Abstract
• Background and Aims The xylem in fruit of a number of species becomes dysfunctional as the fruit develops, resulting in a reduction of xylem inflow to the fruit. Such a reduction may have consequential effects on the mineral balance of the fruit. The aim of this study was to elucidate the dynamics and nature of xylem failure in developing apples (Malus domestica) showing differing susceptibilities to bitter pit, a calcium‐related disorder.
• Methods Developmental changes in xylem functionality of the fruit were investigated in ‘Braeburn’ and ‘Granny Smith’ apples by using a dye infusion technique, to stain the vasculature along the path of dye movement. The vascular bundles were clearly visible in transverse section when fruit were sectioned equatorially. The intensity of staining of the vascular bundles in the fruit was recorded at regular intervals throughout the season. Tissue containing dysfunctional bundles was fixed and embedded in wax for subsequent sectioning and examination.
• Key Results As the season progressed, an increasing proportion of vascular bundles failed to show any staining, with the most marked change occurring in the primary bundles, and in nearly all bundles with increasing distance from the stalk end of the fruit. Decreased conductance in the primary bundles of ‘Braeburn’ occurred earlier than in ‘Granny Smith’. Microscopy revealed that the xylem in vascular bundles of the fruit suffered substantial damage, indicating that the mode of dysfunction was via the physical disruption of the xylem caused by expansion of the flesh.
• Conclusions Results support the view that the relative calcium deficiency of apple fruit is due to a progressive breakdown of xylem conductance caused by growth‐induced damage to the xylem strand in the bundle. The earlier onset of xylem dysfunction in the cultivar more susceptible to bitter pit suggests that the relative growth dynamics of the fruit may control the occurrence of calcium‐related disorders.
Key words: Malus domestica, apple, apoplastic dye, xylem functionality, vascular bundles, calcium, bitter pit
INTRODUCTION
The vascular bundles in apple (Malus domestica) are the principal means by which nutrients are conveyed to the growing tissues of the fruit. The vascular bundles are arranged within two distinct systems, ‘cortical’ and ‘carpellary’, in regard to their position and role within the fruit. The cortical vascular system has ten primary (sepal and petal) bundles that bend around the carpels, giving off a skeleton of traces that ramifies towards the skin. The carpellary vascular system is composed of ten ventral and five dorsal bundles that anastomose with each other. The paired ventral bundles emerge from the stele after the separation of primary bundles to form a ring along the ventral lobes of the carpels, giving off lateral traces to the ovules. Dorsal bundles diverge from the primary (sepal) bundles, swinging around the locules and terminating together with the ventral bundles in the fused styles of the pistil (MacArthur and Wetmore, 1939; MacDaniels, 1940).
The conducting tissues within the vascular bundles are the xylem and phloem. However, the xylem in fruit from a number of species becomes dysfunctional as the fruit develops (Düring et al., 1987; Findlay et al., 1987; Lang, 1990; Creasy et al., 1993; Lang and Ryan, 1994; Wolswinkel et al., 1999; Dražeta et al., 2001). In wine grapes, xylem dysfunction coincides with the sudden resumption of berry growth after the onset of ripening (Düring et al., 1987; Findlay et al., 1987) while the phloem remains functional (Findlay et al., 1987). Here, the cause of xylem dysfunction may be due to growth‐induced damage of the bundles as the fruit expands.
Progressive breakdown of conducting tissue in apple has been attributed to the failure of a number of vessels in the xylem (Lang and Ryan, 1994), resulting in a reduction in the rate of xylem inflow to the fruit and a shift in the relative contributions of xylem and phloem saps to fruit growth (Lang, 1990). This change may have consequential effects on the balance and amounts of fruit minerals and thus on fruit quality. Calcium, a xylem‐mobile element (Marschner, 1986), shows a progressive decline in its rate of accumulation during the season (Wilkinson, 1968; Jones et al., 1983). Furthermore, the sudden drop in fruit calcium concentration in apples coincides with the onset of rapid fruit growth (Jones et al., 1983). These lines of evidence indicate that the change in xylem function and thus mineral composition of the fruit may be associated with mechanisms that control fruit growth.
The aim of this study was to elucidate the dynamics and nature of xylem failure in developing apple fruit using a water‐soluble dye as an indicator of functionality coupled with a conventional light microscopic examination of dysfunctional bundles. Two apple cultivars were tested, these having different susceptibilities to bitter pit (Dražeta et al., 2001). It was hypothesized that differences in bitter pit susceptibility might be explained in terms of differences in xylem functionality and access of developing fruit to calcium.
MATERIALS AND METHODS
Plant material
The study used mature trees of Malus domestica Borkh. ‘Braeburn’ and ‘Granny Smith’ apple cultivars from a private organic orchard on the outskirts of Palmerston North, New Zealand. The trees were planted on ‘MM 106’ rootstock in three‐row blocks alternating with pollinator blocks. The trees had not been managed according to standard commercial practices and the fruit were not selectively thinned. The flowering period was briefer than usual, with full bloom starting about 3 d earlier in ‘Granny Smith’ than in ‘Braeburn’.
