Abstract
• Background and Aims Some frost-tolerant herbaceous plants droop and wilt during frost events and recover turgor and posture on thawing. It has long been known that when plant tissues freeze, extracellular ice forms. Distributions of ice and water in frost-frozen and recovered petioles of Trifolium repens and Escholschzia californica were visualized.
• Methods Petioles of intact plants were cryo-fixed, planed to smooth transverse faces, and examined in a cryo-SEM.
• Key Results With frost-freezing, parenchyma tissues shrank to approx. one-third of their natural volume with marked cytorrhysis of the cells, and massive blocks of extracellular icicles grew under the epidermis (poppy) or epidermis and subepidermis (clover), leaving these layers intact but widely separated from the parenchyma except at specially structured anchorages overlying vascular bundles. On thawing, the extracellular ice was reabsorbed by the expanding parenchyma, and surface tissues again contacted the internal tissues at weak junctions (termed faults). These movements of water into and from the fault zones occurred repeatedly at each frost/thaw event, and are interpreted to explain the turgor changes that led to wilting and recovery. Ice accumulations at tri-cellular junctions with intercellular spaces distended these spaces into large cylinders, especially large in clover. Xylem vessels of frozen petioles were nearly all free of gas; in thawed petioles up to 20 % of vessels were gas-filled.
• Conclusions The occurrence of faults and anchorages may be expected to be widespread in frost-tolerant herbaceous plants, as a strategy accommodating extracellular ice deposits which prevent intracellular freezing and consequent membrane disruption, as well as preventing gross structural damage to the organs. The developmental processes that lead to this differentiation of separation of sheets of cells firmly cemented at determined regions at their edges, and their physiological consequences, will repay detailed investigation.
Key words: Cell separation, cryo-SEM, Eschscholzia californica, extracellular ice, frost resistance, Trifolium repens, vessel embolisms
INTRODUCTION
It is a common observation, in gardens subject to light frosts followed by warm days that some herbaceous plants are killed immediately by the first frost, while there are others that are resistant to frosts. The frosted leaves and/or stems of these latter plants droop before dawn, and then recover their upright posture when the temperature rises above freezing. This drooping and recovery cycle can occur repeatedly with no obvious ill effect on plant vigour. Reported here is an investigation into the distributions of ice and water in the tissues, which underlie these changes in turgidity. The process of freezing of plant organs has been much researched. In water below the freezing point ice crystals grow on nucleation sites, attracting water from regions where it is still liquid. The attractive force of the ice crystal, measured by its chemical potential relative to that of liquid water, is considerable, and increases as the temperature of the ice falls. For every 1 °C temperature drop the chemical potential of ice falls by 1·16 × 103 J kg−1 (Beck et al., 1984).
When plant tissue freezes in a natural frost (termed frost-freezing in this study) the temperature falls slowly, at a rate of approx. 1 °C h−1. Ice nucleation occurs first in the water with the lowest solute content (water in the intercellular apoplast and in the xylem–lumen apoplast) and the crystals grow as the temperature falls further, extending to other tissues and attracting water from solutions with more solutes. It was established by several observers in the early nineteenth century, and confirmed by many subsequent studies (for review, see Levitt, 1972) that the ice crystals grow outside the cells, while the cells shrink as water leaves them. The further the temperature falls, the greater the accumulations of extracellular ice, and the more dehydrated are the cells. Illustrations of this phenomenon from a very early study (Prillieux, 1869) are reproduced in Fig. 1. In these frosted petioles, the large ice masses accumulated in spaces created by separation of intact epidermal or epidermal and subepidermal cell layers from underlying shrunken cells. The surface layers bulged outward by the ice remain anchored to internal tissues at three angles of the petiole. In those plants that recover from a night-time frost, when the temperature rises above the freezing point and the ice melts, water must return to the cells, and the tissues of the petiole must adjust back to their original configurations. At some temperature the loss of cellular water produces permanent damage to cell membranes and organelle structure, ice forms within the cells, and when the leaf thaws the cells are dead. The difference between the frost-sensitive and frost-tolerant herbaceous species lies in the fact that the cell membranes of the former are disrupted at a higher sub-zero temperature. Plants that acclimate to frosts do so by lowering the temperature at which the membrane disruption occurs.
Fig. 1.
