Abstract
• Background and Aims The objective of this research was to examine the effects of differences in light spectrum on the stomatal conductance (Gs) and dry matter production of lettuce plants grown under a day/night cycle with different spectra, and also the effects on Gs of short-term exposure to different spectra.
• Methods Lettuce (Lactuca sativa) plants were grown with 6 h dark and 18 h light under four different spectra, red–blue (RB), red–blue–green (RBG), green (GF) and white (CWF), and Gs and plant growth were measured.
• Key Results and Conclusions Conductance of plants grown for 23 d under CWF rose rapidly on illumination to a maximum in the middle of the light period, then decreased again before the dark period when it was minimal. However, the maximum was smaller in plants grown under RB, RGB and GF. This demonstrates that spectral quality during growth affects the diurnal pattern of stomatal conductance. Although Gs was smaller in plants grown under RGB than CWF, dry mass accumulation was greater, suggesting that Gs did not limit carbon assimilation under these spectral conditions. Temporarily changing the spectral quality of the plants grown for 23 d under CWF, affected stomatal responses reversibly, confirming studies on epidermal strips. This study provides new information showing that Gs is responsive to spectral quality during growth and, in the short-term, is not directly coupled to dry matter accumulation.
Key words: Lactuca sativa, lettuce, light-emitting diode (LED), light quality, stomatal conductance
INTRODUCTION
During long-term space missions, higher plant photosynthesis and transpiration could be used in artificial, controlled ecological systems to provide food for the crew as well as recycling water, and carbon dioxide and oxygen in the air (Mackowiak and Wheeler, 1996). One scenario for cultivating plants in space transit vehicles, or in settlements on a planet's surface, involves the use of electric (‘artificial’) light sources (Sager and Wheeler, 1992). Among the lighting technologies considered are light-emitting diodes (LEDs): they are small in mass and volume, have a solid-state construction, are safe (e.g. do not use an arc-discharge approach), and have a long operating life (Bula et al., 1991; Barta et al., 1992).
Before LEDs can be accepted as a light source for growing plants in space their growth and development under the light spectra produced by LEDs must be analysed (Goins, 2002). Several species of plant could be used; lettuce is favoured because of its versatility as a fresh salad crop, its adaptability to controlled-environment cultivation, and its low growth habit with a defined shoot shape (Tibbitts and Alford, 1982; Knight and Mitchell, 1988; Wheeler et al., 1994). Several studies with lettuce using LEDs have been reported (Bula et al., 1991; Hoenecke et al., 1992; Goins et al., 1998, 2001), but none measured the spectral effects on stomatal conductance, which is the focus for this study. Stomatal regulation of gas exchange by leaves is of great importance, but the long-term effects of light quality on stomata is poorly understood.
Stomatal movements can be affected by various environmental factors, including plant water status, CO2 concentration and light (Raschke, 1975). For example, bright light and low concentrations of CO2 stimulate opening, whilst high CO2 concentration even in bright light, cause closure (Scarth, 1932; Raschke, 1975). Thus light has often been suggested to exert an indirect effect on stomata via a lowering of the CO2 concentration by photosynthesis. However, both in intact leaves and in isolated epidermis (epidermal strips) stomata respond directly to light (McDonald, 2003). Stomata of leaves of Xanthium strumarium, Gossypium hirsutum, Phaseolus vulgaris and Perilla frutescens opened in light, in the absence of CO2 fixation (Sharkey and Raschke, 1981b). Sharkey and Raschke (1981a) also measured the wavelengths of light that were most effective in opening stomata in the lower epidermis of detached leaves of X. strumarium. Blue light (430–460 nm) was nearly ten times more effective than red light (630–680 nm) in producing a conductance of 0·15 mol m−2 s−1. The response spectrum of stomatal reactions to red light corresponded to that of CO2 assimilation, and inhibitors of photosynthetic electron transport eliminated the stomatal response to red light. Also, the capacity of guard-cell protoplasts to respond to blue light provided evidence for a specific light response of stomata, independent of any other component of the leaf (Zeiger and Hepler, 1977). Thus, the red-light response is caused by light absorbed by chlorophyll, but the blue-light effect is independent of photosynthesis (Karlsson, 1986; Taiz and Zeiger, 1998; Assmann and Shimazaki, 1999).