Temporal changes in xylem functionality
Functionality of fruit xylem was recorded at weekly intervals until mid‐season (113 and 116 d after full bloom; DAFB) and then fortnightly until harvest (183 and 200 DAFB). Average‐sized fruit without visible blemishes were selected. Whole fruit‐bearing spurs were collected from the orchard around dawn when transpiration would be negligible and hence tree water potential close to soil water potential. Fruit were held in closed polyethylene bags pending investigation later the same day. On each occasion, 15 fruit of each cultivar were assessed for xylem functionality.
Xylem function in the fruit was examined using a simple dye‐infusion technique to stain the vasculature along the path of dye movement. To avoid air embolism of the xylem, each fruit was cut from the spur at the base of the stalk. This was carried out under distilled water prior to immersion in a small vial containing a dye solution. The apoplastic dye (1 % w/w aqueous acid fuchsin) was drawn up into the fruit through the stalk for 2 h under standard transpiration conditions (approx. 22 °C and 65 % RH) with a brisk airflow from two fans to remove boundary layer effects.
Fruit were sectioned equatorially using a microtome blade to minimize tissue damage and to produce a smooth cut surface. The vascular bundles were clearly visible in the transverse sections and were scored for dye intensity (D) under a dissecting microscope (Wild M3Z) as 0 = nil, 1 = trace, 2 = medium and 3 = high. Because developmental changes in xylem functionality may differ between the classes of vascular bundles, the presence of dye was assayed separately for the ten primary, five dorsal and ten ventral bundles. For a particular class of bundle an averaged D value was computed for each fruit from the number of scores obtained at any one time.
For each bundle type and cultivar, a model was chosen which described the approximate shape of the curve of changing D with time. Each model was fitted by non‐linear, least‐squares, using PROC NLIN (SAS, 2000). For each model, F‐tests were applied to determine whether selected features of the shape of the fitted curve were significant, and whether differences between the fitted curves for each cultivar could be adequately described by a simple difference in time scales.
Exposure time
Preliminary staining trials conducted early in the season showed satisfactory dye distribution in the bundles of the fruit after approx. 2 h. However, as changes in xylem functionality might alter the amount of dye appearing later in the season and so change the optimum period of exposure, dye accumulation was assessed as a function of time. For this, 50 fruit of each cultivar were chosen in the age range of 93–104 DAFB and infiltrated with dye for 30, 60, 120, 240 and 480 min. The three classes of vascular bundles were assessed for D and averaged separately for each fruit as described above.
Spatial changes in xylem functionality
Longitudinal dye accumulation in the fruit was investigated in ten fruit of each cultivar at a more advanced stage (160–163 DAFB). Dye‐infiltrated fruit were sliced transversally using a domestic food slicer to form a contiguous series of approx. 2·5 mm thick tissue discs, starting from the calyx end. Due to a slight variability in fruit size, different numbers of discs were obtained from each fruit. D was assessed in each disc, although not all classes of bundles were visible in the discs obtained near the ends of the fruit.
PROC GLM (SAS, 2000) was used to initially evaluate the significance of any longitudinal gradient in D for each bundle class and cultivar and then, secondly, to test whether the gradients differed between bundle classes for each cultivar and between cultivars for each bundle class. The high natural variability between fruit was taken into account by including a fruit effect within each cultivar.
Anatomy of xylem breakage
Several fruit were collected at 85 and 141 DAFB for microscopic study. Fruit from both collection dates were infiltrated with dye and cut transversally into three, approximately equal, discs: disc ‘A’ was from near the stalk end of the fruit, ‘B’ was from the middle and ‘C’ from near the calyx end. In each disc, a randomly chosen dysfunctional (unstained) bundle was excised with a small amount of attached flesh so as to produce an elongated sample.
Fruit picked at 85 DAFB was used to identify structural changes in the xylem. Each sample was soaked for several minutes in an excess of phloroglucin solution (2·5 g in 50 ml of 70 % v/v aqueous ethyl alcohol) and flooded afterwards with concentrated HCl to visualize lignified vascular elements. This procedure yields distinctively red‐stained xylem strands embedded within a reasonably transparent parenchyma. The bulk of the flesh was carefully dissected away working under a dissecting microscope (Wild M3Z). The excised bundle was mounted in 80 % w/w glycerol on a glass slide and examined with a compound light microscope (Microlux‐11).
Fruit picked at 141 DAFB were used to investigate the nature of xylem disruption by a more sophisticated light microscopic procedure. To prepare a dysfunctional bundle for fixation, intercellular spaces of the sample were vacuum infiltrated with water, which rendered the parenchyma reasonably transparent and suitable for dissection. Samples were trimmed under the dissecting microscope (Wild M3Z) until the bundle was exposed with a very thin layer of surrounding flesh. It was then cut transversally every 4–5 mm.