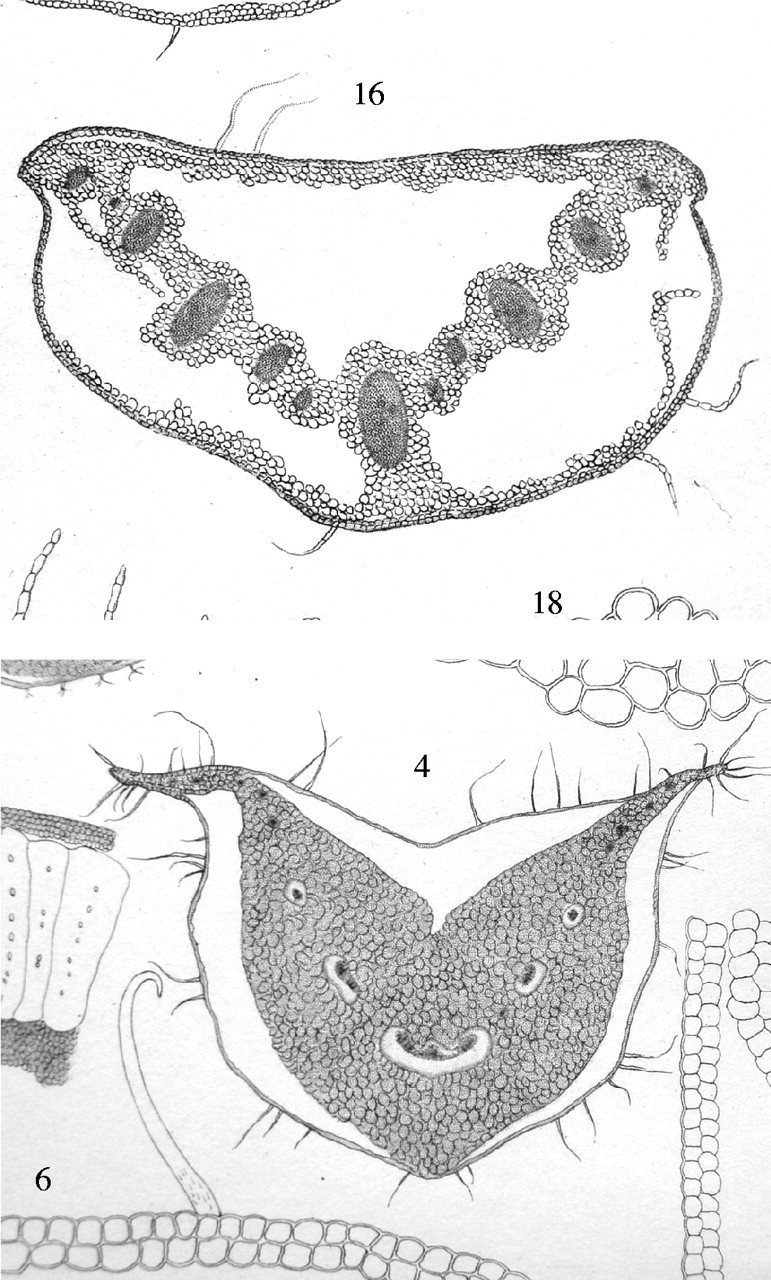
Photographs of two plates of drawings from Prillieux (1869) of transverse sections of frost-frozen petioles of Chelidonium quercifolium (upper drawing) and Symphytum tauricum (lower drawing). In both petioles large masses of ice had grown, drawing water from the shrunken parenchyma cells. The ice masses (seen here as spaces) remained within the distended intact outer tissue, which consisted of a few cell layers in C. quercifolium and a single epidermal layer in S. tauricum. Each petiole has three anchor points, where ice does not penetrate and the epidermis remains attached to the cortical tissues.
Much research published during the last two decades which has attempted to visualize extracellular ice has concentrated on the movements of water and ice growth in bud tissues of woody plants (e.g. Ishikawa and Sakai, 1985; Ashworth et al., 1988, 1989; Ashworth, 1990). Here the ice accumulates in the outer bud scales, which are often dead, leaving the tender young tissues in the centre of the bud dehydrated but undamaged. The process has come to be called extra-organ freezing (Ishikawa and Sakai, 1985), a term inappropriate to the freezing illustrated in Fig. 1.
Two techniques have been used to observe the intercellular spaces occupied by the ice in originally frosted material to avoid the difficulties of direct observation of the ice masses. (1) Fixation in formaldehyde at −5 °C (termed freeze fixation) followed by critical point drying and examination in the scanning electron microscope (SEM) at room temperature (e.g. Ashworth et al., 1989; Ashworth, 1990). (2) Room temperature SEM following freeze-substitution (e.g. Ashworth et al., 1988; Ashworth, 1990). Direct observation of the extracellular ice masses was achieved at very low resolution in woody plants by Ishikawa and Sakai (1985) in thick sections of still frozen tissue observed with polarizing optics. Also, as part of extensive physico-chemical studies of freeze tolerance in Afroalpine ‘giant rosette’ plants (Beck et al., 1984) and the herbaceous species Pachysandra terminalis (Zhu and Beck, 1991) extracellular ice deposits were observed in intercellular spaces by optical microscopy of hand-cut sections of frost-frozen tissues. Cryo-SEM (CSEM) allows the examination of frost-frozen and unfrozen tissues at full hydration at approx. −160 °C and potentially much higher resolution (Ashworth et al., 1988; Pearce, 1988, 2001; Pearce and Ashworth, 1992). In those latter studies segments of grass leaves were quench frozen at very low temperature (termed cryo-fixation in the present study). These frozen specimens were fractured under vacuum in the preparation chamber of the CSEM. This fracturing is random and does not permit precise choice of the part to be examined, and produces an irregular surface which yields images whose anatomical structure is often difficult to interpret. Extracellular ice was identified by the fact that it sublimed more quickly than frozen cell contents when the temperature of the specimen was raised to −50 °C. Techniques for cryo-fixing intact parts of plants in situ in the field have been developed in which flat surfaces on the frozen specimens are planed in any chosen plane, yielding unambiguous structural images where ice can be recognized by its low electron emissivity (McCully et al., 2000; Ball et al., 2002, 2004).
In a recent study with cryo-fixed and cryo-planed frost-frozen leaves of snow gum, Eucalyptus pauciflora (Ball et al., 2004), massive accumulations of ice formed in parenchyma/fibre tissues above and below the mid-vein. Mesophyll cells shrank to thin plates, and ice filled their intercellular spaces, displacing nearly all the gas that had been there. In non-acclimated leaves the distortions produced by the ice extended also to disruption of the cambium, and remained when the tissue had thawed. Mesophyll cells also did not regain their original volume, and water remained in intercellular spaces. These leaves eventually die. In acclimated leaves, all water released from the thawed ice returned completely to the cells. Mesophyll cells regained their turgid shape, intercellular spaces were again filled with gas, and the zones above and below the mid-vein closed to a barely distinguishable line of intercellular spaces. These changes were repeated nightly during the winter whenever there was a frost and the leaves remained healthy.