The objectives of this investigation were: (a) to characterize the pattern of stomatal conductance in lettuce plants developed under different spectral environments; and (b) to investigate the effect of changes in the spectral environment on stomatal conductance. The results will be relevant for the understanding of the responses of stomata to light spectra, and for the design of life support systems for space travel.
MATERIALS AND METHODS
Cultural conditions
Lettuce seeds (Lactuca sativa L. ‘Waldmann's Green’) were planted into plastic pots (7 cm tall, 164 mL capacity, two seeds per pot) containing horticultural vermiculite and Canadian sphagnum peat moss (Terra-Lite® Agricultural Mix, The Scotts Co., Marysville, OH, USA). Studies were done in a growth chamber (Conviron PGW-36, Pembina, ND, USA; 7·8 m3 interior plant growth volume), where 16 pots were arranged in a 0·3-m2 tray under each light treatment. Lighting treatments were systematically rotated between replications to minimize edge and position effects within the growth chamber, and pots were systematically rotated every other day. Seven days after planting (DAP), the seedlings were thinned to one plant per pot. The air temperature, relative humidity, and CO2 concentrations for all treatments were maintained at 21 ± 0·3 °C, 70 ± 4·1 % and 1200 ± 48·9 µmol mol−1 (0·12 kPa), respectively. Fresh half-strength modified Hoagland's nutrient solution (Hoagland and Arnon, 1950; Mackowiak et al., 1989) was added as needed to the bottom of each tray to supply nutrients and replenish evapotranspiration water losses.
Light treatments
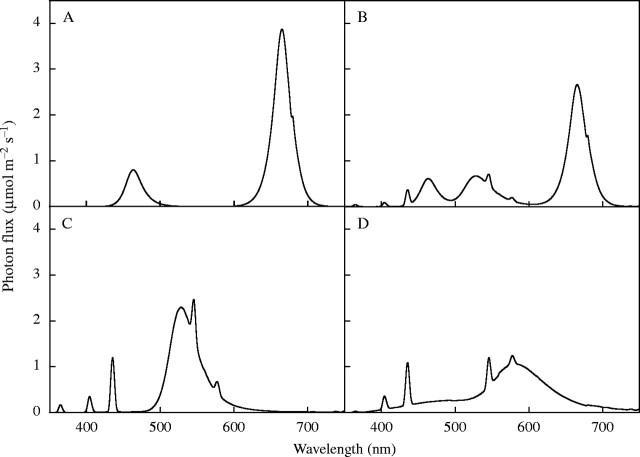
The four light sources were (1) red and blue LEDs (RB), (2) red and blue LEDs with green fluorescent lamps (RGB), (3) green fluorescent lamps (GF) and (4) cool white fluorescent lamps (CWF). Their spectra (Fig. 1) were measured from 300–1100 nm, at 2 nm steps, with a spectroradiometer (LI-1800; LI-Cor, Lincoln, NE, USA). Contributions of blue (400–500 nm), green (500–600 nm), red (600–700 nm), far-red (700–800) and total photosynthetic photon flux (PPF, 400–700 nm) were determined from bandwidth integration. From the spectrometric data for each light treatment, the yield photon flux (YPF) (Sager et al., 1988), the quantum ratios of red, far-red, blue, and the calculated amount of phytochrome in Pfr form relative to total phytochrome at photoequilibrium (Pfr/Ptotal) (Sager et al., 1988) were determined. Short-wave (280–2800 nm) and thermal long-wave (2800–50 000 nm) radiation were measured with Eppley PSP and PIR radiometers (Eppley Laboratories, Newport, RI, USA) (Table 1).
Fig. 1.