The specimens were preserved separately in sealed glass vials containing half‐strength Karnovsky’s fixative (3 % v/v glutaraldehyde and 2 % w/v formaldehyde in 0·1 m phosphate buffer at pH 7·2). Secondary fixation was in 1 % osmium tetroxide using the same buffer. Specimens were dehydrated in a graded acetone series, and infiltrated and embedded in Procure 812 epoxy resin. Cured resin blocks were trimmed and longitudinal sections (1 µm thick) were cut from the middle of the bundle, heat‐mounted on glass microscope slides and stained with 0·1 % toluidine blue. Slides were examined and photographed using a Zeiss Axioplan compound light microscope with attached MC 100 camera system.
RESULTS
Exposure time
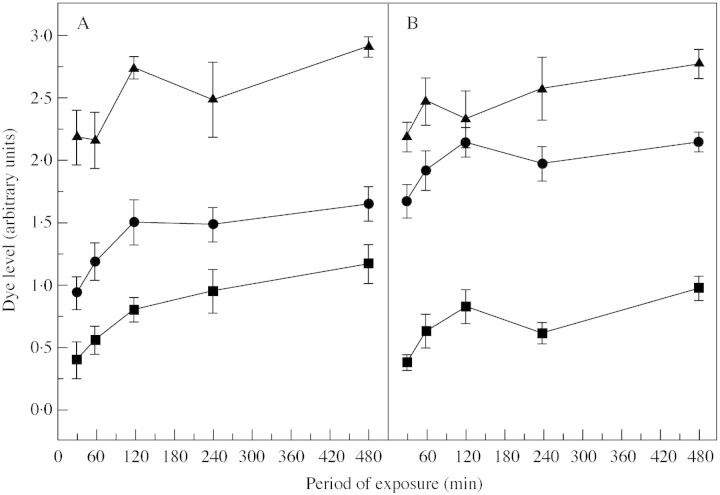
Dye infusion induced a rapid spread of dye throughout the functional portions of the vascular network, enabling an assessment of functionality for the range of exposure times used. In all bundle classes, D initially rose but essentially reached a plateau after 120 min (Fig. 1). Thus any differences in D can be taken to reflect a physical property of the bundle itself rather than of the measuring technique. Accordingly, different bundle classes accumulated different amounts of dye. In both cultivars, the dorsal bundles appeared more functional than the primary or ventral bundles. Furthermore, the intensity of dye in the primary bundles of ‘Braeburn’ was less than in ‘Granny Smith’.
Fig. 1. Dye accumulation in the vascular systems of (A) ‘Braeburn’ and (B) ‘Granny Smith’ as a function of the period of exposure. The points are mean values (n = 10) for the primary (circles), dorsal (triangles) and ventral (squares) vascular bundles of the fruit with the corresponding s.e. indicated by the vertical bars. Fruit were assessed at 93 DAFB (‘Braeburn’) and 104 DAFB (‘Granny Smith’).
Temporal changes in xylem functionality
Changes in dye accumulation were observed initially in the anastomosing vasculature, a dense network of fine bundles that scatter across the flesh and terminate just beneath the skin. Early in the season, dye spread freely through the numerous fine traces leading to a somewhat diffuse appearance. Later, the fine vascular network was less strongly coloured. The reduction in colour took place before any noticeable change in the functionality of the primary bundles and followed a similar pattern in both cultivars, although it was more pronounced in ‘Braeburn’ (Fig. 2).

Fig. 2. Transverse sections of dye‐infused ‘Braeburn’ (left) and ‘Granny Smith’ (right). Fruit were assessed at 64 and 67 DAFB, respectively. The degree of coloration in fine vascular anastomoses indicates a difference in vascular function between the two fruit.
