Abstract
• Background and Aims Leymus chinensis is an economically and ecologically important grass that is widely distributed across eastern areas of the Eurasian steppe. A major problem facing its propagation by man is its low sexual reproductivity. The causes of low fecundity are uncertain, largely because many aspects of the reproductive biology of this species remained unknown or incomplete. This study aims to address some of these issues.
• Methods Pollen dispersion, pollen viability, pollen longevity and pistil receptivity were studied in a representative, natural population of L. chinensis growing in Inner Mongolia.
• Key Results Flowering of L. chinensis occurred at the end of June and lasted for 5 d. Pollination peaked between 1600 h and 1700 h, and about 56·1 % of the total pollen grains were released at this time. Pollen density was highest towards the middle of flowering spikes and lowest at the bottom over the 5 d measurement period. Pollen viability (62·4 %) assessed using TTC was more accurate than using IKI (85·6 %); 50 % of pollen arriving on stigmas germinated. Pollen remained viable for only 3 h and the pollen : ovule ratio was 79 333 : 1. Pistil receptivity lasted for only 3 h and, overall, 86·7 % of pistils were pollinated. Within the spike, the relative fecundity of different positions was middle > lower > upper throughout the period of pollination; daily variation of fecundity was similar to that of the pollen flow. The spikes that opened on the day of highest pollen density exhibited the highest fecundity (36·0 %). No seeds were produced by self‐pollination.
• Conclusions The data suggest that low pollen viability, short pollen longevity and short pistil receptivity all appear to contribute to the low seed production typical of this important forage crop.
Key words: Leymus chinensis, pollen dispersion, pollen viability, pistil receptivity, fecundity, laser scanning confocal microscopy
INTRODUCTION
Leymus chinensis (Aneurolepidium chinensis), a member of the family Gramineae, is a perennial rhizome grass (Kuo, 1987). Due to its excellent stress tolerance (Koyama, 1987; Yin et al., 1993; Xiao et al., 1995), grasslands dominated by L. chinensis are widely distributed at the eastern end of the Eurasian steppe, from North Korea westward to Mongolia and northern China, and north‐westward to Siberia. Early spring emergence and rapid growth, high palatability and herbage production make the grasslands ideal for grazing and forage production (Li et al., 1983). Furthermore, because of its high vegetative propagation and abundant horizontally creeping rhizomes, L. chinensis is used as a soil‐binding plant to protect soil from desertification in the arid areas of northern China.
Because L. chinensis is an economically and ecologically important grass, the species has received considerable attention (Wang, 1984). However, a major problem facing its propagation is its low sexual reproductivity. To better understand the causes of the species’ low fecundity, several investigations have been conducted (Wang, 1984, 1998; Huang et al., 2002); however, these have focused on the effects of ecological factors. Few investigations (Ma et al., 1984; Zhao and Tu, 1993) have dealt with its sexual reproduction. It has been reported that seed production under natural conditions can be <40 % (Wang, 1984). Seed production by L. chinensis may be influenced by climate (Guo and Zhu, 1994; Gao et al., 1999; Yang et al., 2000), nutrient uptake (Yang, 1989; Wang, 1998) and vegetative growth (Wang, 1998, Wang and Ripley, 2000), as well as by disturbance by men and animals (Yang and Zhu, 1988; Yang and Zhu, 1989; Wang, 2000). However, the causes of low fecundity are uncertain, largely because many aspects of the reproductive biology of this species remained unknown or incomplete. The objectives of this investigation therefore were to measure the pattern of pollen dispersion, pollen viability and pistil receptivity during the pollination period under natural conditions, and to evaluate the effects of these factors on seed production.
MATERIALS AND METHODS
Site description
The study site was located south of the Xilin River basin, Inner Mongolia, China (43°32′58′′N, 116°40′34′′E), approx. 1265 m a.s.l. It has a temperate, semi‐arid climate with a mean annual rainfall and temperature of 350 mm and 0·3 °C, respectively (Chen, 1988). The permanent plot of L. chinensis belongs to the Inner Mongolian Grassland Ecosystem Research Station and it has been free from grazing since it was fenced in 1979.