Spectral distribution of light from (A) red and blue LEDs (RB), (B) red and blue LEDs with green fluorescent lamps (RGB), (C) green fluorescent lamps (GF) and (D) cool white fluorescent lamps (CWF). Spectra were recorded at the top of the plant canopy with a spectroradiometer.
Table 1.
Spectral data for red and blue LEDs (RB), red and blue LEDs with green fluorescent lamps (RGB), green fluorescent lamps (GF) and cool white fluorescent lamps (CWF)
| Treatment |
|||||||
|---|---|---|---|---|---|---|---|
| Parameter |
RB |
RGB |
GF |
CWF |
|||
| Photon flux (µmol m−2 s−1) | |||||||
| PPF (400–700 nm) | 150 ± 7·1 | 150 ± 9·2 | 150 ± 3·6 | 150 ± 12·3 | |||
| Blue (400–500 nm) | 24 | 23 | 15 | 29 | |||
| Green (500–600 nm) | 0 | 36 | 129 | 76 | |||
| Red (600–700 nm) | 126 | 92 | 6 | 45 | |||
| Far-red (700–800 nm) | 2 | 2 | 2 | 7 | |||
| Yield photon flux* | 130 | 127 | 122 | 134 | |||
| Fraction (%) | |||||||
| PPF | 100 | 100 | 100 | 100 | |||
| Blue | 16 | 15 | 10 | 19 | |||
| Green | 0 | 24 | 86 | 51 | |||
| Red | 84 | 61 | 4 | 30 | |||
| Ratios | |||||||
| Red : far-red | 63 | 46 | 3 | 6 | |||
| Blue : red | 0·2 | 0·3 | 2·5 | 0·6 | |||
| Blue : far-red | 12 | 12 | 8 | 4 | |||
| Calculated Pfr/Ptotal* | 0·86 | 0·86 | 0·80 | 0·83 | |||
| Irradiance (W m−2) | |||||||
| 280–2800 nm | 28 | 33 | 39 | 41 | |||
| 2800–50 000 nm | 2 | 13 | 134† | 16y | |||
Spectra were recorded at the top of the plant canopy with a spectroradiometer.
Calculated according to Sager et al. (1988).
A clear Plexiglas barrier was placed under the CWF lamps but no barrier was used for GF lamps so as to maintain a PPF of 150 µmol m−2 s−1.
For RB treatments, nine LED arrays (Snap-Lite™; Quantum Devices, Inc., Barneveld, WI, USA) equipped with red gallium-aluminium-arsenide (GaAlAs) and blue gallium-nitride (GaN) LEDs were installed. Each array contained 150 red and 75 blue individual diodes. For RGB treatments, four green fluorescent lamps (F15T8/G; Interlectric Corp., Warren, PA, USA) were mounted around the nine arrays of red and blue LEDs, green light supplying 24 % to the total PPF. For GF treatments, six green fluorescent lamps (F15T8/G, Interlectric Corp., Warren, PA, USA) were used, green light providing 86 % of the total PPF. For CWF treatments, ten cool white fluorescent lamps (F15T12-CW; General Electric Co., Cleveland, OH, USA), with a 3·5-mm-thick Plexiglas heat barrier, provided 51 % of the total PPF in the green region of the spectrum. A vestibule made of black, opaque plastic prevented outside light from entering the growth area with LED arrays and fluorescent lamps.
Lighting for all treatments was 18 h photoperiod (18 h light/6 h dark) with approximately equal PPF at 150 µmol m−2 s−1 (9·7 mol m−2 d−1). Photosynthetic photon flux was measured at the top of the plant canopy with a quantum sensor (LI-190SA; LI-Cor) calibrated with a spectroradiometer (LI-1800; LI-Cor). As the plant canopies grew closer to the light banks, PPF was maintained by adjusting the height of the pots.
Plant measurements and porometry
Stomatal conductance (Gs) measurements were taken with a steady-state porometer (LI-1600; Li-COR), calibrated by the manufacturer. Four of the youngest fully expanded leaves per treatment were used for all measurements, with readings from the abaxial side of the leaves.