The dye level in the primary bundles was at the maximum measurable value of 3·0 early in the season. After this, coloration declined exponentially with time (Fig. 3A), so that a model was chosen of the form
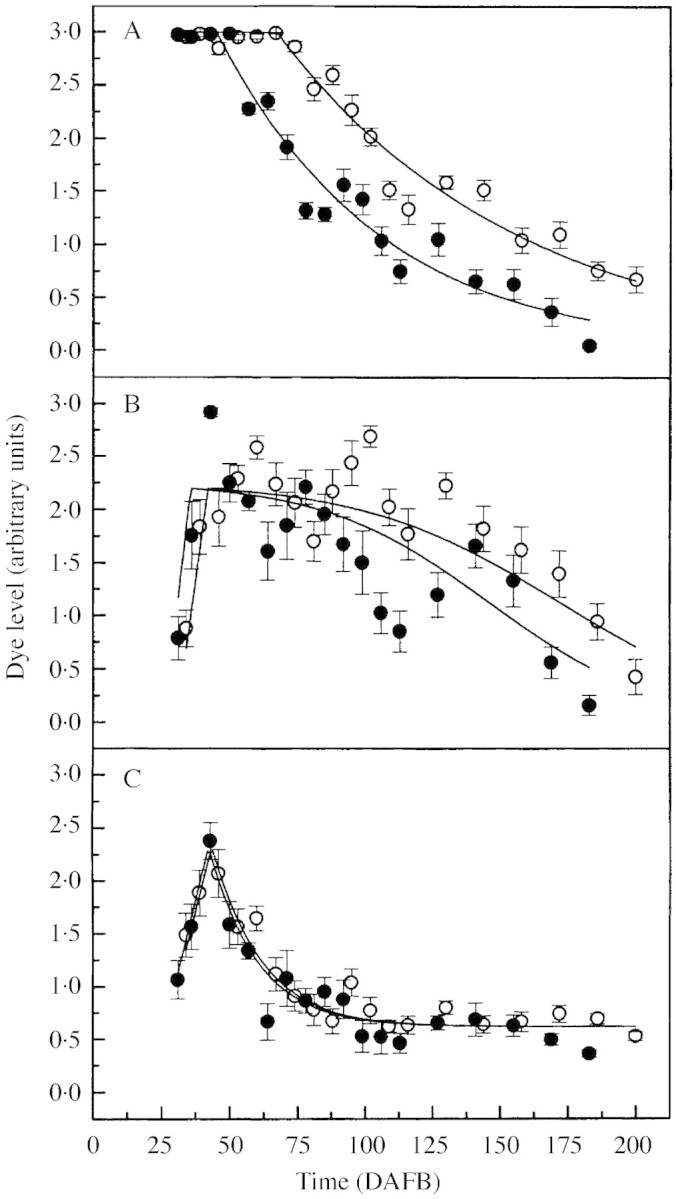
Fig. 3. Dye level in (A) the primary, (B) dorsal and (C) ventral vascular bundles of ‘Braeburn’ (solid circles) and ‘Granny Smith’ (open circles) as a function of fruit age (DAFB). The mean dye level for each time period was obtained by assessing 15 fruit per cultivar. Vertical bars represent s.e.
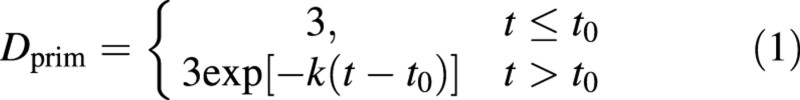
where t0 (the time, DAFB, when D first drops below 3·0) and k are parameters to be fitted. The exponential decay parameter k can be related to a ‘half‐life’ (t½), the time interval for D to be halved from any point on an exponential curve by

For both cultivars, the decrease in D in the primary bundles with time was highly significant (P < 0·001), i.e. k ≠ 0, and, by harvest, the fruit had lost most of its xylem function. Differences in the extent of xylem dysfunction between the two cultivars were well described by a single time‐scaling factor, with the difference being highly significant (P < 0·001), and with ‘Braeburn’ taking just 0·67 times as long as ‘Granny Smith’ to decay at any given D. Using this scaling factor, fitted parameter values were estimated at t0 = 67 DAFB and t½ = 61 d for ‘Granny Smith’, and t0 = 45 DAFB and t½ = 41 d for ‘Braeburn’. The sudden collapse in xylem function of the primary bundles occurred earliest in ‘Braeburn’, whereas in ‘Granny Smith’ function continued for longer and the fruit still retained some functionality at harvest.
The dye level in the dorsal bundles (Fig. 3B) rose initially to a maximum value (A), but then followed a somewhat complex path. The conductance in both cultivars oscillated markedly but the xylem remained fairly functional throughout the season. As there was no obvious mechanism by which bundle conductance and, therefore, D could increase around day 125, this fluctuation was ignored and the path was described by a declining logistic curve
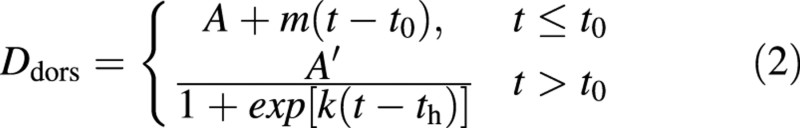
where A, t0 (the time, DAFB, at which D starts to decay), m (the slope up to the maximum D), th (the time, DAFB, when D drops to half of A′), and k are parameters to be fitted. To ensure continuity, the upper asymptote of the logistic curve A′ = 1 + exp[k(t0 – th)]. For a logistic, parameter k can be related to the times (DAFB) at which D drops to two‐thirds or one‐third of A′ (t⅔ or t⅓), by
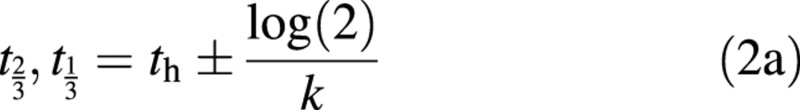
As was the case for the primary bundles, the drop in D in the dorsal bundles was significant (P < 0·001), and using separate values for every parameter in each cultivar provided no significant improvement over using a simple scaling factor. The difference in time scales was significant (P < 0·05), with the fitted scale factor of 0·85. With this scaling factor, fitted parameter values for ‘Granny Smith’ were m = 0·19, t0 = 42 DAFB, and th = 172 DAFB (with t⅔ and t⅓ 25 d before and after this), and for ‘Braeburn’ were m = 0·23, t0 = 36 DAFB, and th = 146 DAFB (t⅔, t⅓ ± 21 d). For both cultivars, A = 2·2.