The snow gum leaves (d. wt approx. 40 %) include tissues with heavy, lignified walls and these relatively rigid structures maintain the form of these leaves over wide ranges of water content (no wilting). Thus the large changes in extracellular ice during frost-freezing are not manifested in changes of leaf form. The frost-tolerant herbaceous plant leaves (approx. 15 % d. wt) that show obvious change in turgidity when frosted/thawed include many fewer thick-walled cells. The form of these leaves is largely maintained by tissue pressure generated in their turgid, thin-walled, highly vacuolated cells and when water is lost from these cells the leaves wilt. For these reasons the phenomenon of shape change is typically shown by herbaceous plants. Prillieux (1869) illustrates the structural changes in petioles of seven herbaceous species, and in stems of a further seven species. This paper reports the changes produced in petioles of such leaves by the freeze/thaw cycle as revealed by the CSEM, the same technique as was used for the snow gum study.
MATERIALS AND METHODS
Plant material
The experimental material and conditions for such an investigation are not wholly under the operator's control. The reacting frost-tolerant plants select themselves because they show the required behaviour. The occurrence of frost of the appropriate temperature, followed by a clear, sunny morning with no lingering fog, must be awaited and utilized. The necessary apparatus must be assembled nearby and held in readiness. These conditions are most easily met in the experimenters' own domestic garden. The site was on the northern slopes of Black Mountain in the Australian Capital Territory where many herbaceous species of plants (e.g. brassicaceous species, hollyhock, broad bean) showed drooping and recovery in frost events. The season was late winter (August) and periodic night-time frosts were still occurring. Sunrise was approx. 06·30 h Eastern Australian Standard Time. Two of the frost-tolerant species that showed this drooping and recovery were chosen: white clover (Trifolium repens L.) and California poppy (Eschscholzia californica Cham.). The lowest temperature reached during frosts was −5 °C. Thawing occurred when the air temperature had risen to approx. +5 °C. Frosted samples were collected at 06·15–06·45 h, thawed samples at 08·30–09·30 h. The appearance of the plants in both frosted and thawed leaves was recorded with a digital camera. Petioles of both frosted and thawed leaves were sampled from different plants of each species.
Cryo-fixation
Pieces 2·7 cm long from the mid-portion of each petiole were cryo-fixed in situ on the plants (both frosted and thawed) using cryo-pliers with heavy polished copper jaws at the temperature of liquid nitrogen (LN2). The pieces were trimmed under LN2 to fit into cryo-vials, and were stored at the temperature of LN2 until examined in the CSEM.
Cryo-microscopy
Preparation of the samples and observation of the preparations followed the procedures given in McCully et al. (2000). In summary, segments from the mid-portion of each sample were cut out under LN2, mounted in stubs with low-temperature Tissue Tek, planed flat in the transverse direction with a cryo-microtome (Reichert-Jung, Vienna) at −80 °C, etched in the column of the CSEM (JEOL6400, JEOL Ltd, Tokyo, Japan) for 1 or 2 min at −90 °C to just reveal cell outlines, sputter-coated with gold, and examined at 15 kV. Images were captured digitally and plates prepared using the program Microsoft Photodraw.
From four to six transverse faces from each of four frost-frozen and four thawed petioles of each species were examined and recorded. Lengths and widths of the epidermal cells of frost-frozen and thawed petioles of poppy were measured on enlarged micrographs, and numbers of vessels (ice-filled and gas-filled) were also counted from the micrographs.
Light microscopy
Transverse sections of petioles of leaves collected after a freeze/thaw event, and in summer from new leaves which had never been frosted were sectioned by hand, stained with toluidine blue (0·05 % aqueous) and micrographs taken with a Zeiss Axiophot microscope on Agfa RSX 200 film.
RESULTS
The external appearance of the two species when frosted and thawed is illustrated in Fig. 2A–D. The leaves of poppy are recumbent on the ground when frosted (Fig. 2A), rigid and brittle and stabilized by the ice within, in shape appearing as when strongly droughted. In contrast, thawed plants (Fig. 2B) are erect and turgid, as when well watered and functioning. The transition between the two states is gradual, and takes place over 0·5 h or so as the plant warms. The frosted clover leaves (Fig. 2C) collapse en masse with their petioles solidified in lax positions. The trifoliate leaflets are closed as in nyctinasty. The view of the same clump approx. 1 h later (Fig. 2D) shows the leaves erect, petioles tumescent, and the leaflets spread wide. The transition between the two states for a single leaf is in fact very rapid. As the clump is observed in the increasing sunshine, individual leaves resurrect quickly and at random. There was a marked colour change in the leaves of both species during each freeze/thaw cycle (cf. Fig. 2A and C with 2B and D).
Fig. 2.