Lettuce grown under different light qualities
Lettuce plants were grown under RB, RGB, GF or CWF for 23 d. Four plants were harvested from each light treatment 21 DAP prior to canopy closure to minimize spectral quality changes. Measurements included leaf area, specific leaf area (SLA), shoot fresh mass (shoot FM) and shoot dry mass (shoot DM). Plant tissue samples were dried in a drying oven for 48 h at 70 °C before weighing. Canopy temperature in each light environment was measured with infrared transducers and logged (Apogee Instruments Model IRTS-P, Logan, UT, USA).
Stomatal conductance was measured 23 DAP on lettuce grown under each light treatment 0·5 h before lights came on, 0·5, 2·5, 5·5, 9, 12·5, 15·5 and 17 h into the light period, and then after 0·5 and 3 h of darkness. During dark measurements green light <1 µmol m−2 s−1 at approx. 15 cm from the lamp was provided by a green LED pen light (LI-Cor). It was assumed not to affect stomatal response (Wheeler and Tibbitts, 1986; Wheeler et al., 1999).
Lettuce exposed to different light qualities
Lettuce plants were grown under CWF for 23 d and then given 24-h exposure to RB, RGB or GF. Stomatal conductance was measured between 2·5 and 3·5 h after lights came on, before the 24-h exposure (23 DAP), after 24-h exposure (24 DAP), and 24 h after returning to the original fluorescent lighting (25 DAP).
Statistical analysis
The experiment was repeated three times with means calculated from four plants per repetition. Using 5 % as the level of significance, statistical analysis was subjected to analysis of variance followed by Duncan's multiple range tests (SPSS Inc., Chicago, IL, USA).
RESULTS
Light quality
The LEDs used in this study had narrow spectral bands (25 nm band width at half peak height) in the red and blue, in contrast to the broad spectral bands of green and cool white fluorescent lamps (Fig. 1). The lower relative weighting of the blue (400–500 nm) and red (600–700 nm), and the higher weighting of the green (500–600 nm) reduced the YPF for the green fluorescent lamps. The red to far-red ratios of the GF and CWF were 3 and 6, respectively, whereas those of RB and RGB were 63 and 46, respectively. The calculated Pfr/Ptotal values for all the treatments were ≥0·80. GF produced more long-wave radiation than other sources (Table 1), attributable to the lack of heat filter. However, this did not significantly affect the average canopy leaf temperature, which was similar between light environments (Table 2).
Table 2.
Influence of light quality on leaf area, specific leaf area (SLA), shoot fresh mass (shoot FM) and shoot dry mass (shoot DM) 21 d after planting, and canopy leaf temperature
Plant growth
Leaf area was largest in plants grown under RGB, followed by CWF, RB and then GF, whereas specific leaf area was greater under GF than RB, RGB and CWF. Shoot FM was highest in plants grown under RGB, followed by CWF, and then RB and GF. Shoot DM was highest in plants grown under RGB, followed by RB and CWF, and then GF (Table 2).
Stomatal conductance
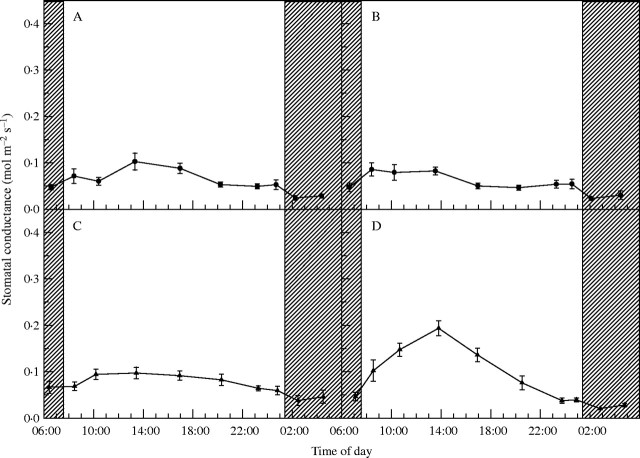
Diurnal patterns of Gs for lettuce leaves grown under different light qualities for 23 d are shown in Fig. 2: those under RB, RGB and GF changed less than under CWF. Under CWF, conductance rose rapidly when the lights came on and then peaked near the middle of the light period, after which it dropped during the late photoperiod before darkness, and was lowest during the dark period (Fig. 2D). The minimum Gs in the dark, as a percentage of the maximum conductances during the light, was 24 % for RB, 27 % for RGB, 39 % for GF and 11 % for CWF.