The dye level for ventral bundles (Fig. 3C) also rose initially to a maximum value (A), but dropped exponentially to a non‐zero final value (B). Hence, a model to describe these characteristics was
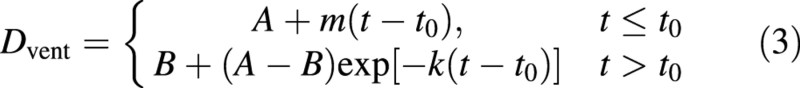
Parameters to be fitted are A, B, m, t0, and the exponential decay parameter k, which is related to a ‘half‐life’ (t½), the time interval for D to halve along exponential curve relative to the final level B by (eqn 1a).
For both cultivars, the drop in D in the ventral bundles with time following t0 was highly significant (P < 0·001), and the final level reached (B) was significantly greater than zero (P < 0·001). Nevertheless, there were no significant differences between parameters for the two cultivars, with the fitted scaling factor (0·97) not differing significantly from 1. For both cultivars, fitted parameter values were approximately A = 2·3, B = 0·6, m = 0·1, t0 = 43 DAFB, and t½ = 12 d.
Spatial changes in xylem functionality
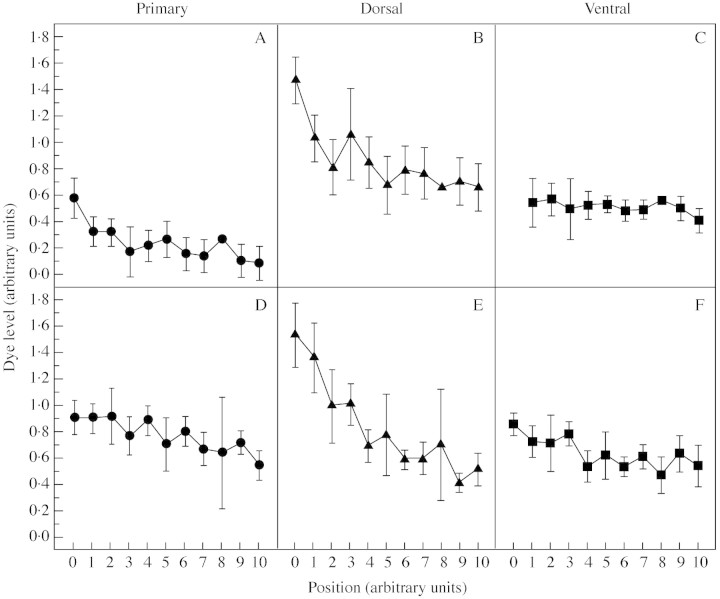
Serial (lengthwise) sectioning of dye‐infused fruit showed a strong spatial gradient in D with increasing distance from the stalk end of the fruit (P < 0·01), except for ventral bundles in ‘Braeburn’ (Fig. 4). Mean dye level is plotted against longitudinal position in the fruit, where ‘0’ refers to the point near the stalk end where the vascular bundles were first apparent, and ‘10’ refers to the last such point close to the calyx end. However, the ventral bundles in ‘Braeburn’ were not clearly distinguishable at the ‘0’ position in the fruit (missing value, Fig. 4C) while some data points in other vascular bundles of the same cultivar were determined only by a single value and lack error bars.
Fig. 4. Longitudinal changes in the functionality of xylem in (A–C) ‘Braeburn’ and (D–F) ‘Granny Smith’, assessed at 160 and 163 DAFB, respectively. Each point represents the means dye level for the primary, dorsal and ventral vascular bundles falling within the same fractional distance between ‘0’ (near the stalk end) and ‘10’ (near the calyx end) of the fruit. Vertical bars represent s.e.
For data pooled across both cultivars, there were significant differences among the gradients for the bundle classes (P < 0·01), with dorsal bundles having the steepest gradient for both. The gradient for dorsal bundles in ‘Granny Smith’ was significantly greater than the gradient for ‘Braeburn’ (P < 0·01), but there were no significant differences in the gradients between cultivars for primary or ventral bundles.
Anatomy of xylem breakage
Microscopic examination of dysfunctional primary bundles in the early stage of fruit growth confirmed a localized area of damage in the xylem tissue (data not shown). The conducting elements of the xylem consisted mainly of vessels organized into parallel columns with spiral thickening of the walls, although occasional annular thickening was also apparent. The most conspicuous damage appeared as an irregular spacing and deformation of the vessel files. Annular structures sometimes collapsed entirely leaving occasional rounded elements completely isolated. At other times, spirals appeared to be overly stretched and partially fragmented. That this characteristic was an artefact of sample preparation is judged unlikely due to the existence of vessels with intact walls adjacent to the deformed and fragmented structures.
