Abstract
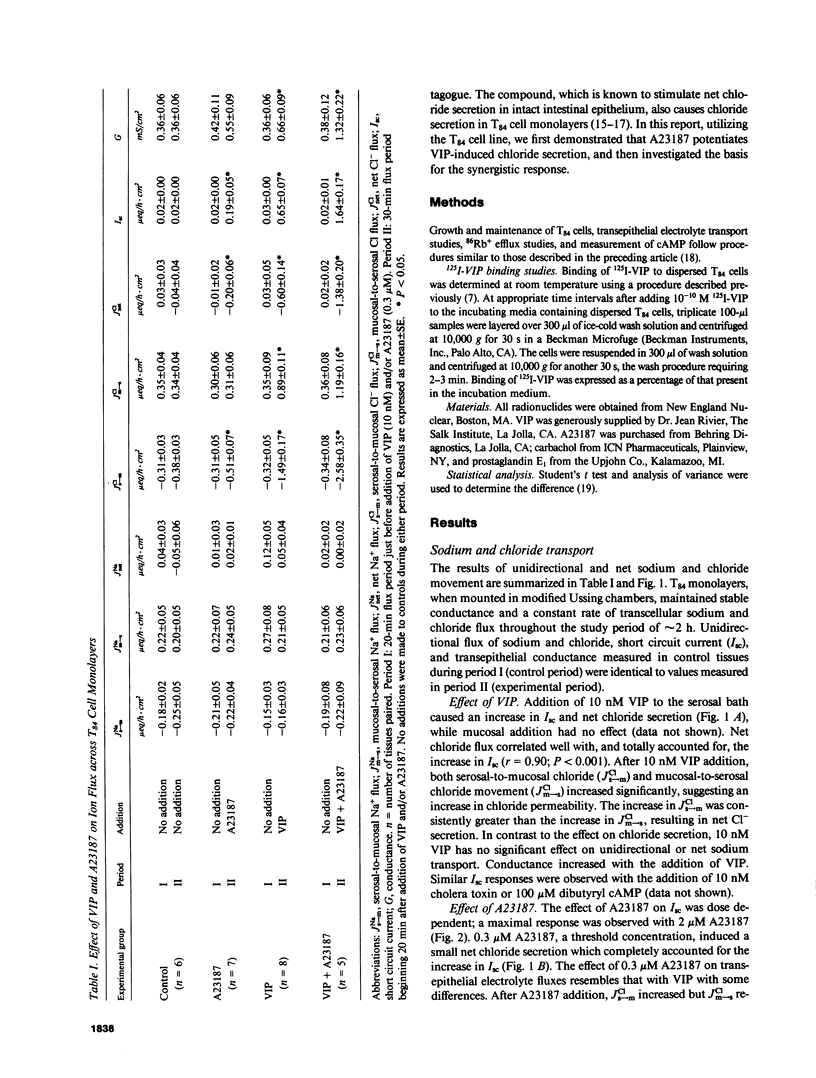
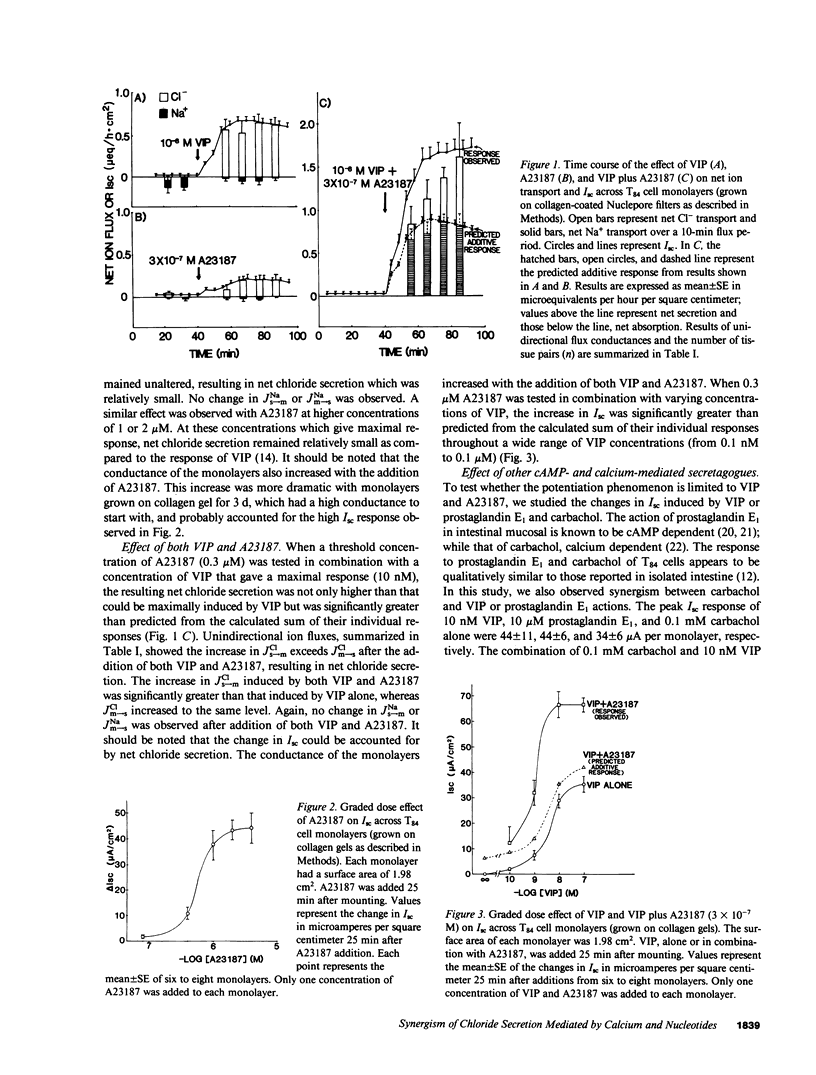
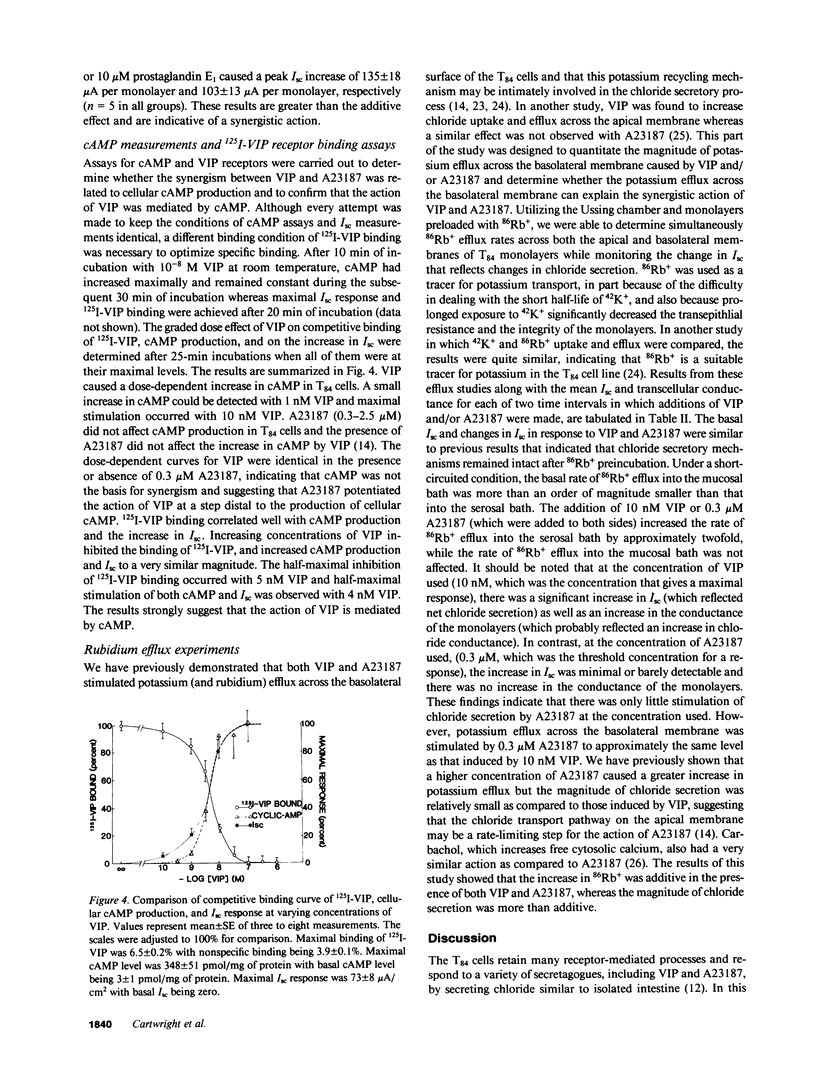
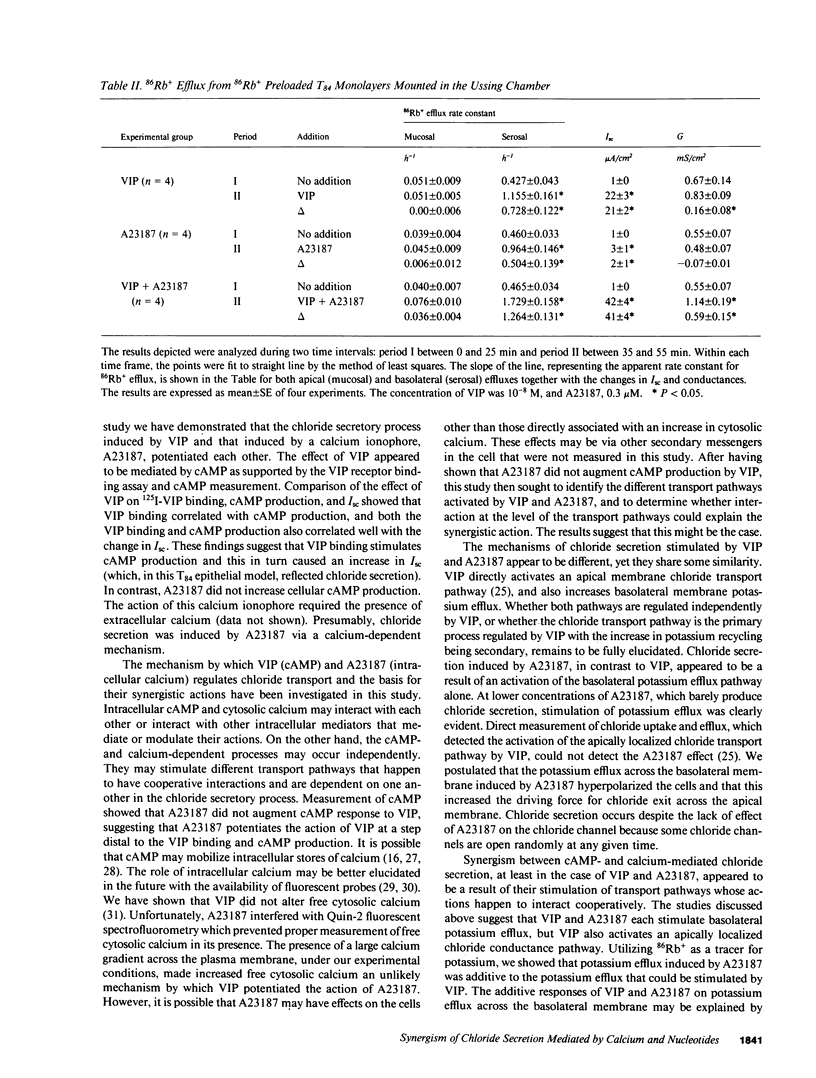
Vasoactive intestinal polypeptide (VIP) and the calcium ionophore A23187 caused dose-dependent changes in the potential difference and the short circuit current (Isc) across confluent T84 cell monolayers mounted in modified Ussing chambers. Both VIP and A23187 stimulated net chloride secretion without altering sodium transport. Net chloride secretion accounted for the increase in Isc. When A23187 was tested in combination with VIP, net chloride secretion was significantly greater than predicted from the calculated sum of their individual responses indicating a synergistic effect. VIP increased cellular cyclic AMP (cAMP) production in a dose-dependent manner, whereas A23187 had no effect on cellular cAMP. We then determined whether VIP and A23187 activated different transport pathways. Earlier studies suggest that VIP activates a basolaterally localized, barium-sensitive potassium channel as well as an apically localized chloride conductance pathway. In this study, stimulation of basolateral membrane potassium efflux by A23187 was documented by preloading the monolayers with 86Rb+. Stimulation of potassium efflux by A23187 was additive to the VIP-stimulated potassium efflux. By itself, 0.3 microM A23187 did not alter transepithelial chloride permeability, and its stimulation of basolateral membrane potassium efflux caused only a relatively small amount of chloride