Fig. 2.
Stomatal conductance of lettuce leaves grown under (A) red and blue LEDs (RB), (B) red and blue LEDs with green fluorescent lamps (RGB), (C) green fluorescent lamps (GF) and (D) cool white fluorescent lamps (CWF) during an 18-h photoperiod. Sequential measurements were taken 23 d after planting. Data represent means ± s.e. of 12 measurements. Within the photoperiod, darkness is indicated by cross-hatching.
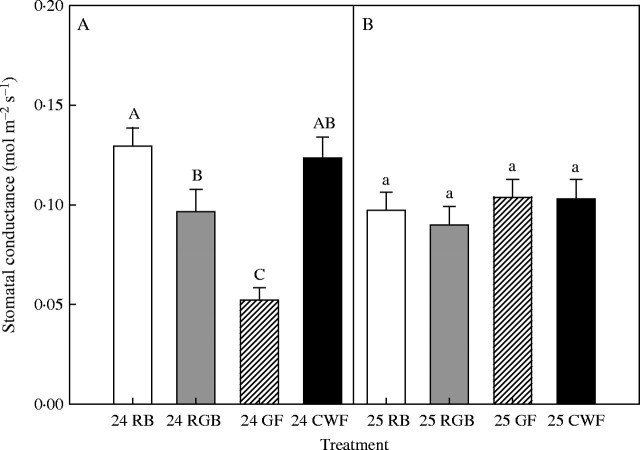
Figure 3 shows Gs of lettuce leaves grown under CWF for 23 d and then exposed for 24 h to RB, RGB or GF. Initial average Gs measured before the 24-h exposure, was 0·09 ± 0·02 mol m−2 s−1. After 24-h exposure, conductance decreased in order RB, CWF, RGB and GF, although the differences between RB and CWF and between RGB and CWF were not statistically significant. Twenty-four hours after returning plants to the fluorescent light under which they were grown, there was no significant difference between any treatments.
Fig. 3.
Stomatal conductance of lettuce leaves grown under cool white fluorescent lamps (CWF) for 23 d and then exposed for 24 h to red and blue LEDs (RB), red and blue LEDs with green fluorescent lamps (RGB) or green fluorescent lamps (GF). Measurements were taken after (A) 24-h exposure (24 DAP) and (B) 24 h after returning to the initial fluorescent lighting (25 DAP). Data represent means ± s.e. of 12 measurements.
DISCUSSION
The DM accumulation for plants grown under RB and CWF was similar. This indicated that normal growth for lettuce could be achieved with only red and blue photons. Yorio et al. (2001) reported similar results for lettuce ‘Waldmann's Green’ when plants were grown with red LEDs and blue fluorescent lamps. Hoenecke et al. (1992) suggested that a blue-photon flux between 15 and 30 µmol m−2 s−1 for 12 h each day would be acceptable for lettuce growth. In the present study, the blue photon flux was 15–29 µmol m−2 s−1, which suggested that the difference in blue photon flux among treatments was negligible.
The major difference between the spectra in the PPF region was the relative ratio of green (500–600 nm) or red (600–700 nm) light rather than that of blue (400–500 nm) light. The Pfr/Ptotal values, which provide an indicator of the expected morphogenic responses common to a wide variety of plants (Sager et al., 1988), were between 0·80 and 0·86 for all treatments. This indicates that the phytochrome photostationary state would not differ significantly between treatments, so it is unlikely that differences in the phytochrome system were responsible for the observed effects. There was very little far-red radiation in the spectra; the greatest amount was from CWF (7 µmol m−2 s−1). Hence, the differences in plant growth appeared to originate from the difference in the amount of green light rather than a change in red light.