Additional structural changes were apparent when longitudinal sections of dysfunctional primary bundle were assessed for xylem damage at an advanced stage of fruit growth. The evidence showed that complete breakages of the xylem strand occurred randomly along the bundle. Cells of parenchyma filled the spaces between files of vessels indicating total discontinuity and collapse in function (Fig. 5A). Within a xylem strand, stretched vessels with spiral thickening were frequent, often sustained in a broken line, reflecting the high degree of strain (Fig. 5B).
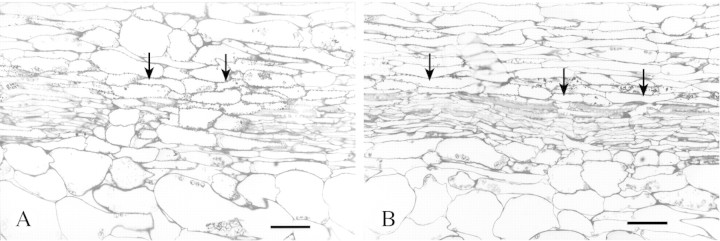
Fig. 5. Longitudinal sections of a primary (sepal) vascular bundle in the fruit of ‘Braeburn’, 141 DAFB. (A) Breakage of the xylem strand. Shattered parts are displaced so that parenchyma cells occupy the resulting void. (B) Ruptured string of vessels. Multiple breaks signify excessive change in length of the stressed walls. The bar represents 50 µm. Arrows indicate the structural changes being described.
DISCUSSION
Changes in xylem functionality
The model developed for the primary and ventral bundles in the fruit provided a good fit to the mean curves for both cultivars (Fig. 3A and C). However, for dorsal bundles, the model could not capture the later rise in D, but otherwise fitted reasonably well (Fig. 3B). The decline in D was significant for all three bundle‐classes. Nevertheless, dysfunction was irregular, with some fruit losing the greater part of their xylem conductance relatively early on. The majority of bundles exhibited reduced dye movement, which indicated random damage to the xylem at any one time.
The most marked change in xylem conductance occurred in the primary bundles (Fig. 3A) that are arguably the major nutrient suppliers of the flesh. The greatest xylem flow of these bundles was obtained at the beginning of the season. The early‐season plateau in dye accumulation might be an artefact of the arbitrary dye assessment that limited the maximum value for D to an integer value of 3. Nevertheless, D in both cultivars exhibited identical trends indicating that the primary bundles were fully functional at the beginning of fruit development. A similar pattern in functionality of the fruit xylem was obtained in other apple cultivars examined using a pressure‐bomb technique (Lang and Ryan, 1994; Dražeta et al., 2001).
As the season progressed, an increasing proportion of primary bundles failed to transport apoplastic dye. For ‘Braeburn’, changes in D were more extreme, and xylem conductance became negligible over the final weeks of fruit development. The sharp decline in D from around 45 DAFB (‘Braeburn’) and 67 DAFB (‘Granny Smith’) closely matches the completion of vessel differentiation (approx. 50 DAFB) in the primary bundles of ‘York Imperial’ apple (Barden and Thompson, 1963). This indicates that cessation of cambial activity in the bundles of the fruit coincides with the onset of xylem dysfunction.
Cortical and carpellary vascular systems varied in the dynamics of xylem breakdown. Dye accumulation was somewhat less disrupted in the carpellary bundles that are apparently less conductive (Fig. 3). Comparing with primary bundles, it is apparent that dorsal bundles are less affected with a progressive but slower loss of xylem function (Fig. 3B) but, similarly, the conductance appears to drop without a lower limit. Changes in D once again occurred more rapidly for ‘Braeburn’ and, although statistically significant, cultivar differences were smaller and less regular.
Ventral bundles exhibited distinctively lower D at most stages throughout the growth period (Fig. 3C). After the initial rise, xylem functionality declined sharply but proceeded with a rather low, yet steady flow following an exponential decay. For most of the fruit evaluated, the ventral bundles accumulated substantially less dye and often appeared unstained.
The lower D in the dorsal and ventral bundles could result from naturally reduced dye uptake through the carpellary network, which develops well below the skin and appears less affected by transpiration. Dorsal bundles that follow the outlines of the carpel bases may undergo seasonal changes in xylem function closely related to the growth rates of adjacent tissue. In contrast, the ventral bundles serve the seeds, which presumably require less nutrient (diminished water influx) than the flesh. This, and the fact that the ventral bundles develop below the carpel bases, may affect dye accumulation in a way that dye spreads slowly throughout the ventral network, which results in consistently lower D when assessed in transverse sections. Simultaneously, the establishment of reproductive structures determines the course of vascular function such that ovule abortion causes degeneration of the supporting ventral bundles (MacArthur and Wetmore, 1939). Considering that seed abortion in developing apples is continuous throughout the season (unpublished data), it seems reasonable that functionality of the ventral bundles is somewhat hampered compared with the other bundles. However, the origin of the functional disturbance at the beginning of the season remains unknown.