(A–D) Plants growing in late winter in Canberra. These and similar plants were used in this study. (A) California poppy at 07·00 h, temperature −4 °C. (B) The same plant at 08·30 h the same day when warmed by the sun, ambient temperature +4 °C. (C) White clover at 07·00 h, temperature −4°C. (D) Same patch of clover on the same day at 08·00 h after brief warming by the sun, ambient temperature +3 °C. (E and F) Hand-cut transverse sections of petioles stained with toluidine blue showing the inherent regions of weakness (faults) between surface tissues and the underlying chlorenchyma at the petiole periphery, and anchorage regions where surface tissues do not detach during extracellular ice formation. (E) California poppy. This petiole was sectioned in early summer and was from a young leaf that had never been frozen. During sectioning the single-layered lower epidermis separated at the fault over the chlorenchyma but not at the anchorage over the vascular bundle at the wing of the petiole. Bar = 100 μm. (F) White clover. This petiole was sectioned in August and was from a leaf that had experienced at least six freezing/thawing events previously. Arrowheads indicate the fault separating the two surface layers from the underlying chlorenchyma. The arrow indicates the anchorage over the vascular bundle. Bar = 100 μm.
The contrast between the internal appearance in the CSEM of frost-frozen and thawed, recovered petioles of both species is dramatic (Figs 3–6). During the frost-freezing the ground tissues of the petioles shrink to a fraction of their normal volume (cf. Fig. 3A with Fig. 3B). Large masses of ice form within the petioles in cavities created as the expanding ice masses separate surface tissues from underlying chlorenchyma on both upper and lower sides of the petioles (Fig. 3A and C–E). The separated surface tissues remain intact and undamaged, completely enclosing the ice deposits. In clover, the overlying surface tissue consists of two layers that adhere tightly to each other (Fig. 3C and D), while in poppy the separated surface tissue is a single epidermal layer (Fig. 3E). The large ice masses are composed of icicle-like units that lie normal to the petiole, some of which frequently detach and are lost during cryo-planing (Figs 3A, C and E and 4A).
Fig. 3.
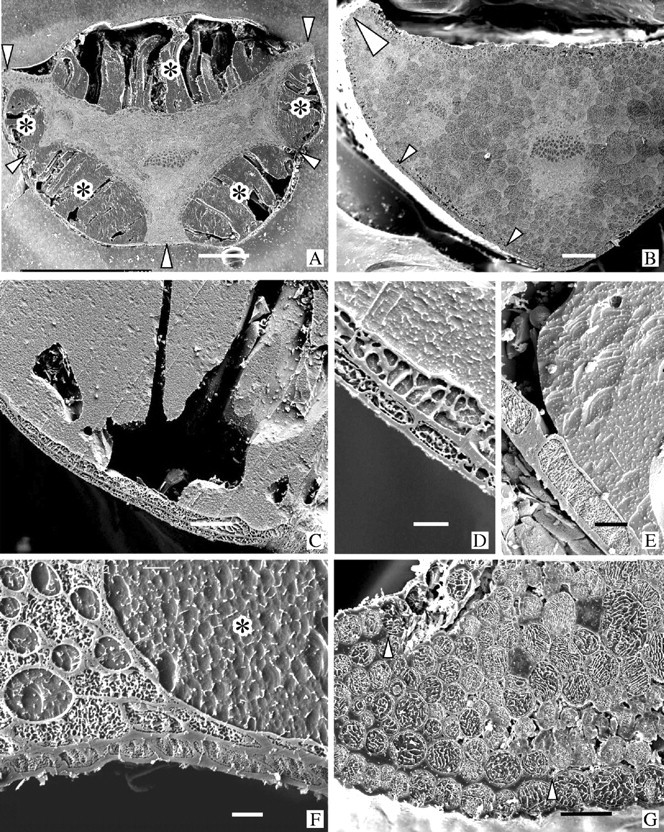
Transverse faces of cryo-planed sections of cryo-fixed petioles viewed in a CSEM. Specimens in A, C–E and F were frost-frozen; B and G were thawed. All were from the plants shown in Fig. 2A–D. In each micrograph the lower epidermis is at the bottom. (A) Poppy. Leaf was frost-frozen as in Fig. 2A. Water has been drawn out of the ground parenchyma which is now much shrunk and distorted, and has accumulated in large deposits of ice (dark grey, typical ones marked by *) on both the upper and lower sides of the petiole where the epidermis has separated from the underlying chlorenchyma. These ice deposits, though incredibly large, are always encompassed on their outer edge by the intact, separated epidermis. During cryo-planing portions of the ice deposits crack, drop out and are lost. Tight anchorages (arrowheads) between epidermis and underlying tissue over the vascular bundles prevent loss of the epidermis. Bar = 500 μm. (B) Poppy. Petiole from the same plant as in A but after recovery as shown in Fig. 2B. The ice has all melted and the extracellular water has been retrieved by the ground parenchyma cells that have recovered their turgor. The epidermis is in place against the underlying cells (small arrowheads). The large arrowhead indicates an anchorage at the petiole wing similar to that in G. Bar = 200 μm. (C) Clover. Surface tissues around the outside of a large extracellular ice deposit from a leaf shown in Fig. 2C. Bar = 50 μm. (D) Clover. Detail of a region similar to C showing the double-layered coherent surface tissue typical of clover. Bar = 10 μm. (E) Poppy. Region similar to that of C and D showing the single epidermal layer characteristic of the separating surface tissue in poppy. Bar = 20 μm. (F) Clover. An anchorage overlying thick-walled cells linking a vascular bundle to the epidermis in a frost-frozen petiole. Note the enlarged and rounded ice-filled intercellular spaces at the tricellular junctions. The asterisk marks a portion of a large peripheral ice deposit as in C. Bar = 10 μm. (G) Poppy. A thawed petiole showing the thick anchorage (arrowheads) at the tip of the wing (large arrowhead in B), and the loose attachment of the epidermis to the underlying cells at the fault (clearest under the three epidermal cells at the lower right). Bar = 50 μm.
