of the lower availability of water and/or nutrients in this area. It has been demonstrated in Eucalyptus plantations that the ‘underground biomass : aerial biomass’ ratio is higher when the availability of water and nutrients is lower (Fabião et al., 1995; Stape, 2002).
A large proportion of the recorded values of soil hydrophobicity in the area of lowest altitude were in Class 6 (24 % ethanol), which represents a high degree of hydrophobicity compared with other studies (Ritsema, 1998). However, higher values of hydrophobicity (Class 7) have already been reported in soils under Eucalyptus stands (Crockford et al., 1991; Doerr et al., 1998). In Portugal, it has been shown that litter decomposition, as well as root exudates of Eucalyptus, is likely to enhance soil water repellency (Doerr et al., 1998). Crockford et al. (1991) also showed that the organic substances in stemflow and possibly litter leachates confer greater repellency on soils of an Australian Eucalyptus forest. An accumulation of root exudates and leachates from the decomposing litter might account for the high values of water repellency observed here in soil microdepressions. The lower values observed in the areas of slight slopes might be a result of the lower residence time of the leachates.
This water repellency observed during the dry season leads to a stagnation of water in the soil microdepressions, and their preferential colonization by the root mat might facilitate the uptake of nutrients released during litter decay. The soil hydrophobicity would then have strong positive effects on stand nutrition during the periods of high water deficit, due to the length of the dry season (4–5 months per year) and the low intensity of rainfall events during this period (generally <2 mm). The monitoring of soil water content in this stand over 3 years showed a quick drying of soil between May and October, which led to the lack of available water for plants down to a depth of 2 m at the end of the dry season (Laclau, 2001).
A seasonal separation of rootlet growth from the growth of foliage shoots is observed in Eucalyptus forests growing on soils of low nutrient status in Australia. This behaviour may be an ecomorphological adaptation to conserve nutrients released from decomposing litter in these forests (Specht, 1996). Even if the marked dry season in Congo led to a decrease in the biomass of the superficial root mat, its presence throughout this period, during which stand growth is slow but not zero, might be an important adaptation to supply water and nutrients to trees.
Nutrient uptake by the root mat inside the forest floor
The mean release of elements over the two years of monitoring the forest floor decomposition represented 46, 3, 4, 19 and 17 kg ha–1 year–1 for N, P, K, Ca and Mg, respectively. Only a direct uptake of nutrients released during litter decay was likely to explain the lack of enrichment of gravitational solutions for these elements (low for P and Mg) during their transfer (Table 3). The development of fine roots inside the plant structures observed on soil thin sections (Fig. 4C and D) might make this direct uptake possible. A similar direct cycling mechanism was observed in the Amazonian rain forest where it was shown that 32P was transferred from labelled leaves to living roots through fungal hyphae (Herrera et al., 1978; St John, 1983).
Various studies in Amazonian forests have suggested that large carbon and nutrient investment in the superficial root mat is an efficient mechanism for conserving the low pools of available elements in the soils, considerably reducing losses by deep drainage. Montagnini and Buscbacher (1989) determined that the rates of N mineralization and nitrification (on a per weight basis) were almost ten times higher in the forest floor invaded by a root mat as compared with the mineral soil under a terra firme Venezuelan rain forest. Moreover, a comparison of soil nitrogen dynamics under tree plantations and primary forest in lowland Amazonia showed that tree species growing in soils with higher N mineralization and N turnover allocated more N to fine roots inside the root mat (Smith et al., 1998). In the present study, the dense network of Eucalyptus roots observed inside the OF horizon (Fig. 4A), from the bottom of the OL horizon (Fig. 4B) to the interface between the OF and A1 horizons (Fig. 4E), behaves as an efficient filter to prevent losses of nutrients by drainage.
The amounts of nutrients taken up inside the forest floor calculated from eqn (2) represented, respectively, 47 %, 4 %, 30 %, 75 % and 59 % of the total uptake of N, P, K, Ca and Mg by the stand during the same period (Laclau et al., 2003b). Even if large amounts of nutrients are taken up from the available reservoir in the mineral soil, in particular for P and K, these results show that the decomposition of the Eucalyptus forest floor in this site is a crucial process for the nutrition of the stands. For this hybrid, totally allochtonous in Congo, the development of a superficial root mat effective in catching the large amounts of nutrients released during litter decay emphasizes a quick ecomorphological adaptation to these savanna soils, which are very poor in nutrients. The detailed study of the biogeochemical cycles of nutrients in this ecosystem showed that many other processes were efficient in this respect: in particular, high nutrient returns to the soil with litterfall, foliar uptake of nitrogen from atmospheric deposits, high internal retranslocations of elements from old tissues to growing organs, and a distribution of roots making it possible to take up water and nutrients in deep layers of soil during the dry season (Laclau et al., 2001, 2003a–c).
