Abstract
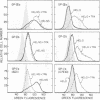
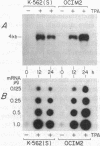
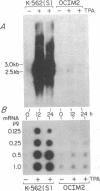
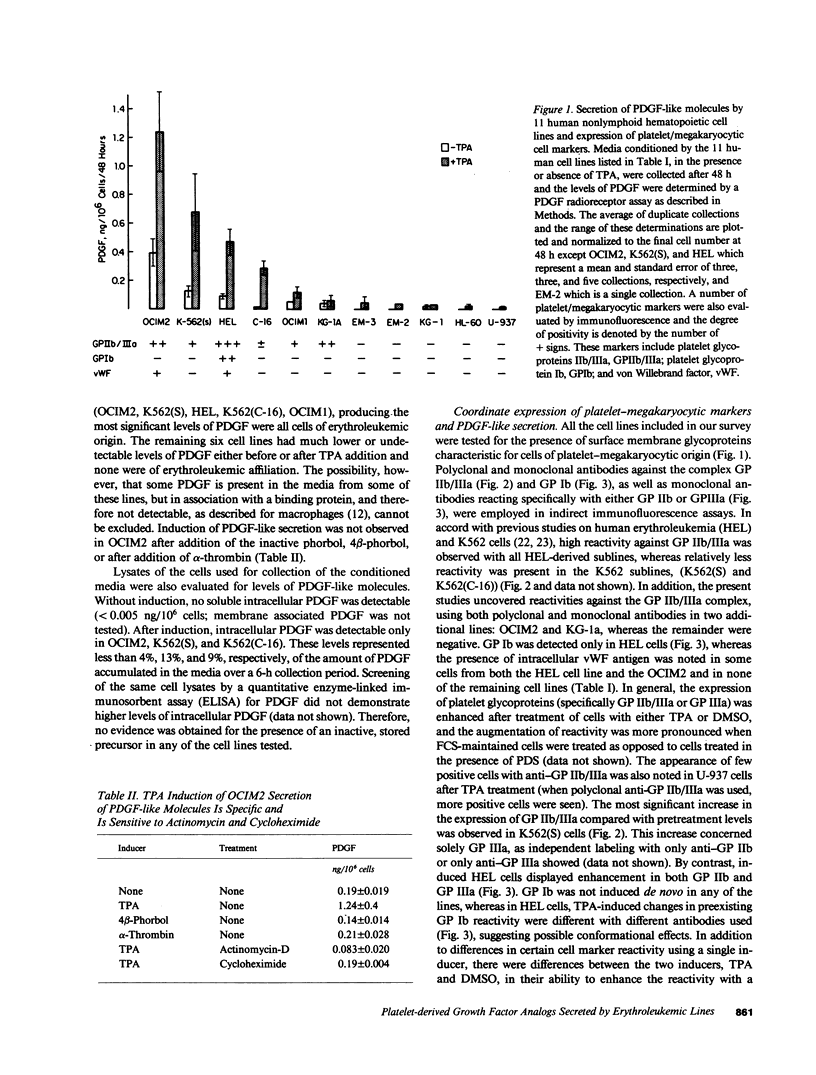
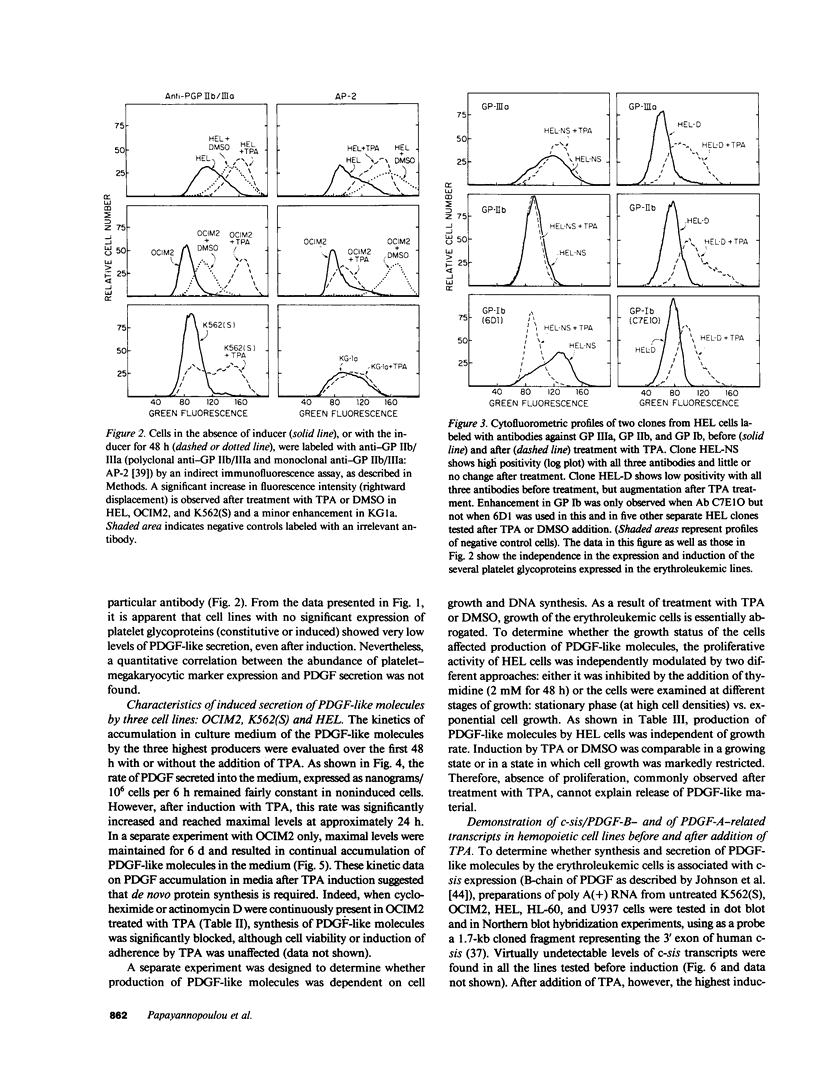
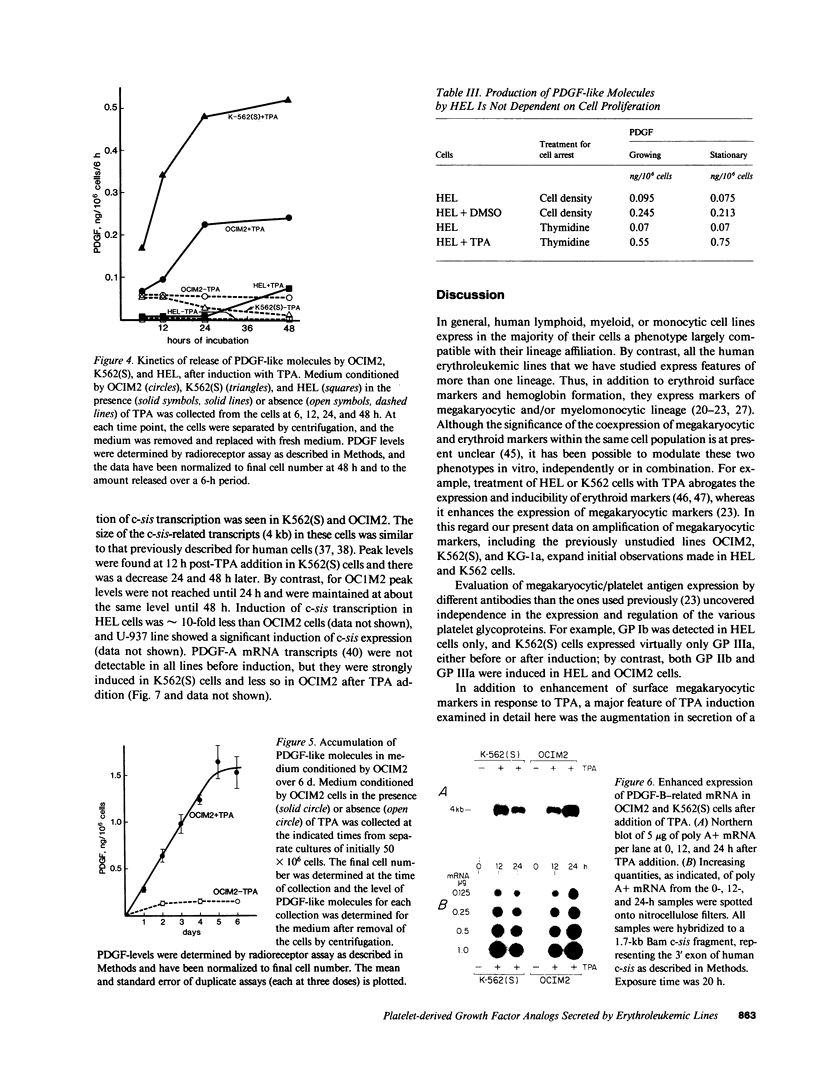
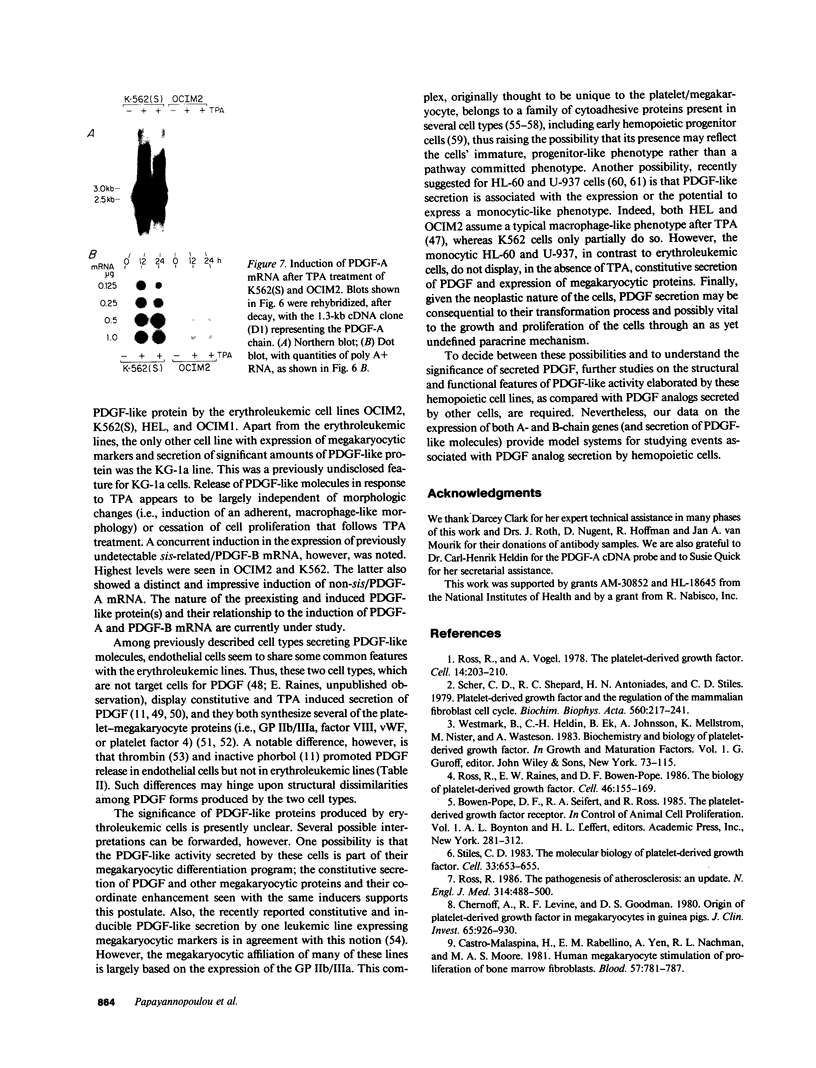
We have examined the constitutive and inducible secretion of platelet-derived growth factor (PDGF)-like proteins in a variety of human hemopoietic cell lines. The highest levels of secreted protein were noted in four human erythroleukemia lines which, in addition to erythroid lineage markers, express one or more megakaryocytic lineage markers. Induction of these lines by 12-O-tetradecanoylphorbol-13-acetate enhanced the expression of megakaryocytic markers and increased secretion of PDGF-like proteins several fold. In concert with these changes, there was significant induction of c-sis/PDGF-B messenger RNA (mRNA) expression in all lines, whereas one line showed significant concurrent induction of PDGF-A mRNA expression. Whether PDGF-like secretion is part of the stem cell-like phenotype displayed by these lines or is secondary to their leukemic transformation remains to be determined. Nevertheless, these lines provide new cellular models for studying the expression and function of PDGF analogs in hemopoietic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bleiberg I., Harvey A. K., Smale G., Grotendorst G. R. Identification of a PDGF-like mitoattractant produced by NIH/3T3 cells after transformation with SV40. J Cell Physiol. 1985 May;123(2):161–166. doi: 10.1002/jcp.1041230203. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Methods for studying the platelet-derived growth factor receptor. Methods Enzymol. 1985;109:69–100. doi: 10.1016/0076-6879(85)09078-4. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns G. F., Cosgrove L., Triglia T., Beall J. A., López A. F., Werkmeister J. A., Begley C. G., Haddad A. P., d'Apice A. J., Vadas M. A. The IIb-IIIa glycoprotein complex that mediates platelet aggregation is directly implicated in leukocyte adhesion. Cell. 1986 Apr 25;45(2):269–280. doi: 10.1016/0092-8674(86)90391-0. [DOI] [PubMed] [Google Scholar]
- Castro-Malaspina H., Rabellino E. M., Yen A., Nachman R. L., Moore M. A. Human megakaryocyte stimulation of proliferation of bone marrow fibroblasts. Blood. 1981 Apr;57(4):781–787. [PubMed] [Google Scholar]
- Chernoff A., Levine R. F., Goodman D. S. Origin of platelet-derived growth factor in megakaryocytes in guinea pigs. J Clin Invest. 1980 Apr;65(4):926–930. doi: 10.1172/JCI109747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cioe L., McNab A., Hubbell H. R., Meo P., Curtis P., Rovera G. Differential expression of the globin genes in human leukemia K562(S) cells induced to differentiate by hemin or butyric acid. Cancer Res. 1981 Jan;41(1):237–243. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Cosgrove L. J., Sandrin M. S., Rajasekariah P., McKenzie I. F. A genomic clone encoding the alpha chain of the OKM1, LFA-1, and platelet glycoprotein IIb-IIIa molecules. Proc Natl Acad Sci U S A. 1986 Feb;83(3):752–756. doi: 10.1073/pnas.83.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. A human onc gene homologous to the transforming gene (v-sis) of simian sarcoma virus. Nature. 1981 Jul 2;292(5818):31–35. doi: 10.1038/292031a0. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Regulation of production of a platelet-derived growth factor-like protein by cultured bovine aortic endothelial cells. J Cell Physiol. 1984 Nov;121(2):298–308. doi: 10.1002/jcp.1041210206. [DOI] [PubMed] [Google Scholar]