Stomatal movement is controlled by various environmental and endogenous factors (Gorton et al., 1993; McDonald, 2003). Light is of major importance, with opening in the light and closing in the dark. However, although the growth chamber lighting in this study was either on or off, under CWF conductance increased gradually in the morning and decreased gradually later in the day. This trend was less marked under RB, RGB and GF.
A possible explanation for the lower Gs in plants grown under RB and RGB, is the relatively monochromatic light. Stomatal conductance is generally smaller with such light, compared with broad-spectrum light source such as CWF, e.g. of lettuce plants grown under red LEDs (Yorio et al., 2001) and of Beta vulgaris grown under red and blue LEDs (Goins, 2002). In addition, since leaf adaptation to spectral quality involved changes in SLA and overall leaf structure, variation in stomatal density per unit leaf area could also have contributed to differences in measured Gs (Schoch et al., 1980). In this study, SLA was greatest in plants grown under GF, i.e. they had the thinnest leaves. Consequently, photomorphogenic responses to light quality could influence overall Gs through altering light capture by leaves, without directly affecting stomatal movement.
Such modifications in leaf morphology in response to different spectral qualities during growth confound the interpretation of the spectral effects. Investigating the effects of spectral quality on stomatal conductance without the morphological modifications is possible by growing plants under the same lighting conditions before temporarily exposing the plants to different light treatments. This was done by growing lettuce plants under CWF for 23 d, then exposing them for 24 h to RB, RGB or GF, and then returning to the initial CWF lighting. The conductance was different after 24-h exposures to different spectral qualities, with maximum conductance under RB and minimum under GF. However, these effects were reversible, since Gs was the same 24 h after returning to the original CWF lighting regardless of previous treatments. It is possible that the temporary changes in spectral quality affected guard cells through photosynthesis.
There are several reports of light quality affecting stomatal movement. Light is perceived by cellular photoreceptor systems stimulating two distinct photosensory pathways. One is the transduction of photosynthetically active radiation by photosynthetic reactions in the guard cell chloroplasts, the other is induced by blue light (Gorton et al., 1993; Taiz and Zeiger, 1998). Experimentally, stomata, which have achieved their steady-state aperture under red light irradiation, open wider when exposed to additional weak blue light (Ogawa et al., 1978; Assmann, 1988). It has been suggested that zeaxanthin, a part of the xanthophyll cycle, may act as the blue light guard cell photoreceptor (Srivastava and Zeiger, 1995; Niyogi et al., 1998; Quiñones et al., 1998; Zeiger and Zhu, 1998; Frechilla et al., 1999).
After 24-h exposure, conductance in plants under RB, with no green light, was higher than under RGB, with a larger proportion of green light, and smallest under GF with the highest fraction of green and the lowest fraction of red light (86 % and 4 % of total PPF, respectively). Therefore, the large Gs under RB may have resulted from lack of green light. Green light reversal of blue-light-stimulated stomatal opening occurs in a number of species, including Vicia faba, Commelina communis, Pisum sativum, Nicotiana glauca, Arabidopsis thaliana, Nicotiana tabacum, Allium cepa and Hordeum vulgare (Frechilla et al., 2000; Talbott et al., 2002). Simultaneous exposure to equal fluxes of blue and green light resulted in approx. 50 % reversal of normal blue light opening. Complete reversal occurred when the flux of green light was approximately twice that of blue light. These results suggested that blue-green reversibility of stomatal opening is a basic photobiological property of guard cells (Talbott et al., 2002). In the present study, the experimental approach was different from Frechilla et al. (2000) and Talbott et al. (2002), who studied stomatal responses of epidermal strips; however, the stomatal responses to spectral quality were similar.