Pollen dispersion
Pollen traps constructed from petroleum jelly on microscope slides were attached to vertical wooden laths facing the direction of the prevailing wind (Mulugeta et al., 1994). Laths were driven into the ground, with the slides at heights of 0·38, 0·45 and 0·52 m above the soil surface, corresponding to the lower, middle and upper positions of the spikes, respectively (two replicates for each position). Pollen was collected daily from 21 to 31 June 2002. From 21 to 25 June, the slides were set at 0800 h and changed at 1800 h. From 26 to 31 June, the slides were set at 0800 h and changed at 1200 h, 1400 h, and then at every hour till 1800 h. The slides set at 1800 h were collected at 0800 h the following day. During the collection periods temperature and relative humidity were recorded. The highest temperature was found between 1400 and 1500 h, and then it gradually declined. Relative humidity was highest between 1400 and 1600 h and declined to a minimum between 1600 and 1700 h, after which it increased again.
Pollen density counts were taken from five random fields of view (field size = 20 mm2) per slide under a microscope (Olympus BH‐2) at ×40 magnification without staining. Leymus chinensis pollen could be identified and distinguished from that of other species by its shape (spherical) and size (30·7–35·2 µm) (Wan and Wei, 1999). The data collected were used to estimate the temporal and spatial distribution of pollen flow.
Pollen viability and longevity
Leymus chinensis began to flower on 26 June, and produced pollen for about 5 d. During the pollination days, fresh pollen was collected daily at 1500 h by placing the anthers in Petri dishes just before anthesis. Pollen was stained with either 1,2,3‐triphenyl tetrazolium choride (TTC) (1·0 % by weight in 50 % sucrose) or iodine–potassium iodide (IKI) (Mulugeta et al., 1994). Freshly harvested pollen was dusted onto a microscope slide with a brush to which four or five drops of stain were added. Then the slide was immediately covered with a coverslip and the edges sealed with nail varnish. Pollen was observed 10 min after staining with IKI, while pollen grains stained with TTC were examined after 15–30 min incubation at 40 °C. The percentage of pollen out of 300–500 grains per slide (three replicates for each staining treatment) that exhibited the appropriate staining reaction was determined using an Olympus Vanox microscope at ×100 magnification. Control experiments were performed using heat‐killed pollen (80 °C for 2 h; Dafni and Firmage, 2000).
Pollen longevity was measured at room temperature. Twenty fresh anthers were selected from ten plants at random; ten were kept on dry blotting paper in Petri dishes and the other ten were kept in Petri dishes containing water‐saturated blotting paper. The viability was then tested by TTC at 0, 1, 2 and 3 h after the anthers dehisced. Pollen longevity was estimated by counting the number of stained pollen grains out of 300–500 pollen grains per slide.
Pistil receptivity, pollination success and pollen tube growth in vivo
To determine the quantity of pollen grains on the pistil and in vivo pollen tube growth, the spikes from which pistils were collected were tagged at six different times (0, 1, 2, 3, 24 and 27 h) from flower opening. After fixation in 2·5 % glutaraldehyde, the pistils were kept in 70 % ethanol, and then transferred to 10 % aqueous sodium sulfite, autoclaved at 160 kPa and 120 °C for 15 min, and stained in decolourized aniline blue (aniline blue WS 0·1 % in aqueous 0·1 m K3PO4, 10 % (w/v) glycerol). Pistils were then squashed in stain and sealed with petroleum jelly to prevent dehydration (Williams et al., 1982). All preparations (30 pistils for each time period) were examined using a laser scanning confocal microscope (Bio‐Rad 1000 laser scanning confocal microscope) with the filter combinations of KP490, KP500, Rfl 510 and LP 528. The pollination success was calculated as the percentage of pollinated pistils after open pollination (Tangmitcharoen and Owens, 1997).
Pollen production and seed set
To study floral morphology, 30 mature flowers were collected and observed using a dissecting microscope (Opton 47 52 00‐9901). Samples for light microscopy were fixed in FAA (formalin–acetic acid–alcohol) and were then sectioned (6–8 µm) after being embedded in paraffin wax (Jensen, 1962). To determine the number of pollen grains per flower, one mature anther per flower was removed from five unopened flowers of five different spikes. The anthers were gently squashed in 1 ml of TTC, the pollen grains transferred to a graticule slide and counted using an Olympus BH‐2 microscope at ×100 magnification. The pollen : ovule ratio was determined by dividing the average number of pollen grains per flower by the average number of ovules per flower. During the anthesis period, ten spikes were tagged every day. The numbers of seeds set were counted 2 months after pollination. Fecundity was calculated by dividing the number of seeds set 2 months after pollination by the total number of flowers at pollination from the ten spikes tagged.