Significant changes in xylem function were established using contiguous sectioning to evaluate D in vascular bundles. The most conspicuous gradient was obtained in the dorsal bundles that on average scored higher D values than the other bundle classes and differed between the two cultivars (Fig. 4B and E). However, dye concentration declined in nearly all bundles with increasing distance from the stalk end of the fruit suggesting gradual disruption of the flow along the bundles. This suggests that tensile stresses created by an expanding fleshy matrix develop evenly along the bundle path so that xylem breakage appears at random as fruit growth continues.
Structure/function character of the vascular tissues
While the lignified conducting cells of the xylem provide good mechanical support, they exhibit low elasticity (Düring et al., 1987). A mechanistic explanation for the reduction in xylem conductance would implicate growth‐induced damage to the xylem strand in the bundle (Düring et al., 1987; Findlay et al., 1987; Lang and Ryan, 1994; Dražeta et al., 2001). Since the magnitude of cell wall deformation is proportional to the time of applied stress (Nobel, 1999), the growth dynamics could principally determine cell functionality in stressed tissue. Consequently, vigorous fruit growth, particularly from the onset of cell expansion, is likely to initiate earlier xylem dysfunction.
However, the function of the phloem remains unchanged relative to the xylem flow (Lang, 1990). The hypothesis that fruit expansion stretches and ruptures the xylem provides no elucidation of the mechanism that maintains phloem function in the bundle. Because phloem tissue is composed of living conducting elements (Esau, 1977), the persistence of functional phloem could be attributed to persistent sieve tube differentiation that bypasses the broken elements [since the phloem differentiates in the absence of the xylem and at lower auxin levels (Aloni, 1980; Aloni and Barnett, 1996)], or to the more extensible cell walls of the sieve tubes (Lee, 1981) with a more prolonged synthesis of cell wall depositions enabling the sieve tubes to accommodate more strain, or to a continuous symplastic pathway through the parenchyma cells that occupy the voids between the broken structures. If fruit growth principally creates stress on conductive tissues it is likely that any one, or some combination, of these features allows continuous phloem uptake into the fruit.
Fruit growth, xylem functionality and calcium nutrition
The timing of xylem dysfunction will affect the mineral composition of the fruit. A steady decline in xylem conductance under constant fruit transpiration must force a shift in the balance of supply to the fruit of xylem‐borne nutrients, notably calcium. Indeed, xylem function decays from relatively early in the season, which mirrors the decline in calcium import (Wilkinson, 1968; Jones et al., 1983). This naturally occurring mechanism could be principally responsible for the increased phloem inflow to the fruit that maintains growth (Lang, 1990).
It has been shown in apple that the direction of xylem movement reverses diurnally such that sap flow is from fruit to tree during the day and from tree to fruit at night (Lang, 1990; Lang and Volz, 1998). Programmed xylem breakdown is likely to reduce diurnal apoplastic backflow of solutes (from fruit to the tree), which promotes the partitioning of assimilates to reproductive sinks, as suggested for grapes (Lang et al., 1986; Lang and Thorpe, 1989; Lang and Düring, 1991). Hence, xylem dysfunction could be seen as minimizing outflowing xylem sap from the fruit but at the expense of reduced import of xylem‐borne minerals, such as calcium, to the fruit. A rather variable timing of xylem dysfunction could, therefore, create high variability in fruit mineral composition. This could explain the high variability observed in the incidence of calcium‐related disorders, such as bitter pit (Dražeta et al., 2001). Hence, the earlier start of xylem dysfunction in ‘Braeburn’ fits with the observation that, of the two cultivars examined, ‘Braeburn’ is the more susceptible to bitter pit.
Furthermore, the reduction in dye accumulation towards the calyx end of the fruit fits with the longitudinal gradient in calcium concentration found in apple fruit (Ferguson and Watkins, 1989). As the fruit expands, progressive xylem dysfunction is likely to reduce calcium uptake and displacement within the fruit, leading to the uneven spatial distribution and imbalance with phloem‐borne minerals. Consequently, the distal regions of the fruit may become relatively more depleted in calcium and more at risk of developing calcium‐related disorders than the proximal zones. Indeed, bitter pit is commonly observed near the calyx‐end of apple fruit (Ferguson and Watkins, 1989). Thus the localized nature and the susceptibility to the disorder fit with the spatial and temporal profile of dye accumulation in the fruit and, by implication, with xylem functionality.