In conclusion, this investigation demonstrated that spectral quality during growth affected the pattern of diurnal stomatal conductance. However, morphological or physiological adaptations probably occurred, since DM accumulation was greatest in plants grown under RGB, although their conductance was smaller than in plants grown under CWF. The observed diurnal fluctuations in Gs caution against the single measurements of photosynthetic rates and/or Gs made during the day, when comparing treatments. In addition, temporary changes in spectral quality affected Gs, in agreement with stomatal responses of epidermal strips. The effects of the different light treatments on Gs reversed after returning to the initial light source. The effects of light spectrum observed on plant production, must be incorporated into the design of spectrally balanced LED systems for supporting plant growth, especially for very specialized applications, such as long-term space missions.
Acknowledgments
This work was performed while the first author held a National Research Council Research Associateship Award at NASA Kennedy Space Center. We thank Johnny Burrows, Larry Koss, Holly Loesel, Joey Norikane, Jessica Prenger and Charles Quincy for their support. Mention of a trade name or proprietary product does not constitute an endorsement, guarantee or warranty by National Aeronautics and Space Administration. Quantum Devices, Inc., Barneveld, WI, USA, holds a patent (No. 5,012,609) on light-emitting diodes as an illumination source for plant growth.
LITERATURE CITED
- Assmann SM. 1988. Enhancement of the stomatal response to blue light by red light, reduced intercellular concentrations of CO2 and low vapor pressure differences. Plant Physiology 87: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki K. 1999. The multisensory guard cell. Stomatal response to blue light and abscisic acid. Plant Physiology 119: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta DJ, Tibbits TW, Bula RJ, Morrow RC. 1992. Evaluation of light emitting diode characteristics for space-based plant irradiation source. Advances in Space Research 12: 141–149. [DOI] [PubMed] [Google Scholar]
- Bula RJ, Morrow RC, Tibbits TW, Barta DJ, Ignatius RW, Martin TS. 1991. Light-emitting diodes as a radiation source for plants. HortScience 26: 203–205. [PubMed] [Google Scholar]
- Frechilla S, Talbott LD, Bogomolni RA, Zeiger E. 2000. Reversal of blue light-stimulated stomatal opening by green light. Plant and Cell Physiology 41: 171–176. [DOI] [PubMed] [Google Scholar]
- Frechilla S, Zhu J, Talbott LD, Zeiger E. 1999. Stomata from npq1, a zeaxanthin-less Arabidopsis mutant, lack a specific response to blue light. Plant and Cell Physiology 40: 949–954. [DOI] [PubMed] [Google Scholar]
- Goins GD. 2002. Growth, stomatal conductance, and leaf surface temperature of Swiss chard grown under different artificial lighting technologies. Society of Automotive Engineers Technical Paper No. 2002-01-2338. [Google Scholar]
- Goins GD, Sager JC, Wheeler RM, Ruffe LM, Yorio NC. 2001. Salad crop production under different wavelengths of red light-emitting diodes (LEDs). Society of Automotive Engineers Technical Paper No. 2001-01-2422. [Google Scholar]
- Goins GD, Yorio NC, Vivenzio H. 1998. Performance of salad-type plants using lighting and nutrient delivery concepts intended for spaceflight. Journal of Aerospace 107: 284–289. [Google Scholar]
- Gorton HL, Williams WE, Assmann SM. 1993. Circadian rhythms in stomatal responsiveness to red and blue light. Plant Physiology 103: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water culture method for growing plants without soil. California Agricultural Experiment Station Circular No. 347. [Google Scholar]
- Hoenecke ME, Bula RJ, Tibbitts TW. 1992. Importance of ‘blue’ photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 27: 427–430. [PubMed] [Google Scholar]
- Karlsson PE. 1986. Blue light regulation of stomata in wheat seedlings. II. Action spectrum and search for action dichorism. Physiologia Plantarum 66: 207–210. [Google Scholar]