Fig. 4.
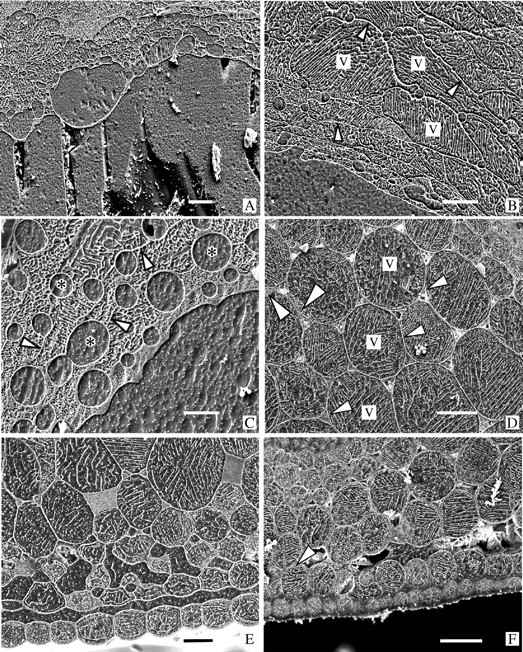
All are cryo-preparations of petioles as in Fig. 3. The lower epidermis is at or beyond the bottom of each micrograph. A, B and C were frost-frozen; D, E and F were thawed. (A) Poppy. The upper surface of a large peripheral ice deposit as in Fig. 3A showing the intersection with the dehydrated and distorted parenchyma cells, and intercellular spaces that have been ballooned out by the accumulating ice during frost-freezing. Bar = 50 μm. (B) Poppy. Parenchyma cells bordering a large ice deposit showing typical variations in size and shape of the cells dehydrated during frost-freezing. Note the clearly defined, ice-filled intercellular spaces. Arrowheads indicate cell walls. v = vacuole. Bar = 50 μm. (C) Clover. A location similar to that in A. In this species the cells tend to be so shrunken by the frost-freezing that some walls are difficult to distinguish (arrowheads). The ice-filled intercellular spaces (*) are usually much larger than those in poppy. Bar = 20 μm. (D) Clover. Fully rehydrated, turgid parenchyma in a recovered petiole. The small triangular intercellular spaces are mostly gas filled (the white material in most of them is ice chips that fell into the spaces during planing). Tonoplasts (small arrowheads) and nuclei (large arrowheads) can be distinguished in some of the cells. v = vacuole. Bar = 50 μm. (E) Poppy. Reabsorption of extracellular water was not quite complete when this thawing petiole was cryo-fixed. The epidermis is still separated from the chlorenchyma by water and, although the inner parenchyma have partly returned to their turgid form, those near the surface are still shrunken and distorted, and intercellular spaces are water-filled. Bar = 30 μm. (F) Clover. Cells in this petiole are back to normal turgidity but a small amount of water is still free in the fault. Arrowhead indicates the beginning of an anchorage which extends beyond the left edge of the micrograph. Bar = 50 μm.
Fig. 5.
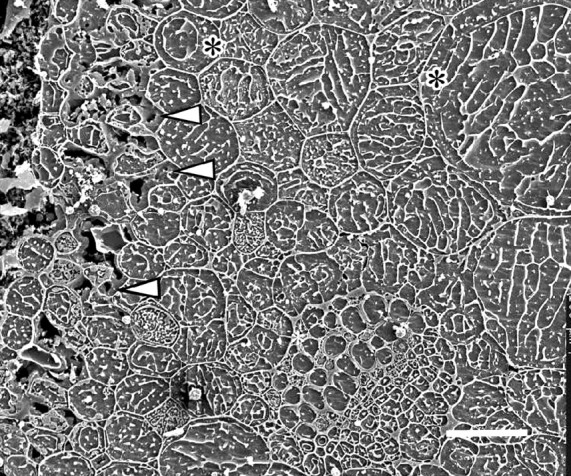
Clover. Cryo-preparation. A partially thawed region of a petiole where the inner parenchyma cells are returning to full turgor but still angular in outline and their intercellular spaces still water-filled. The outermost chlorenchyma cells (left of micrograph) are still very shrunken and distorted. Arrowheads indicate residual water in the fault region. The xylem vessels (lower right of centre) are all water-filled. A wide, watery space between the vacuole and cell wall (*) is often seen in cells at this stage of rehydration. Bar = 50 μm.
Fig. 6.