secretion. However, in the presence of an increased transepithelial chloride permeability induced by VIP, the effectiveness of A23187 on chloride secretion was greatly augmented. Our studies suggest that cAMP and calcium each activate basolateral potassium channels, but cAMP also activates an apically localized chloride channel. Synergism results from cooperative interaction of potassium channels and the chloride channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Lemp G. F., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on small intestinal epithelial cells. Am J Physiol. 1980 Mar;238(3):G190–G196. doi: 10.1152/ajpgi.1980.238.3.G190. [DOI] [PubMed] [Google Scholar]
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Collen M. J., Sutliff V. E., Pan G. Z., Gardner J. D. Postreceptor modulation of action of VIP and secretin on pancreatic enzyme secretion by secretagogues that mobilize cellular calcium. Am J Physiol. 1982 Apr;242(4):G423–G428. doi: 10.1152/ajpgi.1982.242.4.G423. [DOI] [PubMed] [Google Scholar]
- Davis G. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Effect of vasoactive intestinal polypeptide on active and passive transport in the human jejunum. J Clin Invest. 1981 Jun;67(6):1687–1694. doi: 10.1172/JCI110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Harms V., Yamashiro D. J., Hughes R. J., Binder H. J., Wright E. M. Preferential binding of vasoactive intestinal polypeptide to basolateral membrane of rat and rabbit enterocytes. J Clin Invest. 