CONCLUSIONS
The dense root mat, whose dry matter can represent up to 500 kg ha–1, contributes efficiently to the quick cycling of nutrients in this ecosystem. It enables, on the one hand, a direct uptake within the decomposing plant structures and also in the solutions which do not exhibit a clear enrichment during the transfer through the holorganic layer, despite the large amounts of elements released during the biodegradation processes. The root mat was active throughout the year to catch the flux of nutrients from the organic matter, avoiding the transfer of dissolved nutrients in leachates toward deep horizons. Moreover, the root mat develops preferentially in microdepressions at the soil surface, where the hydrophobicity during the dry season greatly slowed down water infiltration and where roots can take up water and nutrients during this period of slow growth. The influence of the root mat and its associated micro‐organisms on the rates of litter decay deserves further investigation in these plantations.
ACKNOWLEDGEMENTS
We acknowledge Jean‐Claude Mazoumbou, Francine Mabounou, Antoine Kinana, Dominique Gelhaye and Stéphanie Cauquil for their contribution to this study. We thank Laurent Vesseyre (IRD) and Gisèle Heral‐Llimous (CIRAD) for performing the chemical analyses. We are also grateful to the founders of UR2PI, Republic of Congo, CIRAD‐Forêt and ECO s.a. for their financial support.
Received: 26 March 2003;; Returned for revision: 18 July 2003; Accepted: 24 October 2003, Published electronically: 28 January 2004
References
- AkimaH.1978. A method of bivariate interpolation and smooth surface fitting for irregularly distributed data points. ACM Transactions on Mathematical Software 4: 148–164. [Google Scholar]
- BabelU.1985. Basic organic components. In: Bullock P, Fedoroff N, Tongerius A, Stoops G, Tursina T, Babel U, eds. Handbook for soil thin section description. Wolverhampton, England: Waine Research Publications, 74–87. [Google Scholar]
- BaldwinDS.1999. Dissolved organic matter and phosphorus leached from fresh and ‘terrestrially’ aged river red gum leaves: implications for assessing river‐floodplain interactions. Freshwater Biology 41: 675–685. [Google Scholar]
- Bernhard‐ReversatF.1982. Measuring litter decomposition in a tropical forest ecosystem: comparison of some methods. International Journal of Ecology and Environmental Sciences 8: 63–71. [Google Scholar]
- Bernhard‐ReversatF.1993. Dynamics of litter and organic matter at the soil‐litter interface in fast‐growing tree plantations on sandy ferrallitic soils (Congo). Acta Oecologica 14: 179–195. [Google Scholar]
- CasenaveF, Valentin E.1989.Les états de surface de la zone sahélienne. Influence sur l’infiltration. Paris: Editions de l’ORSTOM. [Google Scholar]
- Chapuis‐LardyL, Contour‐Ansel D, Bernhard‐Reversat F.2002. High‐performance liquid chromatography of water‐soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Science 163: 217–222. [Google Scholar]
- ChuyongGB, Newbery DM, Songwe NC.2002. Litter breakdown and mineralization in a central African rain forest dominated by ectomycorrhizal trees. Biogeochemistry 61: 73–94. [DOI] [PubMed] [Google Scholar]
- CrockfordH, Topalidis S, Richardson DP.1991. Water repellency in a dry sclerophyll eucalypt forest – measurements and processes. Hydrological Processes 5: 405–420. [Google Scholar]
- CuevasE, Medina E.1988. Nutrient dynamics within Amazonian forests II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76: 222–235. [DOI] [PubMed] [Google Scholar]
- DoerrSH, Shakesby RA, Walsh RPD.1998. Spatial variability of soil hydrophobicity in fire‐prone Eucalyptus and pine forests, Portugal. Soil Science 163: 313–324. [Google Scholar]
- DuchaufourP, Bonneau M.1959. Une nouvelle méthode de dosage du phosphore assimilable dans les sols forestiers. Bulletin AFES 4: 193–198. [Google Scholar]
- FabiãoA, Madeira M, Steen E, Käterer T, Ribeiro C, Araùjo C.1995. Development of root biomass in a Eucalyptus globulus plantation under different water and nutrient regimes. Plant and Soil 168: 215–223. [Google Scholar]
- FitpatrickEA, Gudmundsson T.1978. The impregnation of wet peat for the production of thin sections. Journal of Soil Science 29: 585–587. [Google Scholar]
- GroveTS, Thomson BD, Malajczuk N.1996. Nutritional physiology of eucalypts: uptake, distribution and utilization. In: Attiwill PM, Adams MA, eds. Nutrition of eucalypts. CSIRO, Australia: 77–108. [Google Scholar]