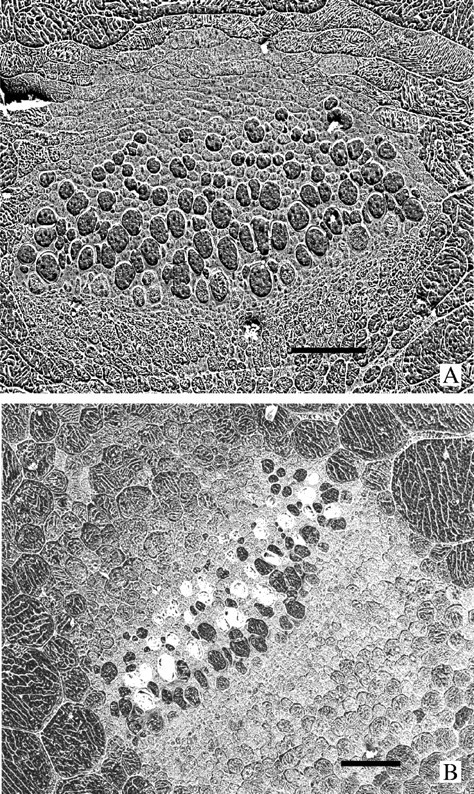
Cryo-preparations. (A) A large vascular bundle in a frost-frozen petiole of a poppy preparation as the one in Fig. 3A. The bundle is surrounded by collapsed parenchyma cells and all the xylem vessels are ice-filled. Bar = 50 μm. (B) A similar vascular bundle in a thawed petiole as seen in Fig. 3B. The surrounding parenchyma cells are rehydrated and many of the xylem vessels are embolized (they appear white because of the ice chips that lodge in the spaces during planing). Bar = 50 μm.
At regions around the petioles that overlie major vascular bundles in both species the surface tissues do not separate from the underlying sclerenchymatous cells and no ice masses form there (Fig. 3A and F). We term these regions ‘anchorages’. In poppy especially, anchorages also retain the epidermis in situ over the outer edges of the wings of the petioles during frost-freezing (Fig. 3A). Clover petioles are frequently partially hollow and ice also accumulates and enlarges the central cavities in frost-frozen material (not illustrated).
In frost-frozen petioles of both species, the thin-walled cells of the internal tissues have shrunk markedly and become very distorted when water is drawn out of them by the growing extracellular ice masses (cf. Fig. 3A with B, and Figs 3F and 4A–C with Figs 3G and 4D). Measurements of the ice and tissue areas in the image of Fig. 3A showed that approx. 70 % of the water in the petiole was in the form of ice, and approx. 30 % remained in the cells. The small intercellular spaces at tricellular junctions, which are triangular in transverse profile in recovered petioles, are much enlarged, rounded and ice-filled in the frost-frozen petioles (cf. Figs 3B and G and 4D with Figs 3F and 4A–C).
The boundary between the peripheral ice deposits and the bounding intact surface tissues is always clearly defined by the thick tangential walls of the cells (Fig. 3C–E). These outer cells shrink to about half their thickness (Table 1), but though they may stretch somewhat to accommodate the expanding ice mass any difference in average width is small (Table 1). At the inner boundaries where the large ice masses abut the thinned-walled, shrunken parenchyma, cells are frequently very irregular, with the immediately adjacent cells especially shrunken (Fig. 4A–C) and, particularly in poppy, intercellular spaces ballooned out by accumulated ice (Fig. 4A). In some regions it is difficult to distinguish some of the thin cell walls from eutectic lines in vacuoles of the shrunken cells (e.g. Fig. 4C).
Table 1.
Changes in dimensions of epidermal cells of poppy on freezing (means ± s.d. with the number of images measured in parentheses)
| Thickness (µm) |
Width (µm) |
Area (µm2) |
|
|---|---|---|---|
| Frozen | 14 ± 3 (8) | 34 ± 6 (8) | 480 ± 140 (8) |
| Thawed | 26 ± 5 (15) | 31 ± 3 (15) | 800 ± 230 (15) |
In the thawed petioles most extracellular water was gone and the shrunken internal tissues had expanded to contact the inner side of the surface layer(s) that had bounded the ice masses. This region of contact was weak and remained as a fault which ice forming in subsequent frosts would again open to accommodate the large extracellular peripheral deposits. There was a clear difference between the degree of cell adhesion at these faults, and at the anchorages (Fig. 3F and G) where the thick tangential walls of the surface tissues remained adhered firmly to the underlying, also thick-walled, cells. This contrast between the anchorages and the faults was particularly clear in hand-cut sections of fresh petioles (Fig. 2E and F). The epidermis in poppy often detached completely from the chlorenchyma during sectioning while remaining firmly attached to the underlying thick-walled cells at the anchorages in both recovered petioles and petioles of leaves which had never been frozen (Fig. 2E). The two outer layers of the clover petioles remained in place better during hand-sectioning but extensive spaces still separated them from the chlorenchyma except at the anchorages (Fig. 2F).
Several transverse faces were examined in which the thawing and tissue readjustment were still incomplete when the petioles were cryo-fixed (Fig. 4E). A thin layer of unresorbed water still separated the detached surface tissue from the underlying chlorenchyma, and the outermost cells of this latter tissue were still shrunken and surrounded by large, water-filled intercellular spaces. More interior parenchyma cells expanded greatly though some were still somewhat angular in outline compared with similar cells when fully turgid in completely recovered petioles (cf. Figs 4E and 5 with Fig. 4D and F). In fully recovered petioles the water was gone from most of the intercellular spaces of the ground parenchyma (Fig. 4D) though occasionally a small amount remained in places in the fault (Fig. 4F). In the fully rehydrated parenchyma cells, the thin peripheral cytoplasm, nuclei and the tonoplast could be distinguished in favourable transverse views of the cells (Fig. 4D and F). In partially rehydrated cells there was frequently a wide, water-filled gap between the vacuole and the cell wall (Fig. 5).