Data analysis
Means (± standard error) were calculated for all the measurements using Excel (2000) or SPSS vs10·0 software for Windows (10·0). Analysis of variance (ANOVA) was used to assess the variation of the data. Duncan’s new multiple range test at P < 0·05 was used to compare the means and determine the significance of differences between variables.
RESULTS
Floral morphology
Leymus chinensis flowers are hermaphroditic and arranged in compound spikes. There were approx. 45 ± 5 spikes m–2, and the plant height ranged from 0·4 to 0·6m with 0·14 ± 0·02 mm spikes at the time of flowering. At the base of each spikelet, there is a pair of sterile glumes that surrounds a series of flowers (five to eight in number). Each flower has a lemma (outer bract) and a palea (inner bract) at its base, and contains two lodicules and one pistil surrounded by three anthers. The mature pistil has two laterally feathery stigmas and a hairy ovary (Fig. 1A). Each anther has four microsporangia arranged in pairs in the two lobes (Fig. 1B). The ovary has one locule, containing one anatropous ovule. The ovary is superiorly positioned with basal placentation (Fig. 1C). The flowers usually open synchronously or basipetally within a spike between 1400 h and 1800 h. Some of the upper flowers begin to open at 1400 h. At 1500 h, several middle flowers begin to open. Most of the middle flowers and lower flowers opened synchronously between 1600 h and 1700 h, while a few flowers open after 1700 h.

Fig. 1. Flower structure and micrograph of the pollen and pollen tube of Leymus chinensis. (A) A complete flower, showing three stamens, one pistil with two feathery stigmas, a lemma (outer bract) and a palea (inner bract). Bar = 2 mm. (B) Transverse section of an anther. Note that there are four microsporangia arranged in pairs in the two lobes of an individual anther. Bar = 150 µm. (C) Longitudinal section of pistil stained with Heidenhain’s iron–haematoxylin. Note that the ovary has one locule and contains one anatropous ovule. Bar = 170 µm. (D) Pollen grains stained by 1, 2, 3‐triphenyl tetrazolium choride (TTC). Red staining (white arrow) indicates that pollen grains are viable, lack of stain indicates that they are not (black arrow). Bar = 45 µm. (E) Pollen grains stained with iodine–potassium iodide (IKI). Stained pollen grains (white arrow) are viable, unstained grains (black arrows) are not. Bar = 45 µm. (F) Heat‐killed pollen treated with TTC: no staining. (G) Heat‐killed pollen treated with IKI: some grains are stained (white arrow). (H) Confocal micrograph of a pollinated pistil, showing pollen grains and pollen tube growth. The pollen grains on the stigma are countable. Bar = 80 µm. (I) Higher magnification of the stigma shown in (H). Pollen grains germinate on the stigma and pollen tubes grow down through the transmitting tissue of the stigma. Callose formed during pollen tube elongation is visualized after staining with aniline blue. Bar = 20 µm. Abbreviations: An, anther; Ib, inner bract; Ob, outer bract; Ov, ovary; St, stigma; Ms, microsporangia; Pg, pollen grain; Es, embryo sac; Ii, inner integument; Oi, outer integument; Pt, pollen tube; Tt, transmitting tissue.
Pollen dispersion
In this study, few plants flowered before 26 June, and no pollen grains were collected from 21 to 25 June. Pollen dispersal began on 26 June. The daily pollen density increased to a peak on 28 June, and then decreased (Fig. 2). After 30 June, <5 % of the spikes in this field produced pollen.
Fig. 2. Mean fecundity and pollen flow during the pollination period. Vertical bars represent the standard deviation of the mean (P = 0·05). Data for pollen density after 30 June is not presented.