1983 Jan;71(1):27–35. doi: 10.1172/JCI110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Mandel K. G., Masui H., McRoberts J. A. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl- cotransport system. J Clin Invest. 1985 Feb;75(2):462–471. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Donowitz M. Ca2+ in the control of active intestinal Na and Cl transport: involvement in neurohumoral action. Am J Physiol. 1983 Aug;245(2):G165–G177. doi: 10.1152/ajpgi.1983.245.2.G165. [DOI] [PubMed] [Google Scholar]
- Fast D., Tenenhouse A. The effect of dibutyryl cyclic adenosine-3'-5'-monophosphate on protein secretion from the rat exocrine pancreas in vitro. Br J Pharmacol. 1976 Dec;58(4):605–612. doi: 10.1111/j.1476-5381.1976.tb08630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A. Active chloride secretion by rabbit colon: calcium-dependent stimulation by ionophore A23187. J Membr Biol. 1977 Jun 30;35(2):175–187. doi: 10.1007/BF01869948. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Walker M. D., Rottman A. J. Effect of A23187 on amylase release from dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1980 May;238(5):G458–G466. doi: 10.1152/ajpgi.1980.238.5.G458. [DOI] [PubMed] [Google Scholar]
- Juzu H. A., Holdsworth E. S. New evidence for the role of cyclic AMP in the release of mitochondrial calcium. J Membr Biol. 1980;52(2):185–186. doi: 10.1007/BF01869125. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Henderson A. Effects of prostaglandins and cholera enterotoxin on intestinal mucosal cyclic AMP accumulation. Evidence against an essential role for prostaglandins in the action of toxin. J Clin Invest. 