In all of the cells of both frost-frozen and thawed petioles the vacuoles contain arrays of white electron-emissive lines. These are eutectic lines of solutes excluded from the vacuolar solution during freezing (either frost-freezing or cryo-fixation) and separated by zones of electron non-emissive (black) ice. They are characteristic of CSEM preparations, and their patterns provide some clues to the concentration and nature of the solutes. The denser concentration of the eutectic lines in the cell vacuoles in the preparations of frosted petioles (Figs 3C–F and 4A–C) compared with that in the thawed (Figs 3G and 4D) and incompletely thawed petioles (Figs 4E and F and 5) are evidence of the higher concentration of solutes produced by the loss of water from the frost-frozen cells.
Any gas-filled (embolized) xylem vessels in recovered petioles form part of the space where ice can grow during frost-freezing and vascular strands of frosted petioles always were less embolized than those of completely recovered petioles (Fig. 6 and Table 2). In those petioles which had been cryo-fixed before complete recovery, vessels were sometimes still completely filled with ice, while some ice remained in the much shrunken peripheral spaces (Fig. 5), suggesting that retrieval of water from the vessels is much slower than from the peripheral deposits of melted ice.
Table 2.
Percentage of vessels containing gas (numbers of vessels counted in parentheses)
| Plant |
Frozen |
Thawed |
Incompletely thawed |
|---|---|---|---|
| Poppy | 2·7 (550) | 20 (273) | 4·8 (229) |
| Clover | 4 (300) | 12 (475) |
DISCUSSION
The appearance of the frosted plants (Fig. 2A and C), as mimicking the form of drought-stressed wilted plants with much reduced turgor, in spite of their structures being made rigid by ice is easily explained. The growth of extracellular ice proceeds slowly as the temperature falls. As water leaves the cells to join the ice crystals in the intercellular spaces, the initial result is a fall in cell turgor and a loss of tissue pressure. At this early stage the tissues are still flexible because there is not enough extracellular ice to make them rigid. The leaves collapse as though droughted.
As the extraction of water and the accretion of ice continue, the leaves become rigid, fixed in their collapsed state by their now large accretions of extracellular ice. These appearances contrast strongly with that of the frosted snow gum leaf, which, because of its large component of cells with rigid cell walls, retains its original form (Ball et al., 2004).
A similar turgor change producing leaf movements was recorded by Beck et al. (1984) in Afroalpine ‘giant rosette’ plants. These plants are subject to frosty nights followed by warm, sunny days. During frosts, the extracellular water accumulates in the very large intercellular spaces of the outer leaves of their central bud where the young tender tissues are enclosed. These authors say ‘The spongy structure of the mesophyll tissue … may be a prerequisite for these leaves to cope with the almost regular nocturnal freezing. It should be noted that the leaves of the Afroalpine ‘giant rosette’ plants, by nyctinastic movements, form a nocturnal ‘night bud’ which insulates the central leaf bud from freezing (Beck et al., 1982). These nyctinastic movements may, at least partially, be caused by the decrease of turgor during freezing of cellular water in the intercellular spaces.’
A second contrast with the frosted snow gum leaves is the degree of distortion produced by the extracellular ice. A frozen snow gum leaf looks just like an unfrozen one except for a slight colour change. While the ice-filled expansion zones at the snow gum mid-veins are impressive (fig. 2a, c and e in Ball et al., 2004), the broken and squashed tissues of the herbaceous petioles encased in masses of ice look as though they have suffered irreparable damage (Fig. 3A). But, in parallel with the changes in the thawed snow gum leaf, the thawed herbaceous petioles return to their fresh, functioning, unfrozen state; their cells regain their volumes and turgor, and fit back together so that it is difficult to see where they ever came apart. Like the snow gum, they can go through this transformation and restoration every night when there is a frost, and resume their activities each day.
The segregation of the ice masses into columnar icicles (Figs 3A and 4A) was not observed in snow gum, but is mentioned by Prillieux (1869), and is illustrated on the left side of Fig. 1, lower drawing, which shows part of a frosted petiole of Althea rosea. Gas bubbles are drawn enclosed in the icicles, and are commented on by Prillieux, but we have not seen any in the cryo-preparations. The icicles are evidence of the manner of growth of the extracellular ice, and suggest that water must leave the wet cell in discrete local patches, rather than as a continuous sheet over the whole cell wall.
The fault – which we would define as a particular region of compact tissue arranged so that by expansion it can accommodate large masses of extracellular ice, and by contraction on thawing can restore its form with no apparent damage – has now been found in leaves of all three species examined by the present method, and can be seen in the other illustrations in Prillieux (1869) like those of Fig. 1. It is tempting to suggest that such faults may be of very general occurrence in frost-tolerant plants, though no doubt differing in the details of form and operation. Two examples of the two-cell-layered outer boundary of such faults may be seen at the right and bottom of Fig. 1, lower drawing, which are drawn by Prillieux from frosted petioles of Symphytum tauricum and Senecio crassifolius. Faults are clearly not universal, as an alternative strategy of ice accumulation in very large intercellular spaces was mentioned above (Beck et al., 1984).
A feature that has not been described before is the anchorage (Figs 3A, F and G and 4F) which restricts the indefinite extension of the fault, and which must very often accompany the occurrence of faults. Without such regions it is easy to see that the epidermal layers of clover and poppy petioles would easily be torn off. The thickened cell walls at the anchorage must be very strong, and would repay detailed investigation.
Unexpected strength is also evident in the cell walls of the parenchyma. The transformation in the size and shape of the intercellular spaces between the frozen state in Fig. 4C and the thawed state in Fig. 4D is very striking. The cell–cell-space junctions (see fig. 2 in Jarvis, 1998) at the corners of the gas-filled space must be very strong to stand the pressure of ice distending the spaces to large cylinders. Jarvis et al. (2003) stress the importance of these junctions: ‘Precisely at these points there are reinforcing zones which … can be distinguished under the electron microscope and which differ in polymer composition from both the primary cell walls and the middle lamella.’