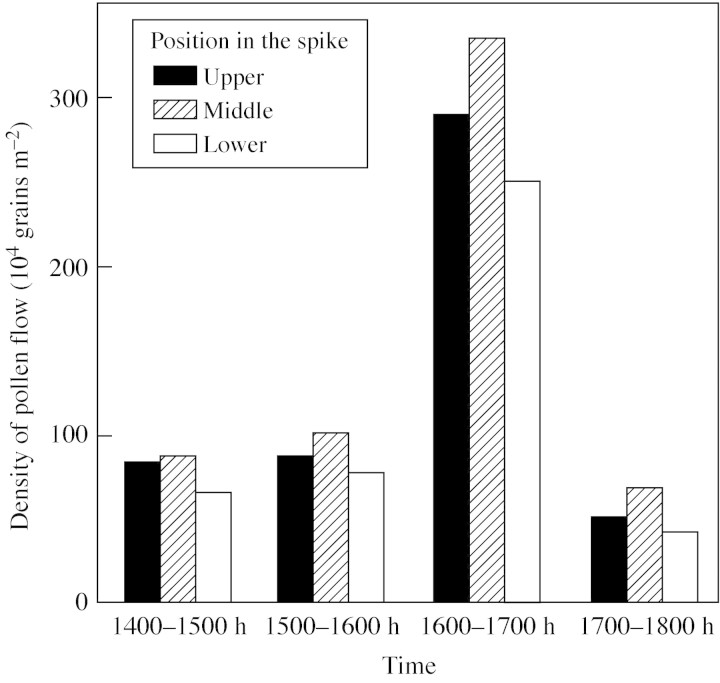
Variability was found in pollen density between different periods of collection within an individual day and between the different positions within a spike, as shown in Fig. 3. The variation in pollen density during each day followed a similar trend at the three different positions within a spike. Pollen dispersal began at 1400 h, but at low density until 1600 h. The peak occurred between 1600 and 1700 h, during which period 56 % of the total pollen liberated that day was obtained (Fig. 3). After 1700 h, pollen density sharply decreased to 55·54 × 104 grains m–2.
Fig. 3. Temporal and spatial distribution of pollen flow. Data show the means of the density of pollen flow (104 grains m–2) over the 5 d of collection period.
Variation was also observed in pollen density between different positions in a spike. For each collection period, the number of pollen grains was highest in the middle portion of the spike and lowest in the upper portion of the spike. The highest pollen density occurred between 1600 and 1700 h at the middle position (330·63 × 104 grains m–2). The lowest pollen density (44·50 × 104 grains m–2) occurred at the lower position between 1700 and 1800 h. The highest mean of pollen density (148·0 × 104 grains m–2) was found in the middle position and the lowest (109·3 × 104 grains m–2) was found in the lower position; that of the upper position was 128·5 × 104 grains m–2.
Pollen viability and longevity
Different staining methods gave different assessments of pollen viability. Viability was 62 ± 5 % when stained with TTC (Fig. 1D), but was 86 ± 3 % when stained with IKI (Fig. 1E). The difference between the two treatments was highly significant (P < 0·05). After fresh pollen was heat‐killed for 2 h at 80 °C, no colour reaction was observed with TTC (Fig. 1F). However, most of the heat‐killed pollen treated with IKI stained in the same manner as fresh pollen (Fig. 1G).
Staining with TTC showed that pollen can survive for about 3 h at room temperature. Pollen viability was about 71 % at the moment of anther dehiscence. When pollen was kept at room temperature in Petri dishes, viability decreased quickly. After 1 h, the viability declined to 56 % in wet conditions and to 44 % in dry conditions; after 2 h it decreased further to 34 % and to 18 %, respectively. Although the viability of the pollen kept in wet conditions decreased more slowly than in dry conditions, under both conditions viability decreased to below 5 % after 3 h (Fig. 4).
Fig. 4. Pollen longevity at room temperature under wet or dry conditions. Data show the means (n = 6) and vertical bars represents the standard deviation of the mean (P = 0·05).
Pollination began when pollen grains landed on the surface of the stigma, which had the appearance of a meadow of finger‐like papillary cells. The pollen germinated in about 3 h, and pollen tubes emerged and grew down the style. The tubes were readily visualized by staining callose with aniline blue (Fig. 1H and I). It was found that approx. 50 % of the pollen germinated under the conditions of the experiment.