- HerreraR, Merida T, Stark N, Jordan CF.1978. Direct phosphorus transfer from leaf litter to roots. Naturwissenschaften 65: 208–209. [Google Scholar]
- JordanCF.1985.Nutrient cycling in tropical forest ecosystems: principles and their implication in management and conservation. New‐York: John Wiley & Sons. [Google Scholar]
- KalbitzK, Solinger S, Park JH, Michalzik B, Matzner E.2000. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Science 165: 277–304. [Google Scholar]
- KingsburyN, Kellman M.1997. Root mat depths and surface soil chemistry in southeastern Venezuela. Journal of Tropical Ecology 13: 475–479. [Google Scholar]
- LaclauJP.2001.Dynamique du fonctionnement minéral d’une plantation d’Eucalyptus. Effet du reboisement sur un sol de savane du littoral congolais; conséquences pour la gestion des plantations. PhD Thesis, Institut National Agronomique Paris Grignon, France. [Google Scholar]
- LaclauJP, Arnaud M, Bouillet JP, Ranger J.2001. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: relationships with the ability of the stand to take up water and nutrients. Tree Physiology 21: 129–136. [DOI] [PubMed] [Google Scholar]
- LaclauJP, Ranger J, Nzila JD, Bouillet JP, Deleporte P.2003a. Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo. 2. Chemical composition of soil solutions. Forest Ecology and Management 180: 527–544. [Google Scholar]
- LaclauJP, Deleporte P, Ranger J, Bouillet JP, Kazotti G.2003b. Nutrient dynamics throughout the rotation of Eucalyptus clonal stands in Congo. Annals of Botany 91: 879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaclauJP, Ranger J, Bouillet JP, Nzila JD, Deleporte P.2003c. Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo. 1. Chemical composition of rainfall, throughfall and stemflow solutions. Forest Ecology and Management 176: 105–119. [Google Scholar]
- MabberleyDJ.1992.Tropical rain forest ecology, 2nd edn. New York: Chapman and Hall. [Google Scholar]
- MontagniniF, Buschbacher R.1989. Nitrification rages in two undisturbed tropical rain forests and three slash‐and‐burn sites of the Venezuelan Amazon. Biotropica 21: 8–14. [Google Scholar]
- NzilaJD.2001. Caractérisation minéralogique des sols ferrallitiques sableux sous plantation d’Eucalyptus et sous savane naturelle de la région de Pointe‐Noire (Congo). Rapport interne UR2PI, Pointe‐Noire, Congo. [Google Scholar]
- NzilaJD, Bouillet JP, Laclau JP, Ranger J.2002. The effects of slash management on nutrient cycling and tree growth in Eucalyptus plantations in the Congo. Forest Ecology and Management 171: 209–221. [Google Scholar]
- QuallsRG, Haines BL, Swank WT, Tyler SW.2000. Soluble organic and inorganic fluxes in clearcut and mature deciduous forests. Soil Science Society of America Journal 64: 1068–1077. [Google Scholar]
- ReisingerO, Toutain F, Mangenot F, Arnould MF.1978. Etude ultrastructurale des processus de biodégradation I‐ pourriture blanche des feuilles de hêtre (Fagus sylvatica). Canadian Journal of Microbiology 24: 725–733. [PubMed] [Google Scholar]
- RitsemaCJ.1998.Flow and transport in water repellent sandy soil. PhD Thesis, Wageningen Agricultural University, The Netherlands. [Google Scholar]
- SmithCK, Assis Oliveira F, Gholz HL, Baima A.2002. Soil carbon stocks after forest conversion to tree plantations in lowland Amazonia, Brazil. Forest Ecology and Management 164: 257–263. [Google Scholar]
- SmithCK, Gholz HL, Assis Oliveira F.1998. Soil nitrogen dynamics and plant‐induced soil changes under plantations and primary forest in lowland Amazonia, Brazil. Plant and Soil 200: 193–204. [Google Scholar]
- SpechtRL.1996. Influence of soils on the evolution of the eucalypts. In: Attiwill PM, Adams MA, eds. Nutrition of eucalypts Canberra, Australia: CSIRO, 31–60. [Google Scholar]
- StapeJL.2002.Production ecology of clonal Eucalyptus plantations in north‐eastern Brazil. D.Sc. Thesis, Colorado State University, USA. [Google Scholar]
- St JohnTV.1983. Response of tree roots to decomposing organic matter in two lowland amazonian rain forests. Canadian Journal of Forest Research 13: 346–349. [Google Scholar]
- ZellerB, Colin‐Belgrand M, Dambrine E, Martin F, Bottner P.2000. Decomposition of 15N‐labelled beech litter and fate of nitrogen derived from litter in a beech forest. Oecologia 123: 550–559. [DOI] [PubMed] [Google Scholar]