1974 Mar;53(3):941–949. doi: 10.1172/JCI107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang W., Domboski L., Krausz Y., Sharp G. W. Mechanisms of synergism between glucose and cAMP on stimulation of insulin release. Am J Physiol. 1984 Dec;247(6 Pt 1):E701–E708. doi: 10.1152/ajpendo.1984.247.6.E701. [DOI] [PubMed] [Google Scholar]
- Racusen L. C., Binder H. J. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology. 1977 Oct;73(4 Pt 1):790–796. [PubMed] [Google Scholar]
- Scarpa A., Malmstrom K., Chiesi M., Carafoli E. On the problem of the release of mitochondrial calcium by cyclic AMP. J Membr Biol. 1976 Oct 20;29(1-2):205–208. doi: 10.1007/BF01868960. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Sheerin H. E., Field M., Said S. I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974 Sep;54(3):536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Calcium and cyclic nucleotide interaction in secretion of amylase from rat pancreas in vitro. J Physiol. 1979 Nov;296:159–176. doi: 10.1113/jphysiol.1979.sp012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. The interaction of histamine with gastrin and carbamylcholine on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):381–389. doi: 10.1172/JCI108948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. W., Dobbins J. W., Binder H. J. Mechanism of cholinergic regulation of electrolyte transport in rat colon in vitro. Am J Physiol. 1982 Feb;242(2):G116–G123. doi: 10.1152/ajpgi.1982.242.2.G116. [DOI] [PubMed] [Google Scholar]