Pistil receptivity
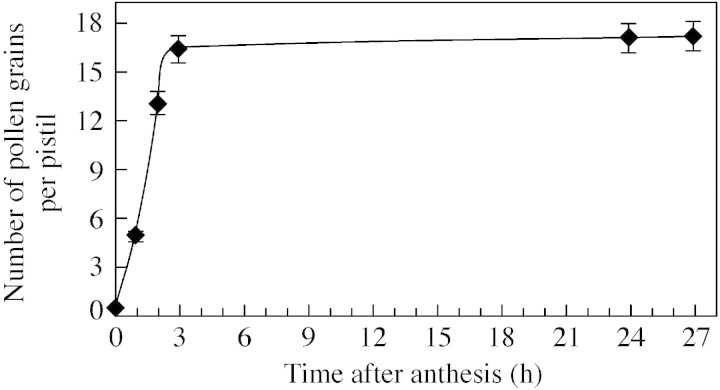
Pistil receptivity lasted for about 3 h after anthesis. Laser scanning confocal microscopy showed that, pre‐anthesis, almost all the stigmatic surface was small and smooth without pollen grains. As the pistil emerged from the bracts, the style elongated and the stigma expanded markedly in size and, finally, became receptive to pollen. The number of pollen grains reaching the stigma (pollination success) gradually increased during the first 3 h of the period of receptivity and than reached a plateau. About 6·7 % of pistils were pollinated at the time of anthesis (1500 h); however, on average, there was less than one pollen grain per pistil at this time. There was a significant increase in pollination between 1500 and 1700 h. At 1800 h approx. 86·7 % of pistils were pollinated with an average of 16·5 pollen grains per pistil. There were no further significant increases thereafter (Figs 5 and 6). Thus, the most effective pollination period was between 1500 and 1800 h in terms of both pistils pollinated and the number of pollen grains per pistils (Figs 5 and 6).
Fig. 5. Variation in the receptivity of the pistil. Data show the mean of pollen grains per pistil (n = 30), and each vertical bar represents the standard deviation of the mean (P = 0·05).
Fig. 6. Variation in the success of pollination. Data show the percentage of pollinated pistils after anthesis.
Pollen production and fecundity
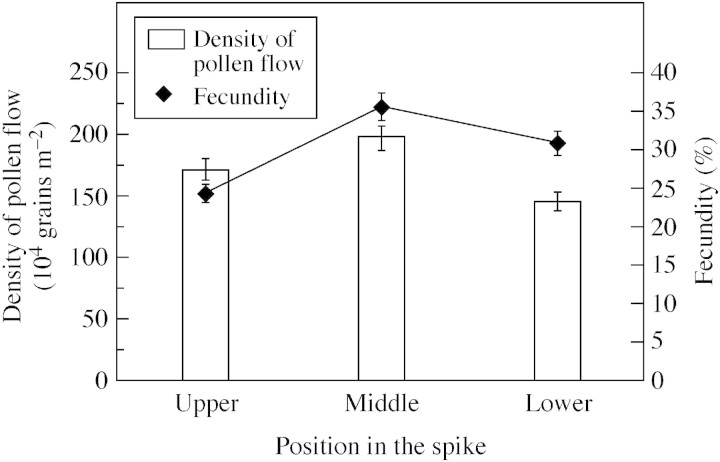
There was not much difference in the number of pollen grains produced per anther between spikes (26 444 ± 1799, n = 10). The pollen : ovule ratio was 79 333 : 1. Variation was observed in fecundity in relation to different densities of pollen flow. Within an individual spike, fecundity was 24·3, 35·5 and 30·8 % for the lowest, middle and upper positions of the spike, respectively (Fig. 7), and correlated with the relative pollen densities at these positions.
Fig. 7. Pollen flow and fecundity at the different positions within the spike. Data show means and vertical bars represent the standard deviation of the mean (P = 0·05).
Over the whole period of pollination (26–30 June), the variation in fecundity tended to be similar to that of pollen flow (Fig. 2). Fecundity varied as the spikes opened from day to day, and the mean value was 30·2 % for all the spikes. It increased from 26 to 28 June, followed by a decline thereafter. After 30 June, fecundity dropped to 16·5 %. No seeds were produced if spikes were covered with bags.
DISCUSSION
It is common for hermaphroditic angiosperms to produce more flowers and ovules than fruits and seeds (Willson, 1979; Bawa and Webb, 1984; Sutherland and Delph, 1984; Sutherland, 1986). To explain the existence of these non‐fruiting flowers, two hypotheses have been put forward (Sutherland, 1987). One proposes that resources other than pollen limit seed production. The other hypothesis proposes that seed plants typically do not receive enough pollen for full fruit‐ or seed‐set. The amount of pollen produced by a flower reflects the probability that a sufficient number of pollen grains will reach a stigma (Cruden, 1977). In the present investigation, pollen production was prolific as shown by the high pollen : ovule ratio (79 333 : 1). According to the definitions proposed by Cruden (1977), L. chinensis should be classified as exhibiting obligate xenogamy (the highest outcrossing level). In addition, the lack of seed production in the spikes covered with bags also supports the view that L. chinensis is normally outcrossing and possesses strong barriers to self‐fertilization.