The formation of faults is a new example of the large class of cell separations that are such a common feature of the formation of plant structure (Jarvis et al., 2003). The stage at which the fault cell separations develop, whether before any frost event (as suggested by the appearance of sectioned, never-frosted poppy petioles as in Fig. 2E), or only after the first frost when the expanding extracellular ice might initiate the separation, would repay further study. Investigation of similar regions in cultivars of the same species grown in frost-free regions would reveal if the ability to develop faults has been inadvertently selected for in the development of frost-tolerant cultivars. Further questions are raised by the continuing life of the one or two cell layers outside the fault once they have lost all direct contact with the underlying tissues. They must be maintained by sideways (paradermal) traffic of water and solutes from the anchorage regions.
The finding that the xylem vessels of frosted plants contain much more water than those of the thawed plants agrees with the report of Ball et al. (2004) for snow gum. A simple explanation would be that gas-filled vessels present in the leaf at night before the frost are just another extracellular space in which ice can accumulate during freezing, and that their small volume is quickly filled with ice drawn from surrounding watery cells. On thawing the expanding cells would withdraw water from the melted ice wherever it was easily available, emptying the vessels and leaving them gas-filled.
We feel we have justified the use of the word ‘management’ in the title. Those frost-tolerant herbs observed by Prillieux (1869) and ourselves have evolved a complicated arrangement of structural strengths and weaknesses which enables them to accommodate large volumes of intercellular ice during some hours of low temperatures, and to return to their unfrozen state and tissue arrangement when the temperature rises above freezing. Questions remain about many details of this management. Where is the weak point of the system which, with colder temperatures and/or longer frost durations, is the first to be permanently put out of action? How far does the water move to and from the ice masses? What special wall properties provide the strong cell attachments at the anchorages and tricellular junctions? What changes of vacuolar solutes accompany the developments of cell adhesion and separation? Some of these questions will be addressed in future papers.
Supplementary Material
LITERATURE CITED
- Ashworth EN. 1990. The formation and distribution of ice within flower buds. Plant Physiology 92: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth EN, Echlin P, Pearce RS, Hayes TL. 1988. Ice formation and tissue response in apple twigs. Plant, Cell and Environment 11: 703–710. [Google Scholar]
- Ashworth EN, Davis GA, Wisniewski ME. 1989. The formation and distribution of ice within dormant and deacclimated peach flower buds. Plant, Cell and Environment 12: 521–528. [Google Scholar]
- Ball MC, Canny MJ, Huang CX, Heady R. 2004. Structural changes in acclimated and unacclimated Eucalyptus leaves during freezing and thawing. Functional Plant Biology 31: 29–40. [DOI] [PubMed] [Google Scholar]
- Ball MC, Wolfe J, Canny M, Hofmann M, Nicotra AB, Hughes D. 2002. Space and time dependence of temperature and freezing in evergreen leaves. Functional Plant Biology 29: 1259–1272. [DOI] [PubMed] [Google Scholar]
- Beck E, Schulze E-D, Senser M, Scheibe R. 1984. Equilibrium freezing of leaf water and extracellular ice formation in Afroalpine ‘giant rosette’ plants. Planta 162: 276–282. [DOI] [PubMed] [Google Scholar]
- Beck E, Senser W, Scheibe R, Steiger HM, Pongratz P. 1982. Frost avoidance and freezing tolerance in Afroalpine ‘giant rosette’ plants. Plant, Cell and Environment 5: 215–222. [Google Scholar]
- Ishikawa M, Sakai A. 1985. Extraorgan freezing in wintering flower buds of Cornus occidentalis Sieb. et Zucc. Plant, Cell and Environment 8: 333–338. [Google Scholar]
- Jarvis MC. 1998. Intercellular separation forces generated by intracellular pressure. Plant, Cell and Environment 21: 1307–1310. [Google Scholar]
- Jarvis MC, Briggs SPH, Knox JP. 2003. Intercellular adhesion and cell separation in plants. Plant, Cell and Environment 26: 977–989. [Google Scholar]
- Levitt J. 1972.Responses of plants to environmental stresses. New York, NY: Academic Press. [Google Scholar]
- McCully ME, Shane MW, Baker AN, Huang CX, Ling LEC, Canny MJ. 2000. The reliability of cryoSEM for the observation and quantification of xylem embolisms and quantitative analysis of xylem sap in situ Journal of Microscopy 198: 24–33. [DOI] [PubMed] [Google Scholar]
- Pearce RS. 1988. Extracellular ice and cell shape in frost-stressed leaves: a low-temperature scanning-electron-microscope study. Planta 175: 313–324. [DOI] [PubMed] [Google Scholar]
- Pearce RS. 2001. Plant freezing and damage. Annals of Botany 87: 417–424. [Google Scholar]
- Pearce RS, Ashworth EN. 1992. Cell shape and localisation in leaves of overwintering wheat during frost stress in the field. Planta 188: 324–331. [DOI] [PubMed] [Google Scholar]
- Prillieux E. 1869. Sur la formation de glaçons a l'intérieur des plantes. Annales des Sciences Naturelles 12: 125–134. [Google Scholar]
- Zhu J-J, Beck E. 1991. Water relations of Pachysandra leaves during freezing and thawing. Plant Physiology 97: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