- Knight SL, Mitchell CA. 1988. Effects of CO2 and photosynthetic photon flux on yield, gas exchange and growth rate of Lactuca sativa L. ‘Waldmann's Green’. Journal of Experimental Botany 39: 317–328. [DOI] [PubMed] [Google Scholar]
- McDonald MS. 2003.Photobiology in higher plants. Chichester: John Wiley & Sons. [Google Scholar]
- Mackowiak CL, Wheeler RM. 1996. Growth and stomatal behavior of hydroponically cultured potato (Solanum tuberosum L.) at elevated and superelevated CO2 Journal of Plant Physiology 149: 205–210. [Google Scholar]
- Mackowiak CL, Owens LP, Hinkle CR, Prince RO. 1989. Continuous hydroponic wheat production using a recirculating system. National Aeronautics and Space Administration Technical Memorandum No. 102784. [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. 1998.Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Ishikawa H, Shimada K, Shibata K. 1978. Synergistic action of red and blue light and action spectra for malate formation in guard cells of Vicia faba L. Planta 142: 61–65. [DOI] [PubMed] [Google Scholar]
- Quiñones MA, Lu Z, Zeiger E. 1998. Genetic variation of stomatal conductance, blue light sensitivity and zeaxanthin content in guard cells of Pima cotton. Physiologia Plantarum 103: 560–566. [Google Scholar]
- Raschke K. 1975. Stomatal action. Annual Review of Plant Physiology 26: 309–340. [Google Scholar]
- Sager JC, Wheeler RM. 1992. Application of sunlight and lamps for plant irradiance in space bases. Advances in Space Research 12: 133–140. [DOI] [PubMed] [Google Scholar]
- Sager JC, Smith WO, Edwards JL, Cyr KL. 1988. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Transactions of the American Society of Agricultural Engineers 31: 1882–1889. [Google Scholar]
- Scarth GW. 1932. Mechanism of the action of light and other factors on stomatal movement. Plant Physiology 7: 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch PG, Zinsou C, Sibi M. 1980. Dependence of the stomatal index on environmental factors differentiation in leaves of Vigna sinensis L. 1. Effect of light intensity. Journal of Experimental Botany 31: 1211–1216. [Google Scholar]
- Sharkey TD, Raschke K. 1981. Effects of light quality on stomatal opening in leaves of Xanthium strumarium L. Plant Physiology 68: 1170–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K. 1981. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiology 68: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Zeiger E. 1995. The inhibitor of zeaxanthin formation, dithiothreitol, inhibits blue-light-stimulated stomatal opening in Vicia faba Planta 196: 445–449. [Google Scholar]
- Taiz L, Zeiger E. 1998.Plant physiology. Sunderland: Sinauer Associates. [Google Scholar]
- Talbott LD, Nikolova G, Ortiz A, Shmayevich I, Zeiger E. 2002. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. American Journal of Botany 89: 366–368. [DOI] [PubMed] [Google Scholar]
- Tibbitts TW, Alford DK. 1982. Controlled ecological life support system – use of higher plants. NASA Conference Publication No. 2231. [Google Scholar]
- Wheeler RM, Tibbitts TW. 1986. Growth and tuberization of potato (Solanum tuberosum L.) under continuous light. Plant Physiology 80: 801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, Mackowiak CL, Sager JC, Yorio NC, Berry WL, Knott WM. 1994. Growth and gas exchange by lettuce stands in a closed controlled environment. Journal of American Society for Horticultural Science 119: 610–615. [PubMed] [Google Scholar]
- Wheeler RM, Mackowiak CL, Yorio NC, Sager JC. 1999. Effects of CO2 on stomatal conductance: do stomata open at very high CO2 concentrations? Annals of Botany 83: 243–251. [DOI] [PubMed] [Google Scholar]
- Yorio NC, Goins GD, Kagie HR, Wheeler RM, Sager JC. 2001. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 36: 380–383. [PubMed] [Google Scholar]
- Zeiger E, Hepler PK. 1977. Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science 196: 887–889. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Zhu J. 1998. Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. Journal of Experimental Botany 49: 433–442. [Google Scholar]