In several xenial species, it has been reported that the pistils do not receive enough pollen for fruit‐ or seed‐set and that the fecundity can be raised by supplemental hand pollination (Bawa and Webb, 1984; Zimmerman and Pyke, 1988; Johnston, 1991). Ma (1984) observed a low density of pollen flow due to asynchronous dehiscence in a cultivated population of L. chinensis, resulting in low fecundity. However, in the current study it was found that dehiscence in the natural population was generally synchronous in terms of pollen dispersion. Over 95 % of the spikes flowered within 5 d between 26 and 30 June, and 56·15 % of the total pollen was concentrated between 1600 and 1700 h in the 4‐h collection period. Correspondingly, the highest fecundity occurred on 28 June, while the lowest fecundity was found in the spikes that flowered after 30 June. The similar trend of variation in both seed‐setting and pollen density indicated that a high fecundity was consistent with a high pollen density on the day of flowering. It was further observed that variation in fecundity correlated positively with pollen density within an individual spike, as both the highest fecundity and highest density of pollen flow appeared half‐way up the spikes (Fig. 7). From this positive correlation, it is deduced that fruit‐ and seed‐set can be limited by pollen load, particularly in the lower spikes and beyond the period 26–30 June. Even in the upper spikes and within the period 26–30 June, the frequency of successful pollination can be lowered when and/or where pollinators are unreliable, since xenogamous species are primarily outcrossers or self‐incompatible (Cruden, 1977).
Pollen viability is considered as an important parameter of pollen quality (Dafni and Firmage, 2000). Staining with IKI and TTC are common techniques used to determine pollen viability (Mulugeta et al., 1994; Shirazi and Muir, 1998; Zhou et al., 1999; Dafni and Firmage, 2000). Ma et al. (1984) reported that pollen viability was about 90 % when tested with IKI, while the fecundity was below 50 %. However, in the study presented here, the pollen viability was 85·6 % and 62·4 % after staining with IKI and TTC, respectively. The difference between these two treatments was highly significant (P < 0·05), demonstrating that it is essential to correlate staining techniques with in vivo methods before trusting either of the pollen staining reactions as an indicator of viability (Fritz and Lukaszewski, 1989; Sedgley and Harbard, 1993). In this study, it was found that IKI staining indicated an unrealistically high viability, whilst the TTC reaction produced a reasonable indication of viability when compared with the germination rate of pollen grains in vivo. Furthermore, this discrepancy was reflected in the colour reaction of heat‐killed pollen. IKI staining clearly failed to distinguish fresh from heat‐killed pollen, whilst TTC staining easily separated the two.
Pollen longevity is another important factor related to fecundity that might limit seed production (Fritz and Lukaszewski, 1989; Dafni and Firmage, 2000). The experiments described here indicated that pollen viability can decline sharply from 70·8 % to <5 % after 3 h. In other words, pollen of this species is short‐lived and pollination may therefore fail if the pollen reaches the pistil after a delay of longer than 3 h. Since L. chinensis is an anemophilous species, low pollen viability could be associated with the high temperatures and low relative humidity typical of this arid area.
In many angiosperm species, pistil receptivity can last for one or several days. A long duration of pistil receptivity helps high pollination success (Nepi and Pacini, 1993; Tangmitcharoen and Owens, 1997; Sornsathapornkul and Owens, 1998; Aleemullah et al. 2000). The results presented here indicate that pistil receptivity of L. chinensis lasted for only about 3 h. Beyond this time, pistils will usually fail to be pollinated no matter how high the viability and the density of pollen. There was a seeming conflict between the higher pollen density and the lower fecundity at the upper position of the spike (Fig. 7). As the flowers at the upper position frequently opened earlier in the day (1400 h) than those in the other positions (1600–1700 h), pollen density was low at that time. Indeed, pollen density increased (1600–1700 h) when the pistil receptivity would have been significantly decreasing. It would appear that the short pistil receptivity is an adverse factor for seed production in this species.