- Fraser J. K., Leahy M. F., Berridge M. V. Expression of antigens of the platelet glycoprotein IIb/IIIa complex on human hematopoietic stem cells. Blood. 1986 Sep;68(3):762–769. [PubMed] [Google Scholar]
- Fukuda M. Tumor-promoting phorbol diester-induced specific changes in cell surface glycoprotein profile of K562 human leukemic cells. Cancer Res. 1981 Nov;41(11 Pt 1):4621–4628. [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., DiCorleto P. E. Cultured endothelial cells do not respond to a platelet-derived growth-factor-like protein in an autocrine manner. Biochim Biophys Acta. 1985 Sep 30;846(3):405–412. doi: 10.1016/0167-4889(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Billing R., Lusis A. J., Sparkes R., Golde D. W. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980 Aug;56(2):265–273. [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Martinet Y., Yamauchi K., Bitterman P. B., Grotendorst G. R., Chytil-Weir A., Martin G. R., Crystal R. G. Spontaneous expression of the c-sis gene and release of a platelet-derived growth factorlike molecule by human alveolar macrophages. J Clin Invest. 1986 Jul;78(1):61–66. doi: 10.1172/JCI112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Lanfrancone L., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Human leukemia cells synthesize and secrete proteins related to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5526–5530. doi: 10.1073/pnas.83.15.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Sariban E., Kufe D., Antoniades H. N. Induction of c-sis gene expression and synthesis of platelet-derived growth factor in human myeloid leukemia cells during monocytic differentiation. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6455–6459. doi: 10.1073/pnas.83.17.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Yokochi T., Chait A., Kannagi R. Human erythroleukemia cell line (HEL) undergoes a drastic macrophage-like shift with TPA. Blood. 1983 Oct;62(4):832–845. [PubMed] [Google Scholar]