ACKNOWLEDGEMENTS
This work was supported by the New Zealand Foundation for Research, Science and Technology (FRST) and the Agricultural and Marketing Research and Development Trust (AGMARDT). We also thank Mr Mike Jarvis, Ms Kathy Murray, Miss Andrea Leonard‐Jones, Dr Stefan Henton, Dr Paul Austin, Mr Doug Hopcroft and Mr Raymond Bennett for their contributions.
Supplementary Material
Received: 14 August 2003;; Returned for revision: 8 October 2003; Accepted: 26 November 2003
References
- AloniR.1980. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 150: 255–263. [DOI] [PubMed] [Google Scholar]
- AloniR, Barnett JR.1996. The development of phloem anastomoses between vascular bundles and their role in xylem regeneration after wounding in Cucurbita and Dahlia Planta 198: 595–603. [DOI] [PubMed] [Google Scholar]
- BardenJA, Thompson AH.1963. Developmental anatomy of vascular tissues in York Imperial apple, with special emphasis on the pedicel. Maryland Agricultural Experiment Station Bulletin A‐131. [Google Scholar]
- CreasyGL, Price SF, Lombard PB.1993. Evidence for xylem discontinuity in Pinot noir and Merlot grapes: dye uptake and mineral composition during berry maturation. American Journal of Enology and Viticulture 44: 187–192. [Google Scholar]
- DražetaL, Lang A, Morgan L, Volz R, Jameson PE.2001. Bitter pit and vascular function in apples. Acta Horticulturae 564: 387–392. [Google Scholar]
- DüringH, Lang A, Oggionni F.1987. Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology. Vitis 26: 123–131. [Google Scholar]
- EsauK.1977.Anatomy of seed plants, 2nd edn. New York: John Wiley & Sons. [Google Scholar]
- FergusonIB, Watkins CB.1989. Bitter pit in apple fruit. Horticultural Reviews 11: 289–355. [Google Scholar]
- FindlayN, Oliver KJ, Nii N, Coombe BG.1987. Solute accumulation by grape pericarp cells. IV. Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries. Journal of Experimental Botany 38: 668–679. [Google Scholar]
- JonesHG, Higgs KH, Samuelson TJ.1983. Calcium uptake by developing apple fruits. I. Seasonal changes in calcium content of fruits. Journal of Horticultural Science 58: 173–182. [Google Scholar]
- LangA.1990. Xylem, phloem and transpiration flows in developing apple fruits. Journal of Experimental Botany 41: 645–651. [Google Scholar]
- LangA, Thorpe MR.1989. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes’ principle. Journal of Experimental Botany 40: 1069–1078. [Google Scholar]
- LangA, Düring H.1991. Partitioning control by water potential gradient: evidence for compartmentation breakdown in grape berries. Journal of Experimental Botany 42: 1117–1122. [Google Scholar]
- LangA, Ryan KG.1994. Vascular development and sap flow in apple pedicels. Annals of Botany 74: 381–388. [Google Scholar]
- LangA, Volz RK.1998. Spur leaves increase calcium in young apples by promoting xylem inflow and outflow. Journal of the American Society for Horticultural Science 123: 956–960. [Google Scholar]
- LangA, Thorpe MR, Edwards WRN.1986. Plant water potential and translocation. In: Cronshaw J, Lucas WJ, Giaquinta RT, eds. International conference on phloem transport, Asilomar, CA, USA, 18–23 August 1985. Phloem transport New York: Alan R. Liss, 193–194. [Google Scholar]
- LeeDR.1981. Elasticity of phloem tissues. Journal of Experimental Botany 32: 251–260. [Google Scholar]
- MacArthurM, Wetmore RH.1939. Developmental studies in apple fruit in the varieties McIntosh Red and Wagener. I. Vascular anatomy. Journal of Pomology and Horticultural Science 17: 218–232. [Google Scholar]
- MacDanielsLH.1940. The morphology of the apple and other pome fruits. New York (Cornell University) Agricultural Experiment Station Memoir 230. [Google Scholar]
- MarschnerH.1986.Mineral nutrition of higher plants. London: Academic Press. [Google Scholar]
- NobelPS.1999.Physicochemical and environmental plant physiology, 2nd edn. San Diego: Academic Press. [Google Scholar]
- SAS.2000.SAS OnlineDoc(rtm), Version 8. SAS Institute, Cary, NC. [Google Scholar]
- WilkinsonBG.1968. Mineral composition of apples. IX. Uptake of calcium by the fruit. Journal of the Science of Food and Agriculture 19: 646–647. [DOI] [PubMed] [Google Scholar]
- WolswinkelP, Ammerlaan A, Koerselman‐Kooij J.1999. Changes in the function of the xylem pathway during the development of fleshy fruits: implications for phloem transport, water movement and fruit growth. In: Heyes J, Offler C, Patrick J, Thorpe M, eds. International conference on assimilate transport and partitioning, Newcastle, NSW, Australia, 15–20 August 1999. Scientific Programme and Abstracts Book, 164. Hamilton, NSW: Lloyd Scott Enterprises Pty Ltd. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.