In summary, the present work has revealed details of pollen dispersal, pollen longevity and pistil receptivity in a natural population of Leymus chinensis. In particular, it has shown that variation in pollen density and fecundity occurs between different positions within individual spikes and also between spikes. In addition, pollen viability, pollen longevity and pistil receptivity were found to be closely related to fecundity levels. The data presented here suggest that these factors would limit the overall amount of seed production by L. chinensis under natural conditions. Further study of megasporogenesis, fertilization and embryogenesis in this species would help towards understanding the causes of its low fecundity.
ACKNOWLEDGEMENTS
We thank the Inner Mongolian Grassland Ecosystem Research Station for providing field and laboratory facilities. We also thank Dr Shen ShuJin and Prof. Hu Yuxi for constructive criticism of the manuscript. This study was founded by the Key Project of the Chinese Academy of Science (KSCX1‐08).
Supplementary Material
Received: 16 April 2003;; Returned for revision: 10 September 2003; Accepted: 3 December 2003, Published electronically: 26 January 2004
References
- AleemullahM, Haigh AM, Holford P.2000. Anthesis, anther dehiscence, pistil receptivity and fruit development in the Longum group of Capsicum annuum Australian Journal of Experimental Agriculture 40: 755–762. [Google Scholar]
- BawaKS, Webb CJ.1984. Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. American Journal of Botany 71: 736–751. [Google Scholar]
- ChenZZ.1988. Topography and climate of the Xilin River basin. Research on Grassland Ecosystem 3: 13–22. [Google Scholar]
- CrudenRW.1977. Pollen–ovule ratios: A conservative indicator of breeding systems in flowering plant. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- DafniA, Firmage D.2000. Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Systematics and Evolution 222: 113–132. [Google Scholar]
- FritzSE, Lukaszewski AJ.1989. Pollen longevity in wheat, rye and Triticale. Plant Breeding 102: 31–34. [Google Scholar]
- GaoLM, Huang YX. Lin SH.1999. Effects of double CO2 concentration on the phenology and growth of Leymus chinensis Environmental Science 20: 25–29. [Google Scholar]
- GuoJX, Zhu TC.1994. Effect of climatic factors on the yield of Aneurolepidium chinense (Trin.) Keng community. Acta Botanica Sinica 36: 790–796. [Google Scholar]
- HongTD, Ellis RH, Buitink J, Walters C, Hoekstra FA, Crane J.1999. A model of the effect of temperature and moisture on pollen longevity in air‐dry storage environments. Annals of Botany 83: 167–173. [Google Scholar]
- HuangZH, Zhu JM, Mu XJ, Lin JX.2002. Advances on the mechanism of low sexual reproductivity of Leymus chinensis. Grassland of China 24: 55–60. [Google Scholar]
- JensenWA.1962.Botanical histochemistry. San Francisco: Freeman. [Google Scholar]
- JohnstonMO.1991. Pollen limitation of female reproduction in Lobelia cardinalis and L. siphilitica Ecology 72: 1500–1503. [Google Scholar]
- KoyamaT.1987.Grasses of Japan and its neighboring regions Tokyo: Kodansha, 48–50. [Google Scholar]
- KuoPC.1987.Flora Reipublicae Popularis Sinicae Beijing: Science Press, 9: 19. [Google Scholar]
- LiCH, Zhao KY, Ye JX.1983. Basic types of pasture vegetation in the Sonnen plain. In: Proceedings of XIV International grassland congress, 15–24 June 1981, Lexington, KY. Boulder, CO: Westview Press, 876. [Google Scholar]
- MaHL, Wan T, Wang FG.1984. Characteristics of seed setting and causes of low seed yields in Aneurolepidium chinense Grassland of China (6): 15–21. [Google Scholar]
- MulugetaD, Maxwell BD, Fay PK, Dyer WE.1994. Kochia (Kochia scoparia) pollen dispersion, viability and germination. Weed Science 42: 548–552. [Google Scholar]
- NepiM, Pacini E.1993. Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Annals of Botany 72: 527–536. [Google Scholar]