- Papayannopoulou T., Yokochi T., Nakamoto B., Martin P. The surface antigen profile of HEL cells. Prog Clin Biol Res. 1983;134:277–292. [PubMed] [Google Scholar]
- Plow E. F., Loftus J. C., Levin E. G., Fair D. S., Dixon D., Forsyth J., Ginsberg M. H. Immunologic relationship between platelet membrane glycoprotein GPIIb/IIIa and cell surface molecules expressed by a variety of cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6002–6006. doi: 10.1073/pnas.83.16.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Gullick W. J., Waterfield M. D., Rozengurt E. Highly purified fibroblast-derived growth factor, an SV40-transformed fibroblast-secreted mitogen, is closely related to platelet-derived growth factor. EMBO J. 1985 Aug;4(8):1945–1949. doi: 10.1002/j.1460-2075.1985.tb03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tabilio A., Pelicci P. G., Vinci G., Mannoni P., Civin C. I., Vainchenker W., Testa U., Lipinski M., Rochant H., Breton-Gorius J. Myeloid and megakaryocytic properties of K-562 cell lines. Cancer Res. 1983 Oct;43(10):4569–4574. [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan P., Perussia B., De Marco L., Wells K., Trinchieri G. Membrane proteins on human megakaryocytes and platelets identified by monoclonal antibodies. Am J Hematol. 1983 May;14(3):255–269. doi: 10.1002/ajh.2830140307. [DOI] [PubMed] [Google Scholar]
- van Mourik J. A., Leeksma O. C., Reinders J. H., de Groot P. G., Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem. 1985 Sep 15;260(20):11300–11306. [PubMed] [Google Scholar]