- SedgleyM, Harbard J.1993. Pollen storage and breeding system relation to controlled pollination of four species of Acacia (Leguminosae: Mimosoideae). Australian Journal of Botany 41: 601–609. [Google Scholar]
- ShiraziAM, Muir PS.1998.In vitro effect of formaldehyde on Douglas fir pollen. Plant, Cell and Environment 21: 341–346. [Google Scholar]
- SornsathapornkulP, Owens JN.1998. Pollination biology in a tropical Acacia hybrid (A. mangium Willd. × A. auriculiformis A. Cunn. ex Benth.). Annals of Botany 81: 631–645. [Google Scholar]
- SutherlandS,1986. Patterns of fruit‐set: what controls fruit‐flower ratio in plants? Evolution 40: 117–128. [DOI] [PubMed] [Google Scholar]
- SutherlandS.1987. Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana Evolution 41: 750–759. [DOI] [PubMed] [Google Scholar]
- SutherlandS, Delph LF.1984. On the importance of male fitness in plants: patterns of fruit‐set. Ecology 65: 1093–1104. [Google Scholar]
- TangmitcharoenS, Owens JN.1997. Floral biology, pollination, pistil receptivity, and pollen tube growth of teak (Tectona grandis Linn f.). Annals of Botany 79: 227–241. [Google Scholar]
- WanT, Wei ZJ.1999.Pollen morphology of the modern plants in the grassland of Inner Mongolia. Beijing: China Agriculture Press. [Google Scholar]
- WangKP.1984. The study of the species differentiations of Leymus chinensis wildrge. Grassland of China (6): 32–36. [Google Scholar]
- WangML.1998. A study on seed production of Aneurolepidium chinense Grassland of China (20): 18–20. [Google Scholar]
- WangRZ.2000. Effect of grazing on reproduction in Leymus chinensis population. Chinese Journal of Applied Ecology 11: 399–402. [PubMed] [Google Scholar]
- WangRZ, Ripley EA.2000. Biomass and energy allocation of Leymus chinensis in the semi‐arid Songnen Plain of northeast China. International Journal of Ecology and Environmental Sciences 26: 107–115. [Google Scholar]
- WilliamsEG, Knox RB, Rouse JL.1982. Pollination sub‐systems distinguished by pollen tube arrest after incompatible interspecific crosses in Rhododendron (Ericaceae). Journal of Cell Science 53: 255–277. [Google Scholar]
- WillsonMF.1979. Sexual selection in plants. American Naturalist 131: 723–738. [Google Scholar]
- XiaoX, Wang Y, Jiang S, Ojima DS, Bonham CD.1995. Interannual variation in the climate and above‐groud biomass of Leymus chinensis steppe and Stipa grandis steppe in the Xilin river basin, Inner Mongolia. China Journal of Arid Environment 31: 283–299. [Google Scholar]
- YangYF.1989. The watering and fertilizing effects to characters of ear organ and grain yield on population of Aneurolepidium chinense Grassland of China (11): 11–15. [Google Scholar]
- YangYF. Zhu TC.1988. A study on seed production of Aneurolepidium chinense population in different ecological conditions. Acta Ecologica Sinica 8: 256–262. [Google Scholar]
- YangYF. Zhu TC.1989. A preliminary study on seed production of Aneurolepidium chinensis population. Acta Phytoecology et Geobotanica Sinica 13: 73–78. [Google Scholar]
- YangYF, Yang LM, Zhang BT, Li JD.2000. Relationship between the fruit‐bearing characters of Leymus chinensis population and annual climatic variation in natural meadow in northeast China. Acta Botanica Sinica 42: 294–299. [Google Scholar]
- YinLJ, Shi DC. Wang P.1993. Growth adaptability and salt tolerance osmoregulation of Aneurolepidium chinense grown on saline grassland. Acta Botanica Sinica 35: 619–625. [Google Scholar]
- ZhaoQL, Tu LZ.1993. The microsporogenesis and the formation of male gametophytes in Leymus chinensis (Trin.) Kitag. Acta Scientiarum Naturalium Universitatis Nei Monggol 24: 55–65. [Google Scholar]
- ZhouSL, Hong DY, Pan KY.1999. Pollination biology of Paeonia jishanensis T. Hong and W.Z. Zhao (Paeoniaceae), with special emphasis on pollen and stigma biology. Botanical Journal of the Linnean Society 130: 43–52. [Google Scholar]
- ZimmermanM, Pyke GH.1988. Reproduction in Polemonium: assessing the factors limiting seed set. American Naturalist 131: 723–738. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.